Introduction
Gliomas originate from glial cells and are the most
common type of intracranial tumor, accounting for 40–60% of brain
tumors (1,2). Glioblastoma multiforme (GBM) is the most
frequently diagnosed intracranial malignant tumor in adults
(3). The prognosis for GBM patients
is invariably poor, even when the patients receive complete
surgical resection combined with chemoradiotherapy (1,4). According
to a previous study, the postoperative median survival time for GBM
patients was ≤15 months, and only 26.5% of patients survived for 2
years after diagnosis (1).
Hypoxia-induced apoptosis and angiogenesis are key
indicators for the diagnosis of GBM. A large hypoxic area in the
GBM tissue is associated with poorer prognosis in GBM patients
(5,6).
Previous studies have indicated that glioblastoma stem cells were
present in the hypoxic area and developed resistance to
chemotherapy and radiotherapy (7). By
contrast, well-differentiated glioblastoma cells were located in
the marginal and well-perfused areas of tumor tissues (8,9). Previous
studies proposed that hyperbaric oxygen stimulation prior to
chemotherapy and radiotherapy may increase the efficacy of the
treatment and improve prognosis, as hyperbaric oxygen may increase
the oxygen content of tumor tissue (10,11).
Hyperbaric oxygen stimulation of GBM patients has previously
resulted in longer median survival rates and reduced side effects
(12–15). Temozolomide (TMZ) is a novel drug for
the treatment of glioma. The cytotoxicity effects of TMZ on
chemotherapy-resistant and chemotherapy-sensitive cells increased
when the cells were also stimulated with hyperbaric oxygen
(16). In vivo, TMZ treatment
combined with hyperbaric oxygen may reduce angiogenesis, increase
apoptosis and inhibit drug resistance in tumor cells (17).
The direct effect of hyperbaric oxygen stimulation
on malignant glioma cells remains unknown. Stuhr et al
(18) proposed that hyperbaric oxygen
may inhibit the growth of subcutaneous transplanted glioma cells in
C57BL/6J mice; however, no cytological experiments were performed
and the subcutaneous transplanted gliomas were quite different from
intracranial gliomas, which originate from glia cells in
situ (19). The influence of
hyperbaric oxygen on non-tumor cells is complicated: Short exposure
to hyperbaric oxygen has been demonstrated to promote tumor cell
proliferation, while long exposure results in apoptosis and
inhibits proliferation (20). In
addition, another previous study demonstrated that hyperbaric
oxygen promoted angiogenesis by inducing oxidative stress under
physiological conditions (21). In
the present study, an intracranial transplanted glioma model in
congenic mice was constructed to investigate the direct effects of
hyperbaric oxygen stimulation on transplanted glioma cells in
vivo.
Materials and methods
Cell culture
The human glioma GL261/GL261-Luc cell line was
obtained from the Division of Cancer Treatment and Diagnosis Tumor
Repository, National Cancer Institute (NCI; Frederick, MD, USA).
This cell line was routinely cultured in RPMI 1640 medium (GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with
heat-inactivated fetal bovine serum (Gibco Life Technologies,
Carlsbad, CA, USA) in a humidified atmosphere with 5%
CO2 at 37°C.
Tumor xenograft assay
Inbred 10-week-old female congenic C57BL/6J mice
(NCI) were maintained under pathogen-free conditions. GL261-Luc
glioma cells were injected into the caudate nucleus of the right
brain hemisphere, as a 5 µl suspension of 5×105 cells
(1×108 cells/ml) in an equal volume of Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA). A total of 12 mice were
divided into experimental (n=6) and control groups (n=6) and
treated accordingly; the two groups were raised in the same
conditions, however, the mice in the experimental group were
exposed to the hyperbaric intervention process. In vivo
bioluminescence imaging (BLI) was applied to measure the tumor
volumes. This study was approved by the ethics committee of Beijing
Sanbo Brain Hospital (Beijing, China).
Hyperbaric oxygen intervention
process
Mice in the hyperbaric oxygen treatment group were
placed into an NG90-ⅢB medical hyperbaric oxygen chamber (Ningbo
Hyperbaric Oxygen Chamber Plant, Ningbo, China) and pressurized to
2.5 atmospheres at a rate of 0.015 MPa/min for 10 min. This
pressure was maintained for 60 min and decompression was performed
at the same rate for 10 min. The hyperbaric oxygen intervention
process was performed daily for 10 days.
Immunohistochemical staining
Following 10 days of exposure to the hyperbaric
oxygen intervention process, the C57BL/6J mice injected with
GL261-Luc glioma cells were sacrificed. Next, the tumors were
removed, fixed in 10% formalin for 24 h, embedded in paraffin and
sectioned at 5 µm thickness. Following rehydration, antigen
retrieval was performed by heating in EDTA (pH 8.0) for 15 minutes,
and endogenous peroxidase activity was blocked using 3% hydrogen
peroxide at 37°C for 10 min. Nonspecific binding was blocked using
3% skimmed milk for 2 h at 37°C. Rabbit anti-human polyclonal Ki-67
(1:500; cat. no. ab15580; Abcam, Cambridge, MA, USA) and rat
anti-human monoclonal CD34 (1:100; cat. no. ab8158; Abcam)
antibodies and TUNEL reagent (cat. no. ab66108; Abcam) were applied
to each slide and incubated at 37°C for 2 h. The slides were then
incubated using an Envision™ kit (Dako, Glostrup, Denmark) for 30
min following 3 washes, while 3,3′-diaminobenzidine (Sigma-Aldrich,
St. Louis, MO, USA) was used as a chromogen. The sections were
counterstained with hematoxylin (Sigma-Aldrich), dehydrated with
graded ethanol and xylene and mounted in neutral balsam (CWBio,
Inc., Beijing, China).
Flow cytometry
GL261 glioma cells (~1×106) were
harvested by 0.25% EDTA and then fixated in pre-cooled 75% ethanol
for 2 h at 4°C. Subsequently, the cells were washed twice with
phosphate-buffered saline (PBS), resuspended in 500 µl PBS with 1
µg/ml RNase A (Life Technologies, Grand Island, NY, USA) and 40
µg/ml propidium iodide (PI; Biotool, Houston, TX, USA), and then
incubated at 37°C for 30 min. An Annexin V/PI kit (Biotool) was
used to detect the externalization of phosphatidylserine in
apoptotic cells and all experiments were conducted according to the
manufacturer's instructions. The cells were immediately analyzed by
flow cytometry (BD Biosciences) with Cell Quest Lysis II software
(BD Biosciences), and 5,000 events were observed.
Statistical analysis
The data from at least three independent experiments
were analyzed using a two-tailed Student's t-test. The data are
presented as the mean ± standard error (SE), in which the SE is
presented as the error bars. P<0.05 was considered to indicate a
statistically significant difference. SPSS software package
(version 14.0; SPSS, Inc., Chicago, IL, USA) was used for data
analysis.
Results
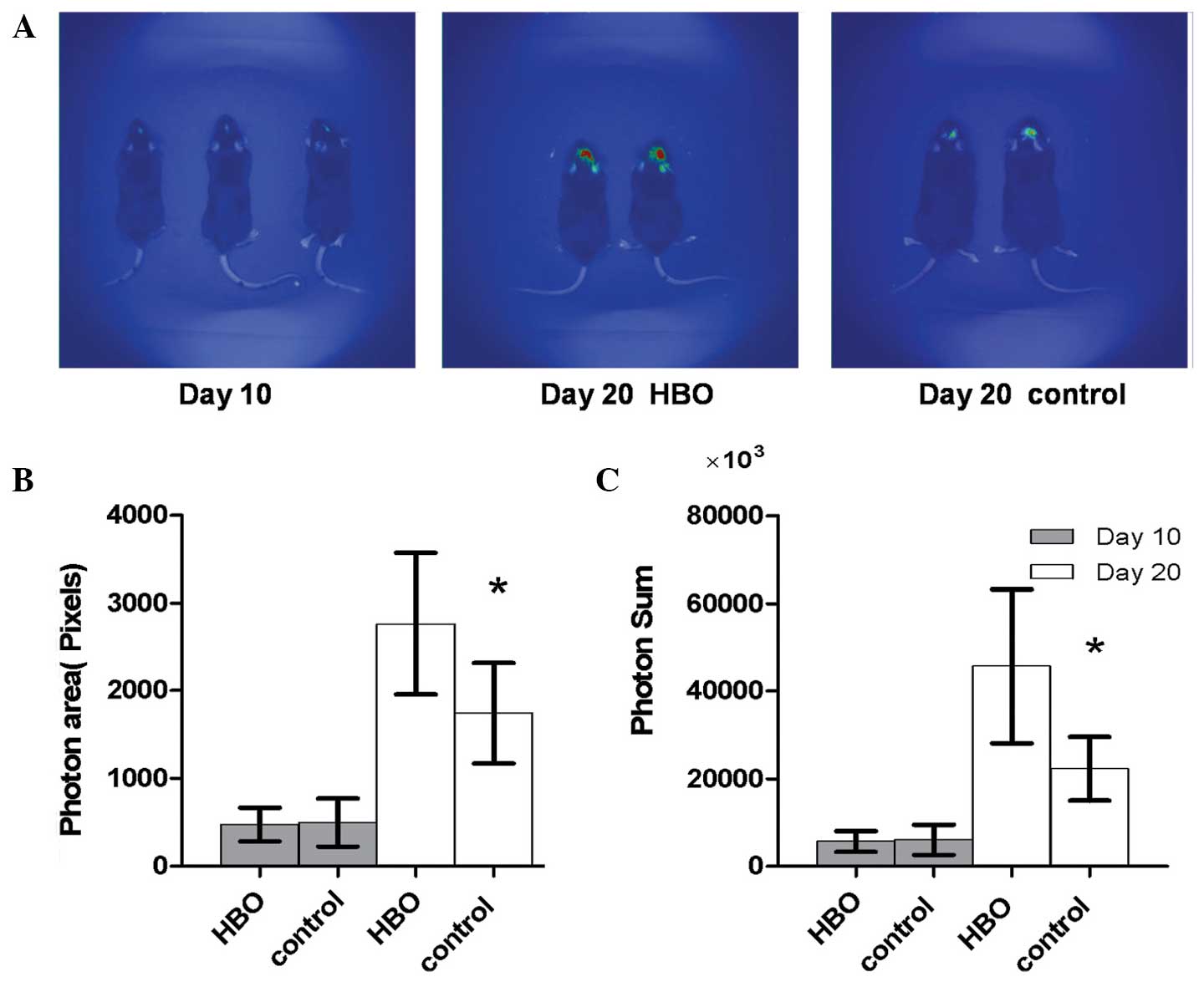
Hyperbaric oxygen promoted growth of
transplanted glioma
After 10 days of GL261-Luc inoculation, BLI was
employed to gain real-time images of the tumors in vivo
(Fig. 1A). The photon area and photon
sum were also determined using the subsidiary software. No
statistically significant difference was identified between the
light-emitting area of the hyperbaric oxygen group and the control
group (476.67±190.46 vs. 498.83±273.73; P=0.872). The difference
between the sum of emitted photons (x1,000) in the two groups was
also not statistically significant (5,677.83±2,346.48 vs.
6,069.17±3,430.37; P=0.822; Fig. 1B and
C).
To investigate the effects of hyperbaric oxygen on
intracranial glioma cells, 6 congenic C57BL/6J mice were subjected
to repeated hyperbaric oxygen stimulation at day 10 after
inoculation. After a further 10 days from the repeated hyperbaric
oxygen treatment, further real-time images were obtained in the
same congenic C57BL/6J mice (Fig.
1A). The light-emitting area of the intracranial glioma in the
hyperbaric oxygen group was significantly increased compared with
the control group (2,767.50±811.44 vs. 1,744.50±572.04; P=0.030).
The sum of emitted photons (x1,000) was also significantly
increased in the hyperbaric oxygen group compared with the control
group (45,642.50±17,613.99 vs. 22,217.83±7,273.73; P=0.013;
Fig. 1B and C).
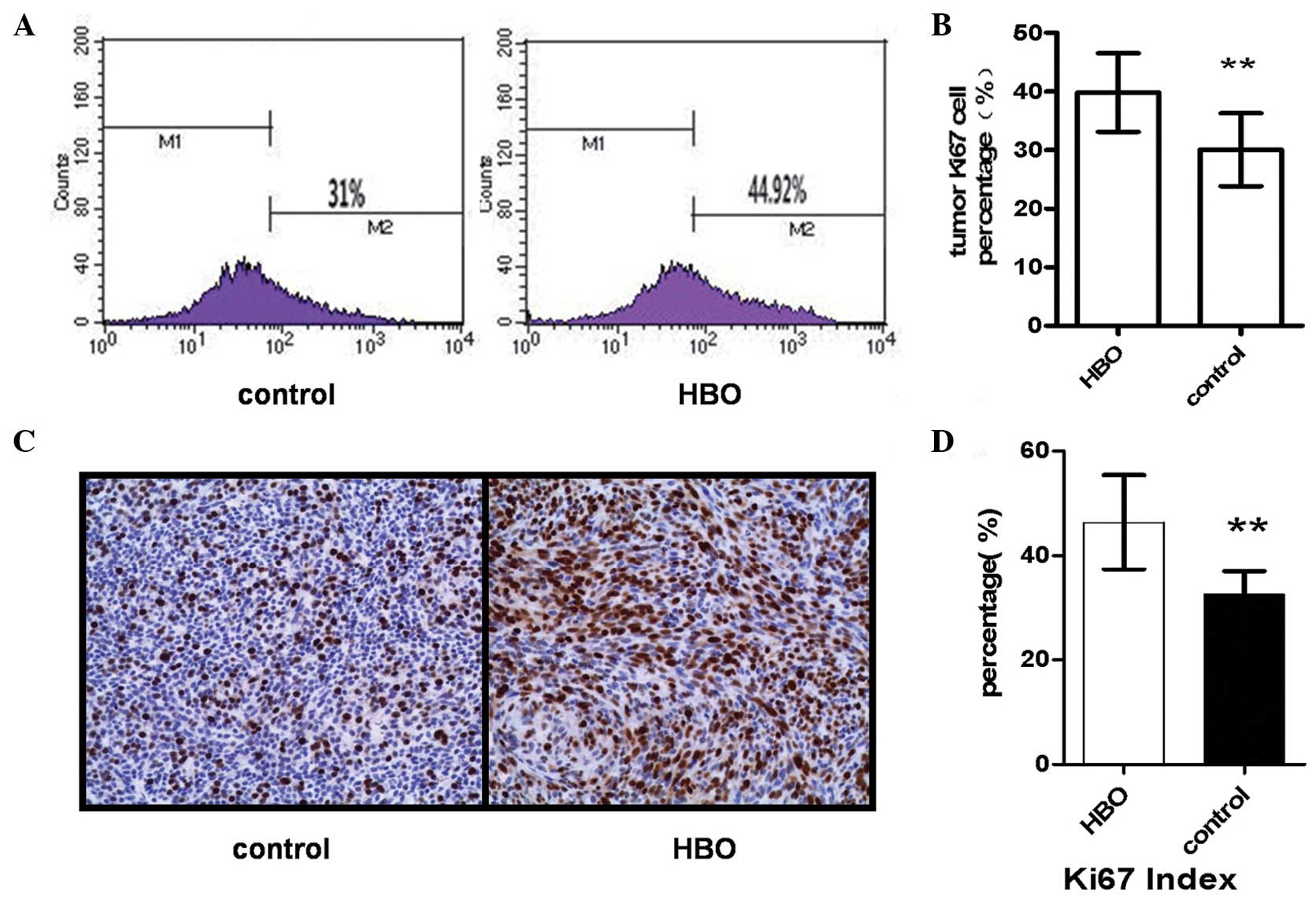
Hyperbaric oxygen promoted glioma cell
proliferation and escape from cell cycle arrest
Flow cytometric analysis indicated that the
proportion of Ki67-positive glioma cells in the hyperbaric oxygen
group was significantly increased compared with the control group
(39.82±6.69 vs. 30.06±6.22; P=0.009; Fig.
2A and B). Immunohistochemical analysis also indicated that the
percentage of Ki67-positive cells was increased in the hyperbaric
oxygen group compared with the control group (46.33±9.03 vs.
32.50±4.42; P=0.007; Fig. 2C and
D).
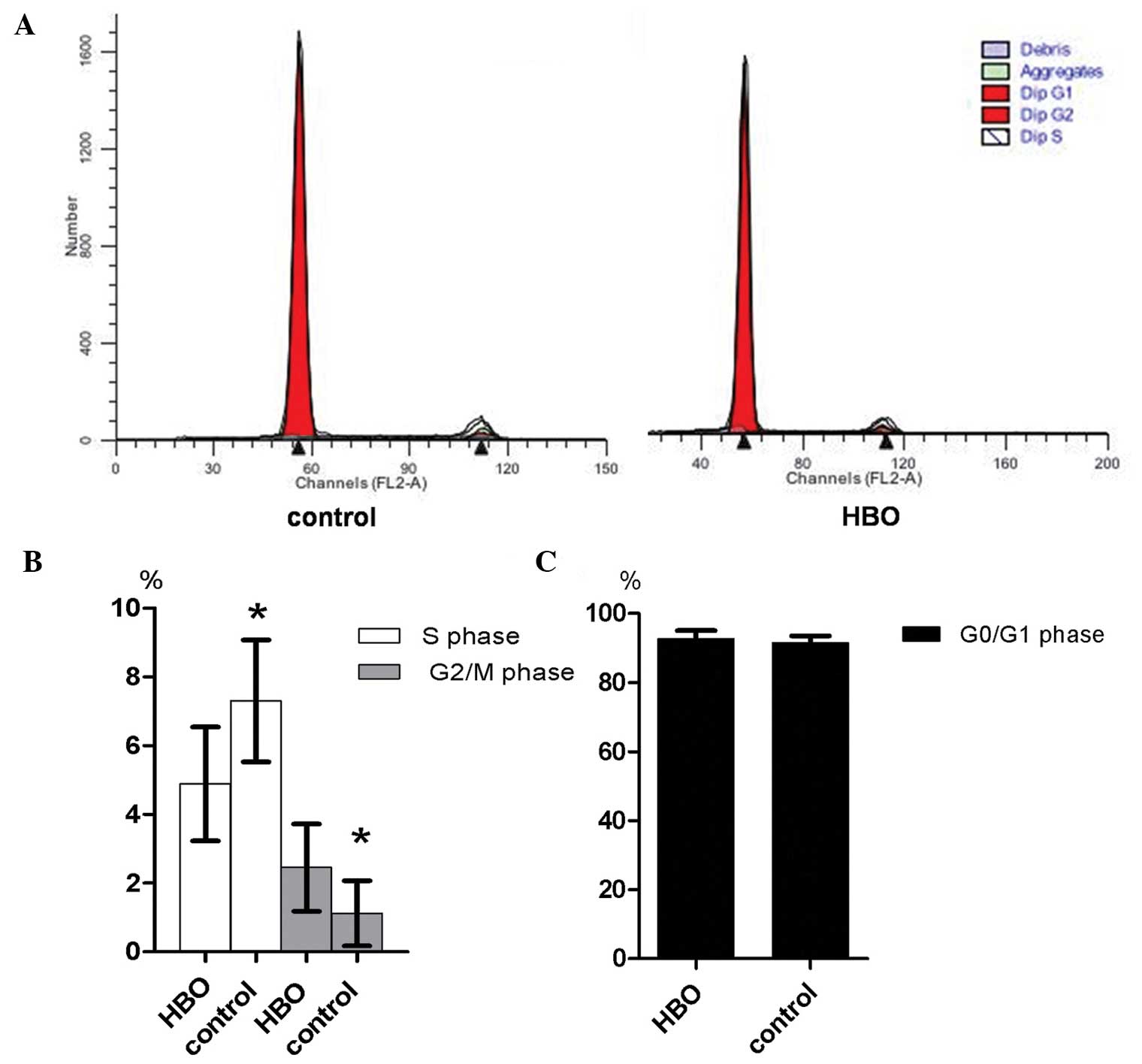
Flow cytometric analysis of the cell cycle status
demonstrated that the majority of glioma cells in the hyperbaric
oxygen and control groups were in G0/G1
phase, while a small number of cells were in G2/M phase
(Fig. 3A and B). The proportion of
glioma cells in S phase of the hyperbaric oxygen group was
significantly reduced compared with the control group (4.88±1.66
vs. 7.30±1.77; P=0.014; Fig. 3C). In
addition, the proportion of glioma cells in G2/M phase
in the hyperbaric oxygen group was significantly increased compared
with the control group (2.45±1.27 vs. 1.12±0.95; P=0.033; Fig. 3C). However, no statistically
significant difference was observed in the proportion of glioma
cells in G0/G1 phase between the two groups
(92.67±2.35 vs. 91.58±1.98; P=0.334; Fig.
3D).
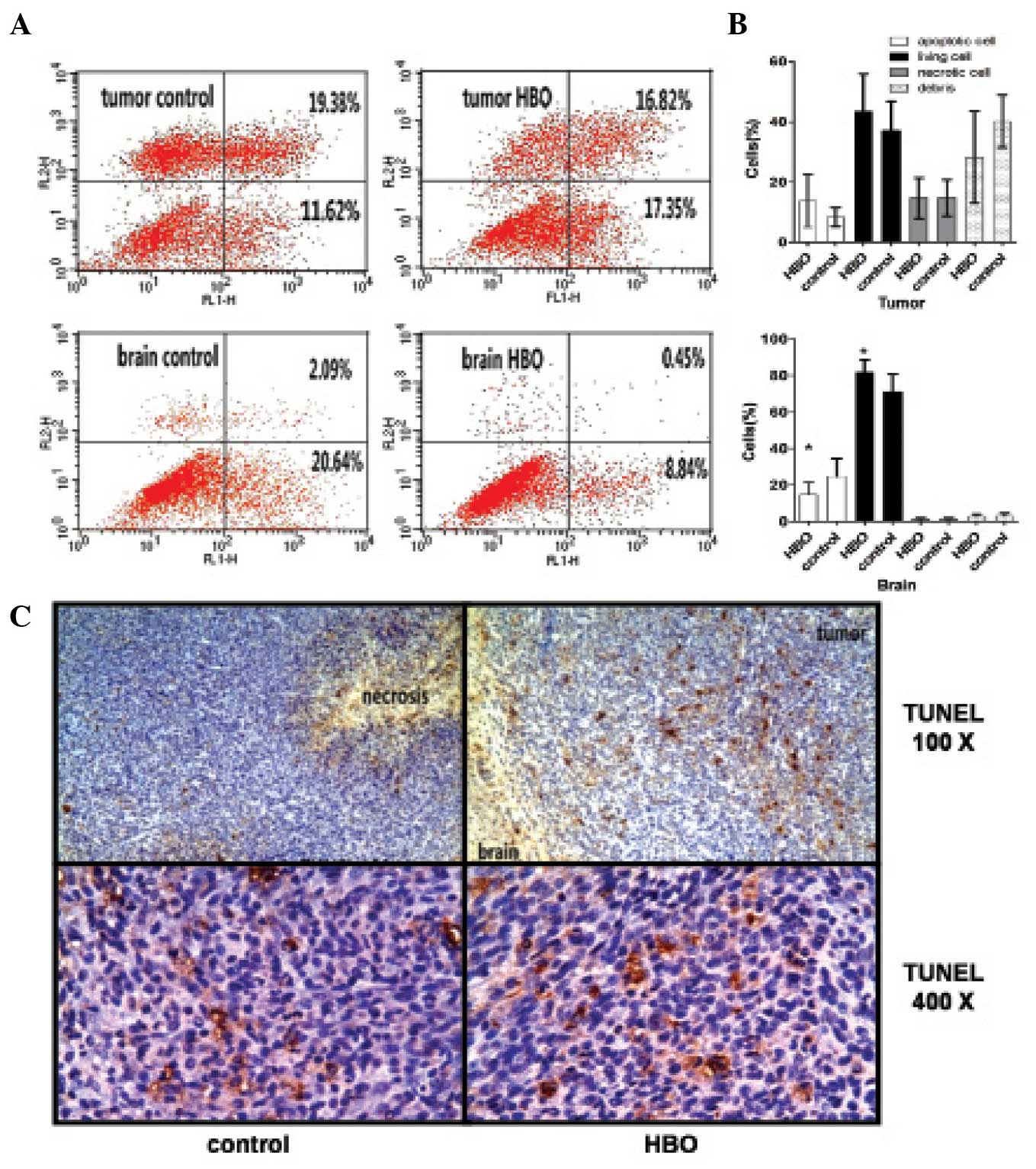
Hyperbaric oxygen inhibited the
apoptosis of glioma cells and promoted angiogenesis in transplanted
glioma
Flow cytometric analysis indicated that the
proportion of apoptotic glioma cells (Annexin V/PI double positive)
in the hyperbaric oxygen group was significantly increased compared
with the control group (13.75±8.84 vs. 8.27±3.19; P=0.023; Fig. 4A and B). By contrast, the proportion
of normal apoptotic brain cells in the hyperbaric oxygen group was
significantly reduced compared with the control group (14.70±6.73
vs. 24.63±9.66; P=0.032; Fig. 4A and
B). Immunohistochemical analysis also indicated that the number
of TUNEL-positive glioma cells in the hyperbaric oxygen group was
increased compared with the control group (Fig. 4C).
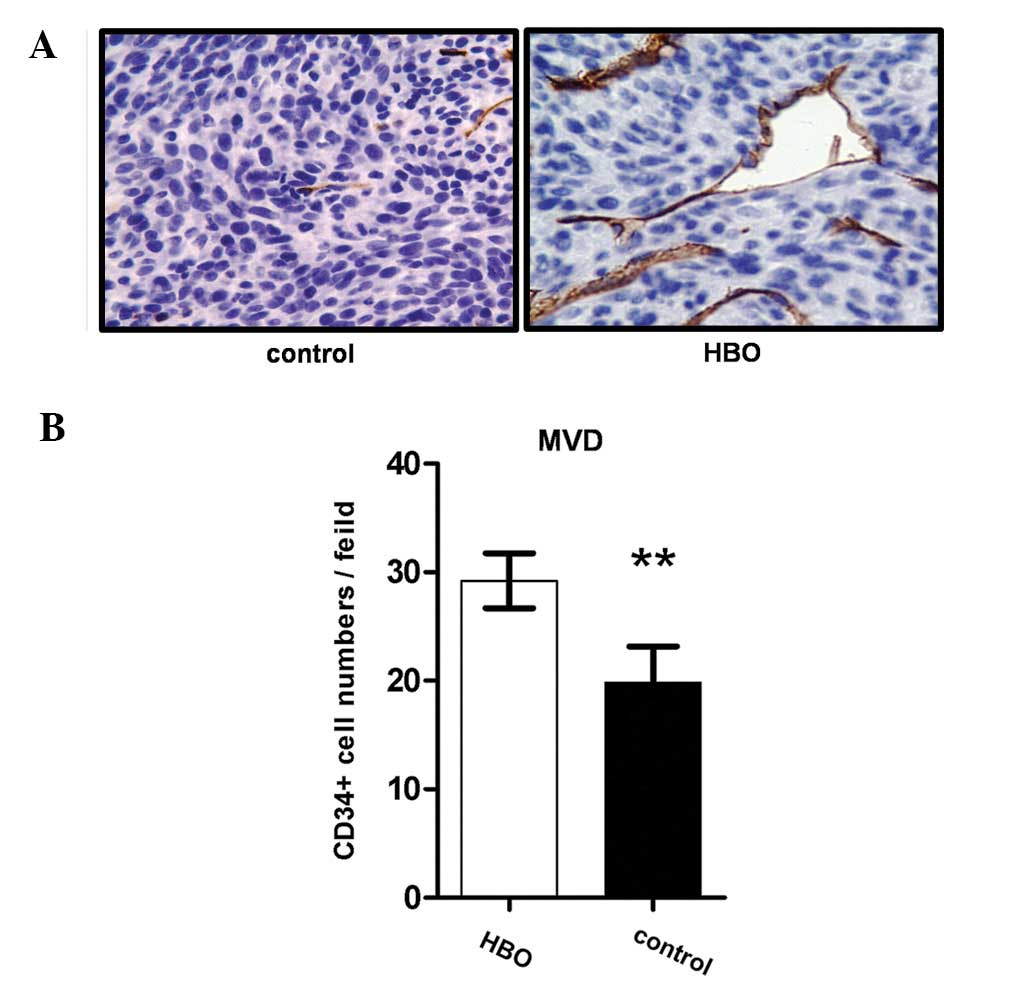
CD34 expression is associated with the angiogenesis
capacity of vascular endothelial cells. Therefore, an increase in
CD34 immunostaining was observed in the hyperbaric oxygen group
compared with the control group (Fig.
5A), indicating that the angiogenesis capacity of the vascular
endothelial cells of the transplanted glioma was increased by
hyperbaric oxygen treatment.
The present study also compared the microvessel
density (MVD) of glioma following hyperbaric oxygen treatment. The
MVD in the hyperbaric oxygen group was significantly increased
compared with the control group (9.18±2.53 vs. 19.80±3.33,
P=0.0003; Fig. 5B), which further
demonstrated that the angiogenesis capacity of vascular endothelial
cells in the transplanted glioma was increased in the hyperbaric
oxygen group.
Discussion
A number of previous studies have indicated that
combining hyperbaric oxygen treatment with chemoradiotherapy may
improve the prognosis for glioma patients and reduce complications
(13–15,22);
however, the specific mechanism by which this occurs remains
unknown. A previous study proposed that hyperbaric oxygen may
induce apoptosis in tumor cells and decrease the vascular density
in subcutaneous transplanted glioma tumors (18). Due to the blood-brain barrier,
numerous therapies that have proven effective for treating
subcutaneous transplanted gliomas, may not be effective in treating
intracranial primary gliomas (19).
Therefore, the present study produced an intracranial transplanted
glioma model in congenic mice to investigate the direct effects of
hyperbaric oxygen stimulation on glioma cells.
In vivo bioluminescent imaging (BLI) was
applied in the present study for the C57BL/6J mice tumorigenicity
assay, in order to continuously and dynamically monitor the
biological function of tumor cells in real-time, without affecting
the physiological function of C57BL/6J mice (23,24). A
previous study indicated that hyperbaric oxygen increased the
oxygen partial pressure of the intracranial tumor and this effect
remained for 40 min following hyperbaric oxygen therapy (11). To avoid the interference of oxygen
partial pressure, BLI was employed 12 h following hyperbaric oxygen
therapy. In the present study, BLI indicated that the
light-emitting area and the sum of emitted photons were increased
in mice treated with hyperbaric oxygen, indicating that hyperbaric
oxygen promoted growth of the intracranial glioma.
Ki67 is expressed in all phases of the cell cycle,
with the exception of the G0 phase, and therefore its
expression is associated with cell proliferation. The results of
immunohistochemical and flow cytometric analyses indicated that the
expression of Ki67 in tumors repeatedly exposed to hyperbaric
oxygen was increased compared with the control group. In contrast
to the subcutaneous model, hyperbaric oxygen also increased the
level of apoptosis in intracranial glioma cells in C57BL/6J mice.
The proportion of TUNEL-positive cells was ≥60% in the control
group, whereas the proportion of Ki67-positive cells was ≤10%. In
addition, in a previous study (15),
for subcutaneous glioma cells, the proportion of apoptotic and
proliferative cells was ≥60% and ≤10%, respectively, whereas in the
present study, the proportion of apoptotic cells in the
intracranial glioma was ≤20%, while the proportion of proliferative
cells was ≥30%. This difference may be due to the differences in
the surroundings of the glioma cells. Therefore, the subcutaneous
microenvironment may delay the growth of glioma cells. The
increased proportion of apoptosis was associated with an increased
capacity of self-renewal, which may have resulted from the glioma
cells adapting to ambient pressure. Furthermore, the decreased
proportion of proliferation may be associated with an adverse
influence on the growth of glioma cells.
The present study also assessed the angiogenesis of
tumor cells. Cells originating from glioma may participate in
angiogenesis; glioma cells have been observed around blood vessels
(25,26), leading to the apoptosis of vascular
cells and the destruction of vascular integrity, resulting in
angiogenesis. The increased density and morphological change of
capillaries is associated with an increased degree of malignancy
and a poorer prognosis (27,28). In the present study, the results
demonstrated that increased MVD was associated with the
proliferation of glioma cells, thus promoting angiogenesis;
however, the opposite was observed in a subcutaneous model
(18) and in breast cancer (29). The aforementioned observations
indicate that the same intervention in different microenvironments
may influence the growth of glioma cells differently (19). In addition, another previous study
indicated that MVD was an effective prognostic factor, although no
direct association was observed between MVD and therapeutic effect
(30).
In conclusion, repeated exposure to hyperbaric
oxygen promoted the proliferation and angiogenesis of intracranial
glioma cells, inhibited apoptosis and prevented cell cycle arrest.
Therefore, hyperbaric oxygen therapy may be a potentially effective
therapeutic option and may improve the prognosis for patients with
glioma.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, et al
European Organisation for Research and Treatment of Cancer Brain
Tumor and Radiotherapy Groups; National Cancer Institute of Canada
Clinical Trials Group: Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson DR and O'Neill BP: Glioblastoma
survival in the United States before and during the temozolomide
era. J Neurooncol. 107:359–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sayegh ET, Kaur G, Bloch O and Parsa AT:
Systematic review of protein biomarkers of invasive behavior in
glioblastoma. Mol Neurobiol. 49:1212–1244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chamberlain MC: Neuro-oncology: A selected
review of ASCO 2014 abstracts. CNS Oncology. 3:321–325. 2014.
View Article : Google Scholar
|
|
5
|
Sathornsumetee S, Cao Y, Marcello JE, et
al: Tumor angiogenic and hypoxic profiles predict radiographic
response and survival in malignant astrocytoma patients treated
with bevacizumab and irinotecan. J Clin Oncol. 26:271–278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jensen RL: Brain tumor hypoxia:
Tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a
therapeutic target. J Neurooncol. 92:317–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Y, Zhou Y, Shingu T, et al: Metabolic
alterations in highly tumorigenic glioblastoma cells: Preference
for hypoxia and high dependency on glycolysis. J Biol Chem.
286:32843–32853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bar EE: Glioblastoma, cancer stem cells
and hypoxia. Brain Pathol. 21:119–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Persano L, Rampazzo E, Della Puppa A,
Pistollato F and Basso G: The three-layer concentric model of
glioblastoma: Cancer stem cells, microenvironmental regulation, and
therapeutic implications. ScientificWorldJournal. 11:1829–1841.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brizel DM, Lin S, Johnson JL, Brooks J,
Dewhirst MW and Piantadosi CA: The mechanisms by which hyperbaric
oxygen and carbogen improve tumour oxygenation. Br J Cancer.
72:1120–1124. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beppu T, Kamada K, Yoshida Y, Arai H,
Ogasawara K and Ogawa A: Change of oxygen pressure in glioblastoma
tissue under various conditions. J Neurooncol. 58:47–52. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beppu T, Kamada K, Nakamura R, et al: A
phase II study of radiotherapy after hyperbaric oxygenation
combined with interferon-β and nimustine hydrochloride to treat
supratentorial malignant gliomas. J Neurooncol. 61:161–170. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kohshi K, Kinoshita Y, Imada H, et al:
Effects of radiotherapy after hyperbaric oxygenation on malignant
gliomas. Br J Cancer. 80:236–241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ogawa K, Ishiuchi S, Inoue O, et al: Phase
II trial of radiotherapy after hyperbaric oxygenation with
multiagent chemotherapy (procarbazine, nimustine, and vincristine)
for high-grade gliomas: Long-term results. Int J Radiat Oncol Biol
Phys. 82:732–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kohshi K, Yamamoto H, Nakahara A, Katoh T
and Takagi M: Fractionated stereotactic radiotherapy using gamma
unit after hyperbaric oxygenation on recurrent high-grade gliomas.
J Neurooncol. 82:297–303. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun S, Lee D, Lee NP, et al: Hyperoxia
resensitizes chemoresistant human glioblastoma cells to
temozolomide. J Neurooncol. 109:467–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu XY, Cao K, Li QY, Yuan ZC and Lu PS:
The synergistic therapeutic effect of temozolomide and hyperbaric
oxygen on glioma U251 cell lines is accompanied by alterations in
vascular endothelial growth factor and multidrug
resistance-associated protein-1 levels. J Int Med Res. 40:995–1004.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stuhr LE, Raa A, Oyan AM, et al: Hyperoxia
retards growth and induces apoptosis, changes in vascular density
and gene expression in transplanted gliomas in nude rats. J
Neurooncol. 85:191–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Biollaz G, Bernasconi L, Cretton C, et al:
Site-specific anti-tumor immunity: Differences in DC function,
TGF-β production and numbers of intratumoral Foxp3+ Treg. Eur J
Immunol. 39:1323–1333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Conconi MT, Baiguera S, Guidolin D, et al:
Effects of hyperbaric oxygen on proliferative and apoptotic
activities and reactive oxygen species generation in mouse
fibroblast 3T3/J2 cell line. J Investig Med. 51:227–232. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Milovanova TN, Bhopale VM, Sorokina EM, et
al: Hyperbaric oxygen stimulates vasculogenic stem cell growth and
differentiation in vivo. J Appl Physiol. (1985)106:711–728.
2009.PubMed/NCBI
|
|
22
|
Kohshi K, Kinoshita Y, Terashima H, Konda
N, Yokota A and Soejima T: Radiotherapy after hyperbaric
oxygenation for malignant gliomas: A pilot study. J Cancer Res Clin
Oncol. 122:676–678. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maes W, Deroose C, Reumers V, et al: In
vivo bioluminescence imaging in an experimental mouse model for
dendritic cell based immunotherapy against malignant glioma. J
Neurooncol. 91:127–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stafford P, Abdelwahab MG, Kim Y, Preul
MC, Rho JM and Scheck AC: The ketogenic diet reverses gene
expression patterns and reduces reactive oxygen species levels when
used as an adjuvant therapy for glioma. Nutr Metab (Lond). 7:74.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Calabrese C, Poppleton H, Kocak M, et al:
A perivascular niche for brain tumor stem cells. Cancer Cell.
11:69–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zagzag D, Amirnovin R, Greco MA, et al:
Vascular apoptosis and involution in gliomas precede
neovascularization: A novel concept for glioma growth and
angiogenesis. Lab Invest. 80:837–849. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deb P, Boruah D and Dutta V: Morphometric
study of microvessels in primary CNS tumors and its correlation
with tumor types and grade. Microvasc Res. 84:34–43. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leon SP, Folkerth RD and Black PM:
Microvessel density is a prognostic indicator for patients with
astroglial brain tumors. Cancer. 77:362–372. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moen I, Jevne C, Wang J, et al: Gene
expression in tumor cells and stroma in dsRed 4T1 tumors in
eGFP-expressing mice with and without enhanced oxygenation. BMC
Cancer. 12:21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Preusser M, Heinzl H, Gelpi E, et al
European Organization for Research and Treatment of Cancer Brain
Tumor Group: Histopathologic assessment of hot-spot microvessel
density and vascular patterns in glioblastoma: Poor observer
agreement limits clinical utility as prognostic factors: A
translational research project of the European Organization for
Research and Treatment of Cancer Brain Tumor Group. Cancer.
107:162–170. 2006. View Article : Google Scholar : PubMed/NCBI
|