Introduction
Multilineage dysplasia and pancytopenia as a
consequence of ineffective hematopoiesis are characteristic
features of myelodysplastic syndrome (MDS), a clonal hematopoietic
stem cell disorder. With an incidence rate of 11–17% of all MDS
cases, primary MDS associated with myelofibrosis is relatively rare
(1). The pathogenesis of the
complication of myelofibrosis in MDS remains to be elucidated. Bone
marrow hypocellularity has been demonstrated to be less common in
MDS with myelofibrosis (12%) compared with MDS without
myelofibrosis (24%) (2). As a
subgroup of MDS, MDS-refractory anemia with excess blasts
(MDS-RAEB) is usually rapidly progressive in clinical evolution.
When myelofibrosis is associated with MDS-RAEB, patients display a
poorer response to chemotherapy and a shortened survival time
(3). However, few cases have been
reported, particularly in Asia, which has limited the progress of
treatment.
Bone marrow fibrosis is variable and typically
graded by the density of reticulin and collagen fibers in marrow
replacement (4). In primary
myelofibrosis, marrow fibrosis is usually progressive and there is
increasing evidence that the bone marrow fibrosis grade has
prognostic significance (5). An
analysis of a large population of patients with primary
myelofibrosis identified risk factors that are highly predictive of
the transformation to AML at any time during the course of
myelofibrosis. Patients with such factors are candidates for more
aggressive therapeutic approaches, including allogeneic
hematopoietic stem cell transplantation (HSCT), or experimental
therapies (6). Decitabine or a FLAG
regimen consisting of fludarabine, cytarabine and
granulocyte-colony-stimulating factor (G-CSF) has been proven to be
effective in the treatment of patients with relapsed and refractory
AML, and high-risk MDS (7,8). These results lead to the use of these
agents in a relapsed patient with MDS with myelofibrosis who
underwent AML transformation, as reported in the present study. The
correlation between the extent of myelofibrosis and the progression
of MDS is also discussed.
Case report
A 28-year-old male presented to The Affiliated
Zhongshan Hospital, Sun Yat-Sen University (Zhongshan, Guangdong)
in December 2008 with malaise that had persisted for 6 months and
gingival hemorrhage that had been apparent for 1 month. An initial
hematological examination showed normocytic anemia, consisting of a
hemoglobin level of 52 g/l (normal range, 120–160 g/l), a white
blood cell count of 2.8×109/l (normal range,
4.0–10.0×109/l) and a platelet count of
46×109/l (normal range, 100–300×109/l). Serum
biochemical analysis was normal on admission and the patient did
not exhibit hepatosplenomegaly, as confirmed by physical
examination and color echography. Bone marrow aspiration revealed
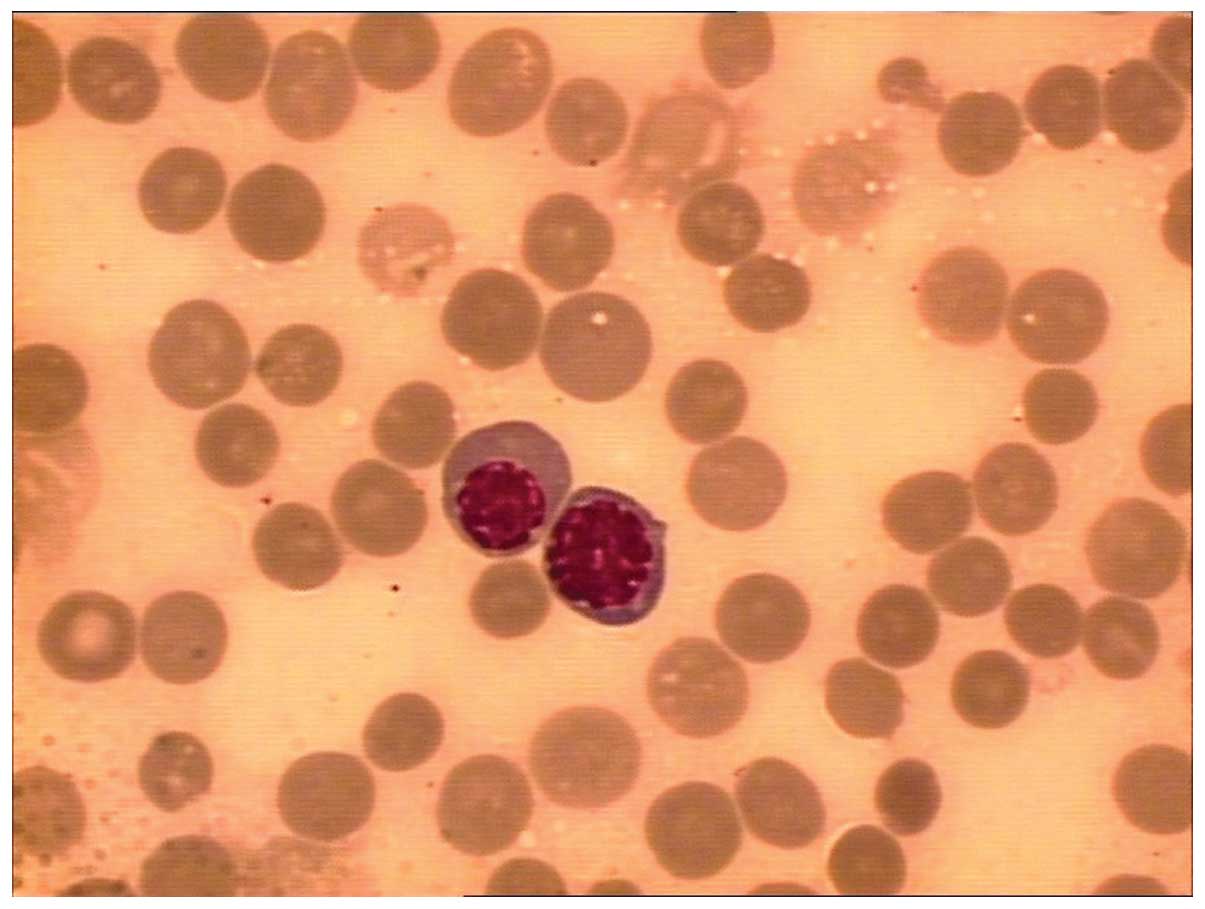
hypocellular marrow with 12% blasts (normal range, <5%; Fig. 1). Cytogenetic analysis of bone marrow
cells revealed a normal male karyotype of 46,XY. Bone marrow biopsy
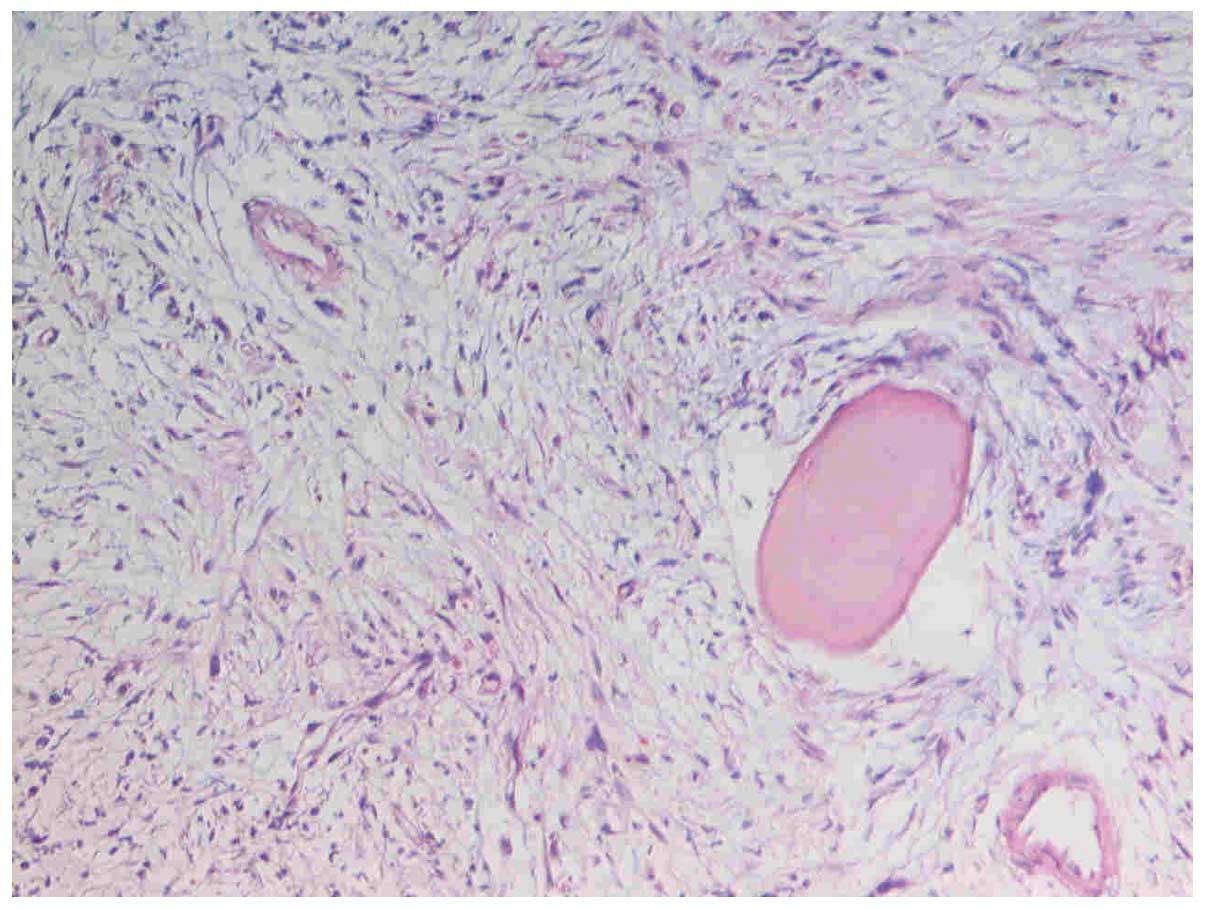
revealed prominent hypocellular marrow with grade IV reticulin
fibrosis (Fig. 2). The patient was
consequently diagnosed with MDS-RAEB associated with myelofibrosis
and started on combination chemotherapy with 8 mg mitoxantrone on
days 1–3 and 0.1 g cytarabine on days 1–5 (MA regimen). Following 2
courses of MA chemotherapy, the patient achieved hematological
complete remission with grade I reticulin fibrosis. The white blood
cell count had decreased to 1.7×109/l when the patient
was ready to received the sixth course of chemotherapy 11 months
later. Bone marrow aspiration revealed slightly hypocellular marrow
with trilineage dysplasia and 21% blasts. Furthermore, biopsy
showed normocellular marrow with no myelofibrosis. FLAG
chemotherapy was administrated as 50 mg fludarabine and 0.3 g
cytarabine on days 1–5, plus 5 µg/kg subcutaneous G-CSF daily from
day 0 until polymorphonuclear neutrophil recovery. The bone marrow
aspiration, performed on day 30 at the end of treatment, showed a
reduction in the percentage of blast cells (1.5%). The patient
achieved a second complete remission, but then relapsed again and
succumbed within the next two months.
Discussion
Differentiating between MDS and AML is dependent on
adequate bone marrow aspirates, which can normally be collected
without difficulty unless there is coexisting myelofibrosis. The
main criterion for this differentiation is the blast percentage in
the bone marrow (9). Myelofibrosis is
a relatively rare finding in primary MDS. In general, myelofibrosis
appears to occur with cytogenetic abnormalities and confer a poor
prognosis, although the recorded survival rates are associated with
the French-American-British subtype (showing a longer survival time
in patients with refractory anemia and chronic myelomonocytic
leukemia compared with those of refractory anemia with an excess of
blasts) (10). The bone marrow smears
of MDS with myelofibrosis are usually normocellular or
hypercellular. However, hypocellular marrow was found in the
present case, which was suggestive of a poor prognosis. When the
disease relapsed and transformed into AML, the survival time was
shortened markedly in clinical evolution, which suggested that
conventional treatment would no longer be effective unless
allogeneic HSCT was performed.
The treatment of MDS with myelofibrosis remains an
unresolved problem. It is the myelofibrosis that makes the
treatment more difficult due to the ease of relapse and
transformation into AML. Patients with relapsed and transformed
disease experience multi-drug resistance, a poor curative effect
and high mortality rates (11). The
synergy between the drugs in FLAG chemotherapy enhance
cytotoxicity, therefore, it is now widely used in refractory and
relapsed acute leukemia, and high-risk MDS treatment (12). In the present case, when the disease
transformed into AML, the patient was administered the FLAG regimen
and achieved remission, and the FLAG regimen showed good
tolerability. However, the second remission time with FLAG was
short, and the induced myelosuppression should also be
considered.
The reason why certain MDS patients develop
myelofibrosis is not well known. The marrow microenvironment and
various cytokines, such as platelet-derived growth factor,
calmodulin, transforming growth factor β and basic fibroblast
growth factor, as well as abnormal megakaryopoiesis, are believed
to be significant with regard to the pathogenesis (2,13). From
the therapeutical course of the present case, it was indicated that
the degree of myelofibrosis maybe not associated with the progress
of MDS. A previous study reported that the level of bone marrow
reticulin showed little correlation with the severity of the
underlying hematological disease (14). Moreover, reticulin fibrosis is often
reversible following therapeutic intervention (15). Thus, the improvement of myelofibrosis
does not reverse the transformation of MDS to AML.
In the present study, a bone marrow aspiration
revealed atypical hypocellular MDS with myelofibrosis. Although MDS
with myelofibrosis is associated with the propensity to transform
into AML and a shortened survival time, the therapeutic response
for myelofibrosis maybe not associated with the progression of MDS.
Conventional chemotherapy exhibits a limited outcome for MDS
patients with myelofibrosis, but allogeneic HSCT or other novel
agents may be of use, and require further analysis.
References
|
1
|
Fu B, Ok CY, Goswami M, Xei W, Jaso JM,
Muzzafar T, Bueso-Ramos C, Verstovsek S, Garcia-Manero G, Medeiros
LJ, et al: The clinical importance of moderate/severe bone marrow
fibrosis in patients with therapy-related myelodysplastic
syndromes. Ann Hematol. 92:1335–1343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akyay A, Olcay L, Kuzu I, Bozdoğan N,
Ünal-İnce E, İleri T, Tükün A and Yürür-Kutlay N: A child with
myelodysplastic syndrome with hypocellular fibrosis. J Pediatr
Hematol Oncol. 32:617–620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orazi A and Czader MB: Myelodysplastic
syndromes. Am J Clin Pathol. 132:290–305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Savona MR: Are we altering the natural
history of primary myelofibrosis. Leuk Res. 38:1004–1112. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lekovic D, Gotic M, Perunicic-Jovanovic M,
Vidovic A, Bogdanovic A, Jankovic G, Cokic V and Milic N:
Contribution of comorbidities and grade of bone marrow fibrosis to
the prognosis of survival in patients with primary myelofibrosis.
Med Oncol. 31:8692014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quintás-Cardama A, Kantarjian H, Pierce S,
Pierce S, Cortes J and Verstovsek S: Prognostic model to identify
patients with myelofibrosis at the highest risk of transformation
to acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 13:315–318.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saunthararajah Y: Key clinical
observations after 5-azacytidine and decitabine treatment of
myelodysplastic syndromes suggest practical solutions for better
outcomes. Hematology Am Soc Hematol Educ Program. 2013:511–521.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ossenkoppele GJ, Graveland WJ, Sonneveld
P, Daenen SM, Biesma DH, Verdonck LF, Schaafsma MR, Westveer PH,
Peters GJ, Noordhuis P, et al: The value of fludarabine in addition
to ARA-C and G-CSF in the treatment of patients with high-risk
myelodysplastic syndromes and AML in elderly patients. Blood.
103:2908–2913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Virchis A, Koh M, Rankin P, Mehta A,
Potter M, Hoffbrand AV and Prentice HG: Fludarabine, cytosine
arabinoside, granulocyte-colony stimulating factor with or without
idarubicin in the treatment of high risk acute leukaemia or
myelodysplastic syndromes. Br J Haematol. 124:26–32. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Varma N and Varma S: Proliferative
indices, cytogenetics, immunophenotye and other prognostic
parameters in myelodysplastic syndromes. Indian J Pathol Microbiol.
51:97–101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marisavljević D, Rolović Z, Cemerikić V,
Bosković D and Colović M: Myelofibrosis in primary myelodysplastic
syndromes: clinical and biological significance. Med Oncol.
21:325–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nokes TJ, Johnson S, Harvey D and
Goldstone AH: FLAG is a useful regimen for poor prognosis adult
myeloid leukaemias and myelodysplastic syndromes. Leuk Lymphoma.
27:93–101. 1997.PubMed/NCBI
|
|
13
|
Huang WH, Li MS, Chu SC, Wang TF, Kao RH
and Wu YF: Thalidomide treatment in a myelofibrosis patient with
leukemia transformation. Int J Hematol. 99:188–192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Della Porta MG and Malcovati L:
Myelodysplastic syndromes with bone marrow fibrosis. Haematologica.
96:180–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Algarni AA, Akhtari M and Fu K:
Myelodysplastic syndrome with myelofibrosis transformed to a
precursor B-cell acute lymphoblastic leukemia: A case report with
review of the literature. Case Rep Hematol.
2012:2075372012.PubMed/NCBI
|