Introduction
Sacrococcygeal teratoma (SCT) is a neoplasm that
arises in the sacrococcygeal region and is derived from multiple
primitive germ layers (1). SCT is the
most frequent congenital solid tumor encountered in fetuses and
newborns, at a prevalence rate of 1 in 20,000–40,000 births
(2) and with a female predominance of
3:1 (female:male) (3,4). The most accepted pathogenetic mechanism
of SCT is the overgrowth and migration of primitive node
multipotent cells into the sacrococcygeal region (1). Other possible mechanisms include the
non-sexual reproduction of germ cells in the gonads or in
extragonadal sites, retention of ‘wandering’ germ cells over the
migration of embryonic germ cells from the yolk sac to the gonads,
and overproliferation of other multipotent embryonic cells
(2). The majority of SCTs are cystic
and benign, but 1–2% of cases are malignant and can be squamous
cell carcinoma, adenocarcinoma, sarcoma and other malignancies
(2,5).
Use of ultrasonography, computed tomography, magnetic resonance
imaging and needle biopsy allows the differential diagnosis of SCT
prior to definitive surgery. Complete surgical resection remains
the preferred treatment modality for SCT and shows a favorable
prognosis in children and young adults (6).
The surgical treatment of older SCT patients is
rarely reported in the current literature and the long-term
treatment outcome remains unknown. Surgical resection of SCT is
normally performed in a single stage; however, the present study
describes multi-stage resection and repair in a middle-aged female
who had suffered from a giant SCT from childhood, and also presents
a literature review of adult SCT. To the best of our knowledge,
this is the first report of multi-staged surgical treatment for
giant SCT in an adult patient.
Case report
A 40-year-old female from a rural region presented
to The First Affiliated Hospital of College of Medicine (December
15, 2011, Zhejiang University, Hangzhou, Zhejiang, China) with a
30-year history of a sacrococcygeal mass. The mass had
progressively increased in size and became infected repeatedly.
Standard medical care was not originally sought due to an
underprivileged socioeconomic status. The patient's condition had
significantly worsened within the last 2 years, with symptoms of
recurrent high fever with spontaneous remission, but no involvement
of the rectum, vagina or urethra. The past medical history and
personal history were clinically insignificant.
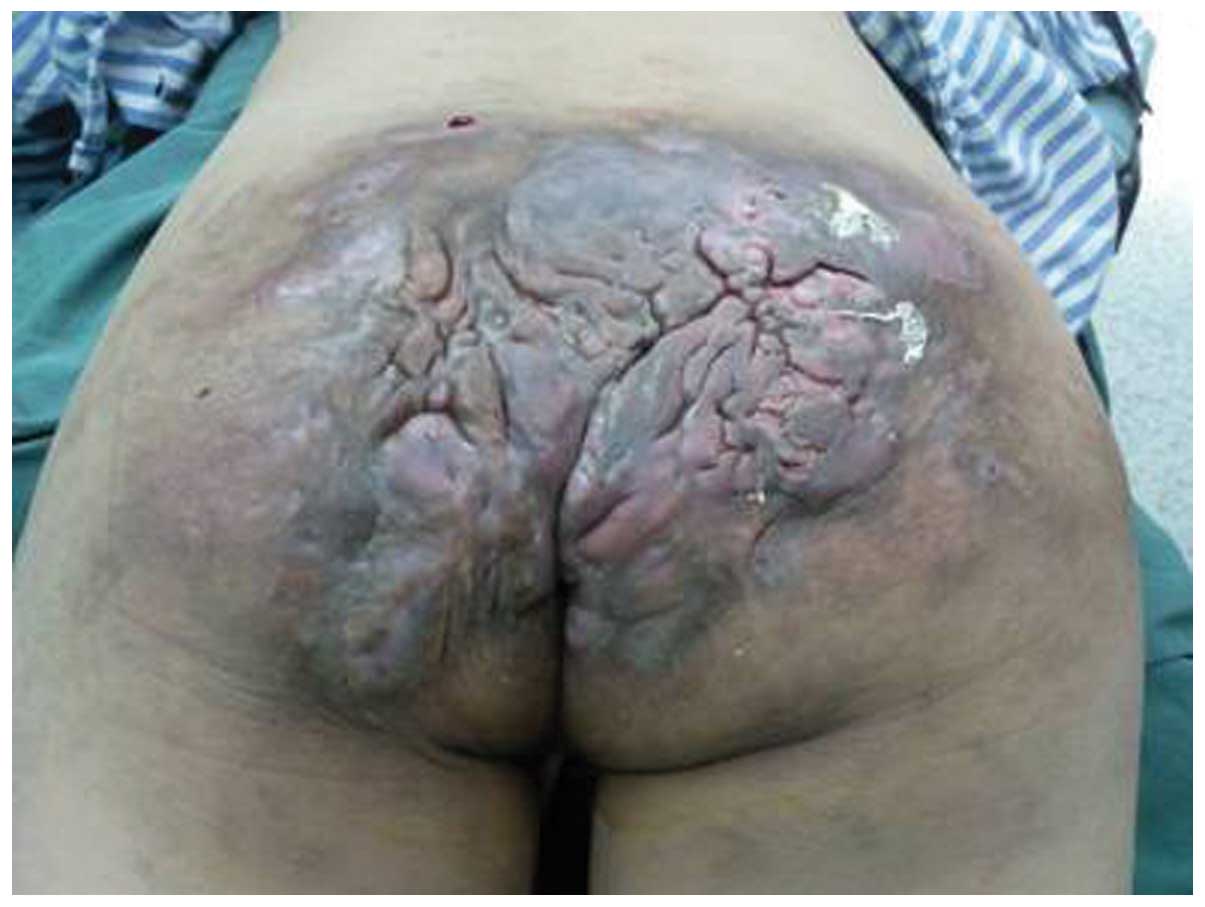
Upon physical examination, a foul-smelling,
pigmented and ulcerated mass was located in the sacrococcygeal
region, with a size of 25×15 cm (Fig.
1). Digital rectal examination identified a rigid irregular
mass palpable behind the posterior rectal wall, while a
transvaginal pelvic examination revealed negative signs.
Hematological testing showed leukocytosis with a white blood cell
count of 18×109/l (normal range, 4–10×109/l)
and a neutrophil percentage of 90% (normal range, 50–70%). Other
laboratory examination results, including clinical biochemistry and
serum tumor biomarkers, such as α-fetoprotein, carcinogenic
embryonic antigen and neuron specific enolase, were within normal
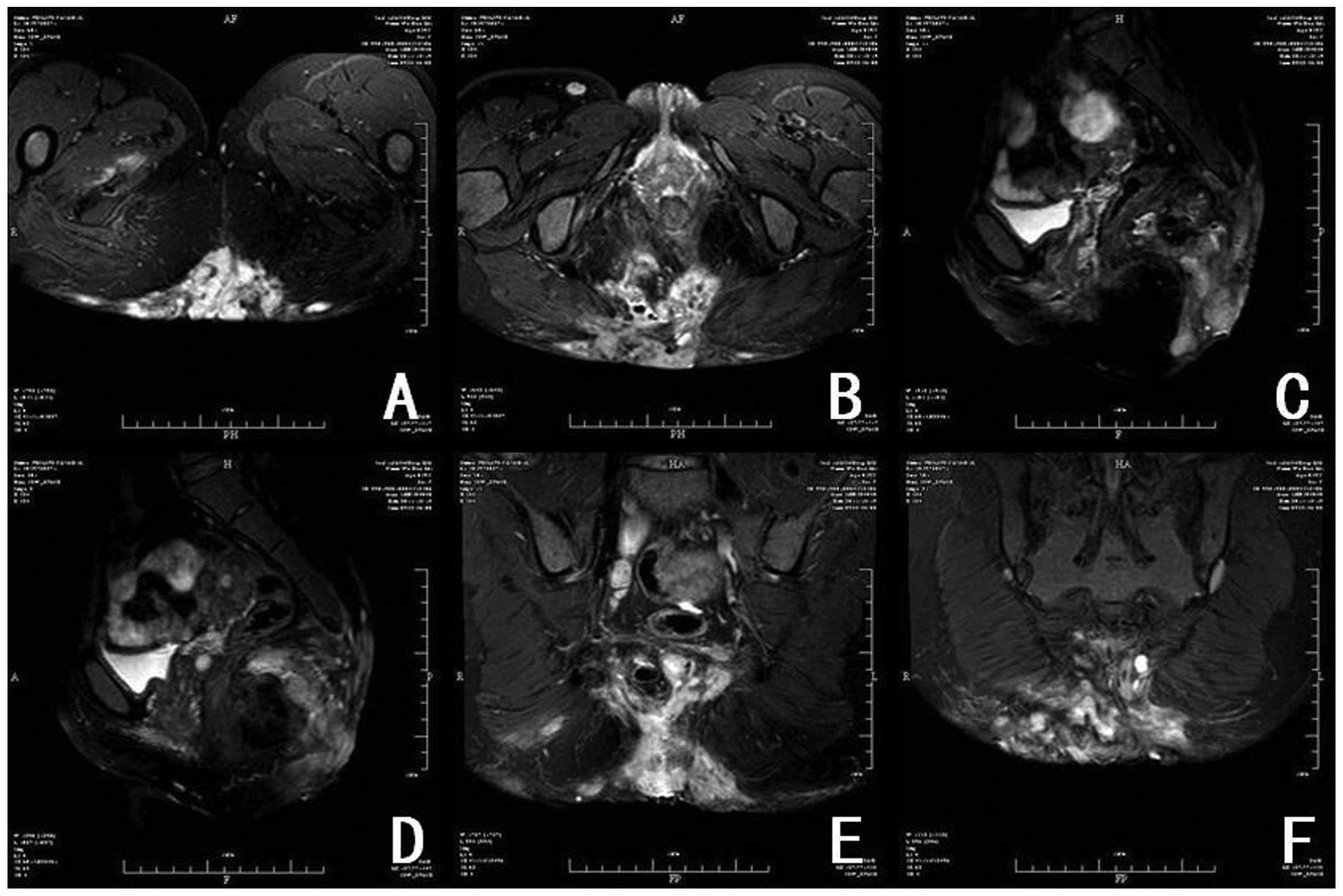
limits. Sacrococcygeal magnetic resonance imaging (MRI) revealed an
abnormal, large, high signal intensity involving the sacrococcygeal
bone and pelvic adipose space, suggesting suppurative inflammation
(Fig. 2). A pre-operative diagnosis
of a sacrococcygeal cyst with complicating hidradenitis suppurativa
was made.
Once the patient provided written informed consent,
definitive surgery was scheduled due to the presence of recurrent
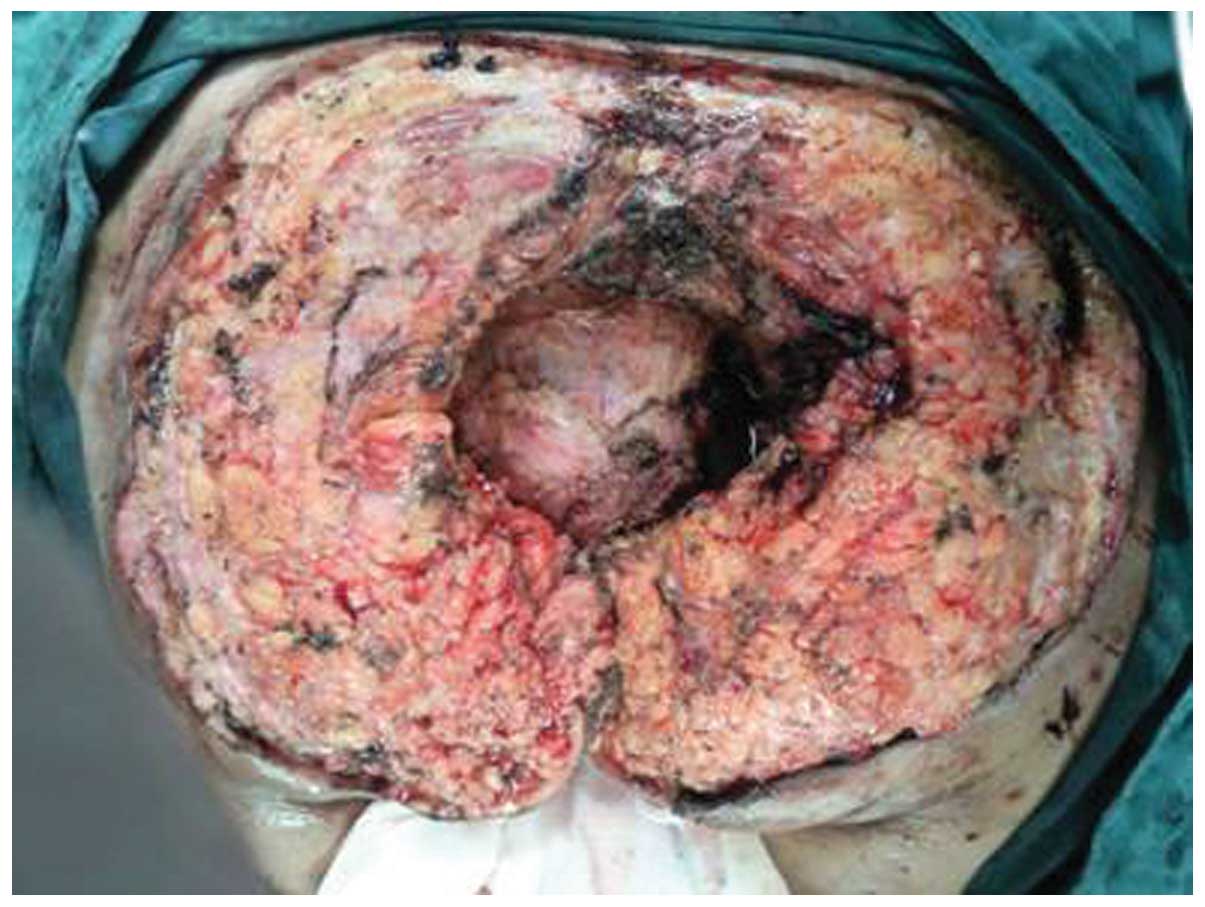
sepsis. Surgical treatment was divided into three stages (Table I). The primary stage included
prophylactic defunctioning loop ileostomy in the right lower
quadrant in case of post-operative rectal leak, and complete
resection of the sacrococcygeal cyst containing the coccyx and part
of the sacrum, with a 1-cm tumor resection margin and preservation
of the anorectum (Fig. 3). During the
surgery, the tumor was found to be extremely large, and to involve
the coccyx and part of the sacrum. The wound surface bled easily
due to the inflammation. Important adjacent structures (rectum,
anus, anal sphincter and autonomic nerve) were adequately
protected. The wound was left unclosed, and the sterile dressing
was changed on alternating days. From 1 week post-primary stage
surgery onwards, the second stage included two sessions of
debridement plus vacuum sealing drainage and two sessions of
debridement plus flap transfer. A free flap was harvested from the
left thigh, and the donor site was resurfaced using a free scalp
flap.
 | Table I.Multi-staged resection and repair of a
huge sacrococcygeal teratoma. |
Table I.
Multi-staged resection and repair of a
huge sacrococcygeal teratoma.
| Stage | Time-point | Surgery | Surgical duration,
min | Blood loss, ml | Blood
transfusion |
|---|
| Primary | 0 | Ileostomy + tumor
excision | 260 | 400 | None |
| Secondary | 1 week | Debridement +
VSD | 210 | 300 | None |
| 2 weeks | Debridement +
VSD | 140 | 200 | None |
|
| 1 months | Debridement + flap
transfer | 270 | 400 | 4.5 units RBCs |
|
| 2 months | Debridement + flap
transfer | 180 | 200 | None |
| Tertiary | 18 months | Closure of
ileostomy | 80 | 50 | None |
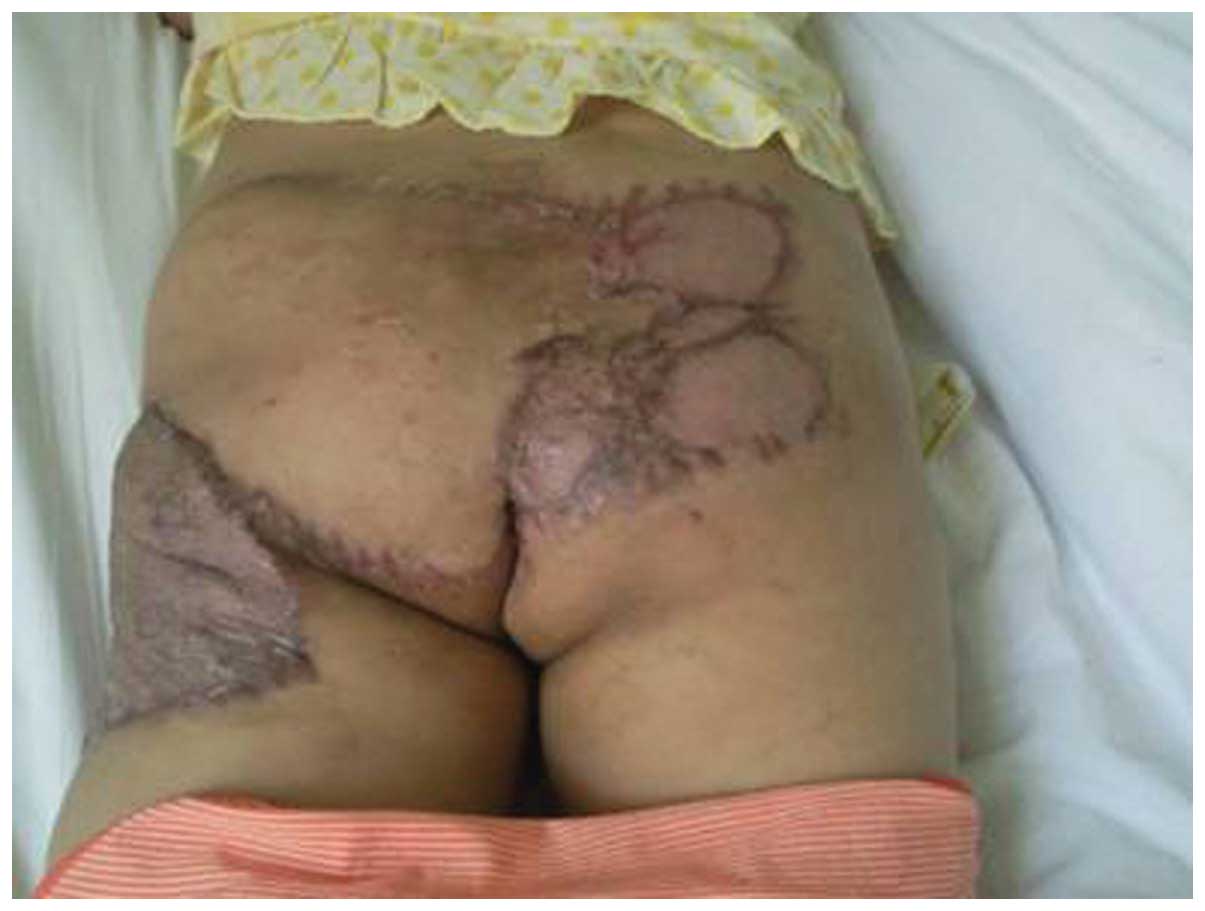
The patient was discharged 2 weeks after the last
session of flap transfer and was followed up at plastic surgery
outpatient clinics. The patient experienced two episodes of mild
skin graft infection, which were resolved after dressing care and
antimicrobial treatment. The sacrococcygeal wound healed well with
minimal scar retraction and mild skin graft ulceration and
pigmentation (Fig. 4). The third
stage was performed 18 months after the primary surgery to close
the ileostomy. The patient experienced frequent defecation, 4–6
times daily, within the first 3 months post-operatively, which was
resolved upon supplementation with a high-fiber diet and continence
care.
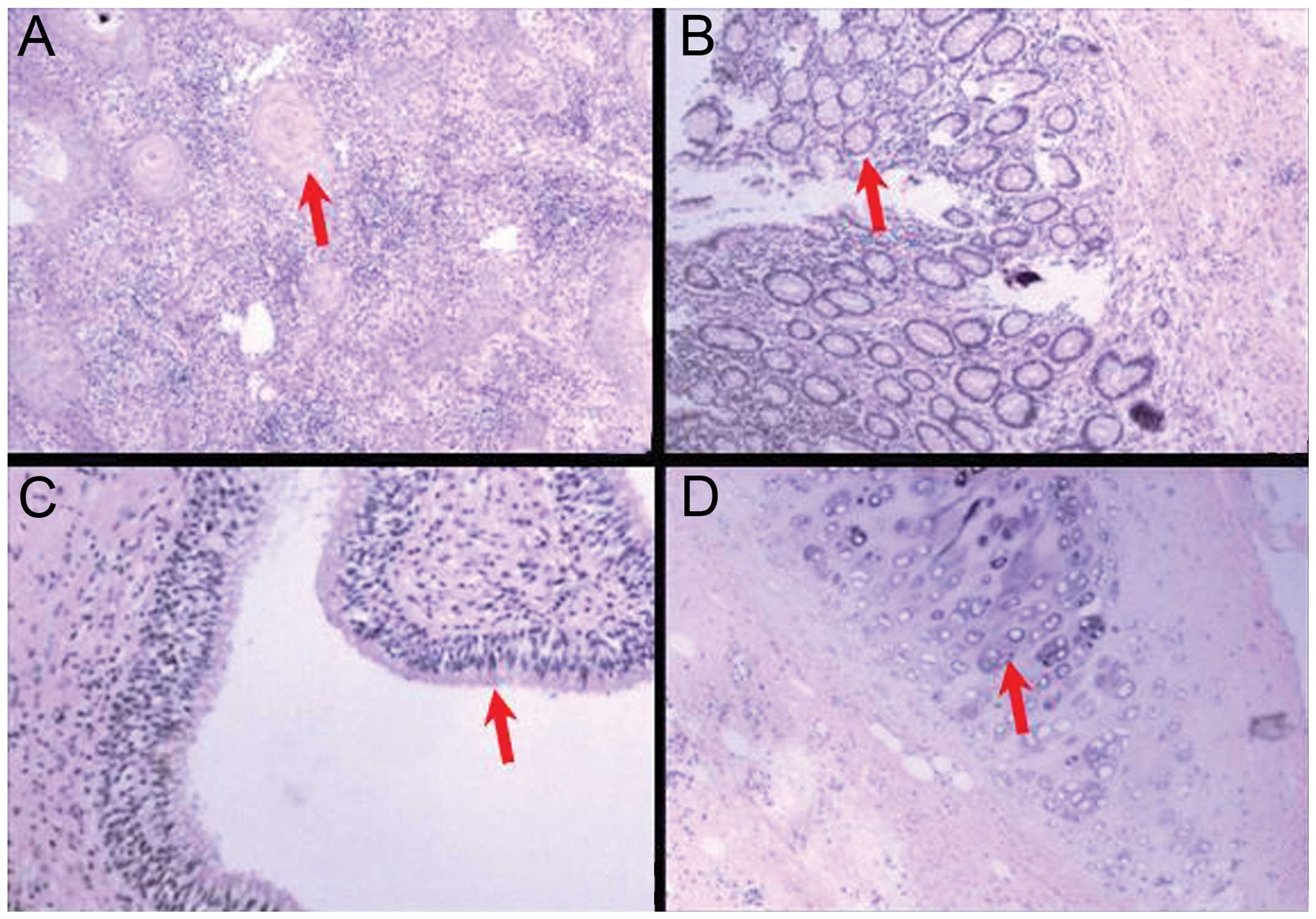
Histological analysis identified the presence of
various types of mature and differentiated tissues derived from the
ecto-, meso- and endodermal layers, consistent with the diagnosis
of mature teratoma (Fig. 5). The
patient had been followed up as scheduled at surgical outpatient
clinics for 12 months until the time of drafting this manuscript.
Follow-up anorectal manometry and defecography showed normal anal
sphincter tone, and good anal and urinary continence. The patient
complained of no clinically significant symptoms and/or signs and
reported an improved quality of life without evidence of local
recurrence after resection.
Discussion
Teratomas can occur in regions throughout the body,
including the gonads, anterior mediastinum, retroperitoneal space
and perineal area, but most frequently involve the sacrococcygeal
region in infancy (7,8). Adult SCT is only occasionally reported
in the current literature, with fewer than 120 isolated cases
(9). The Altman classification system
is normally used to describe the anatomy of SCT relative to the
body (10), as follows: Type I,
predominantly external with a minimal presacral component,
projecting from the sacrococcygeal region and distorting the
buttocks; type II, predominantly external with a significant
intrapelvic component; type III, predominantly intrapelvic with a
small external buttock mass; and type IV, also called presacral
teratoma, entirely internal without any external or buttock
component. Type III is the most common type in adults (10), but in the present case, the patient
had remained untreated since the initial occurrence as a teenager,
and the SCT was classified as type II according to pre-operative
MRI and intraoperative exploration.
Adult SCT may be asymptomatic or minimally
symptomatic on the initial presentation and may be incidentally
identified during routine physical or imaging examination in the
majority of cases. SCT-associated symptoms depend on the size and
location of the tumor, and include lower back pain, defecation or
urinary difficulty, incontinence and venous engorgement of the
lower limbs (1,10). External compression and displacement
of the vagina, uterus or rectum may also be found on pelvic and
rectal examination (1,11). If a complicating infection is present,
SCT can present as a sacrococcygeal or perineal fistula or abscess.
The SCT in the present case exhibited gradual predominant external
growth, which resulted in roughening and pigmentation of the
overlying skin (12). Complicating
septic cellulitis and osteomyelitis caused a recurrent fever.
The evaluation of tumor biomarkers, such as
α-fetoprotein, carcinoembryonic antigen and human chorionic
gonadotropin, is useful for the differential diagnosis of malignant
teratoma and the surveillance of post-operative recurrence
(1,13). However, levels of all these markers
were within the normal limits for the patient in the present case,
consistent with a 30-year history and excluding the possibility of
malignancy. Medical imaging examinations, including transvaginal
and transrectal ultrasonography, computed tomography and MRI
(1,5),
are useful for delineating the anatomical location and gross
pathology, and for determining the optimal surgical approach
(10). MRI is superior to computed
tomography in terms of specificity and accuracy for visualizing a
soft-tissue mass, such as SCT, containing abundant fat and fluid
(1,14). MRI in the present case revealed a
large retrosacral soft-tissue mass involving the sacrococcygeal
bone and accompanied by complicating suppurative inflammation.
Needle biopsy, either transrectal or percutaneous, is seldom used
for the pre-operative assessment of SCT due to the safety concerns
of tumor cell seeding and puncture site infection (1), and as use of currently available medical
imaging techniques can ensure an accurate and safe pre-operative
assessment of SCT. However, Pendlimari et al (15) suggested that pre-operative biopsy may
be useful in selected patients if neoadjuvant chemoradiation
therapy is deemed beneficial and necessary. In the present case,
the pre-operative evaluation revealed a localized sacrococcygeal
soft-tissue mass with complicating septic inflammation, for which
definitive complete excision was indicated. The differential
diagnoses consisted of other uncommon adult benign or borderline
malignant sacrococcygeal soft-tissue tumors or a congenital
malformation, including neurofibroma, a giant cell tumor of the
sacrum or a tailgut cyst (1,2,7).
Complete surgical excision remains the mainstay
definitive modality for the treatment of SCT. Early surgical
intervention normally results in a favorable long-term treatment
outcome and good quality of life. However, the definitive surgery
was delayed in the present patient due to an underprivileged
socioeconomic status. Multiple surgical approaches have been
reported depending on the anatomical classification of SCT,
including an anterior abdominal approach, a retroanal approach, the
combined access approach and the recently emerging
laparoscopic-assisted approach (10).
For SCT types II and III, the posterior trans-sacral approach with
preservation of the rectum is used most often, while an additional
abdominal incision may be required if the SCT extends into the
pelvis and the retroperitoneal space to a certain extent (7). Concomitant excision of the coccyx is
usually performed to eliminate the risk of tumor recurrence, which
is reportedly 30–40% without coccygectomy (2,9). Massive
bleeding is the major surgical morbidity for the surgical excision
of SCT; thus, meticulous dissection between the presacral fascia
and the rectal fascia aids in the prevention of excessive blood
loss and iatrogenic ureteral injuries (16). Other common surgical morbidities (31%)
include bladder dysfunction (15%), fecal incontinence (7%) and
dysesthesia (7%), as the nervous plexuses innervating the bladder
and rectum, such as the pelvic splanchnic nerves, are frequently
injured (16). Transcatheter arterial
embolization may be useful for reducing blood loss during the
excision of a large-sized tumor (2).
In the present patient, complete excision of the SCT was performed
with concomitant removal of the coccyx and part of the sacrum to
minimize the risk of tumor recurrence. Moreover, a prophylactic
ileostomy was performed to prevent rectal leakage and wound
contamination. The patient underwent successful serial surgeries
with minimal morbidities and remained free of recurrence at the
final follow-up prior to preparation of this study.
Mature SCT has an extremely low potential for
malignancy in adults, whereas immature teratoma, particularly that
containing germ cell components, is likely to be malignant and
carry a risk of recurrence. It remains unknown whether extended
resection and adjuvant chemoradiation therapy are beneficial due to
the rarity of malignant SCT and the lack of a standard management
protocol (7). It is recommended that
malignant SCT should be treated by a multidisciplinary surgical
team at an advanced oncological center.
In conclusion, SCT more often occurs in newborns and
is only rarely observed in adults, presenting as a gradually
enlarging sacrococcygeal cystic mass. The diagnosis of adult SCT
mainly depends on a combination of medical imaging examinations,
particularly high-resolution MRI; however, the incorporation of
other medical imaging techniques and tumor biomarker tests may aid
in the differentiation and exclude the possibility of malignant
SCT. Early complete excision is the preferred definitive modality
of treatment for SCT, and multi-staged excision and reconstruction
resulted in successful and safe treatment in the present case.
Mature SCT is rarely malignant in adults, and close follow-up is
essential for the detection of early recurrence.
References
|
1
|
Luk SY, Tsang YP, Chan TS, Lee TF and
Leung KC: Sacrococcygeal teratoma in adults: case report and
literature review. Hong Kong Med J. 17:417–420. 2011.PubMed/NCBI
|
|
2
|
Afuwape OO, Ogundoyin OO, Ogunlana DI and
Adeleye AO: Adult sacrococcygeal teratoma: A case report. Ghana Med
J. 43:40–42. 2009.PubMed/NCBI
|
|
3
|
Goto S, Suzumori N, Obayashi S, Ozaki Y
and Sugiura-Ogasawara M: Two cases of prenatally diagnosed
sacrococcygeal teratoma type I with different clinical features.
Congenit Anom (Kyoto). 53:92–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paramythiotis D, Papavramidis TS,
Michalopoulos A, et al: Chronic constipation due to presacral
teratoma in a 36-year-old woman: A case report. J Med Case Rep.
4:232010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Golas MM, Gunawan B, Raab BW, Füzesi L and
Lange B: Malignant transformation of an untreated congenital
sacrococcygeal teratoma: A amplification at 8q and 12p detected by
comparative genomic hybridization. Cancer Genet Cytogenet.
197:95–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okada T, Sasaki F, Cho K, et al:
Management and outcome in prenatally diagnosed sacrococcygeal
teratomas. Pediatr Int. 50:576–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ng EW, Porcu P and Loehrer PJ Sr:
Sacrococcygeal teratoma in adults: Case reports and a review of the
literature. Cancer. 86:1198–1202. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho SH, Hong SC, Lee JH, et al: Total
laparoscopic resection of primary large retroperitoneal teratoma
resembling an ovarian tumor in an adult. J Minim Invasive Gynecol.
15:384–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wishnia SC, Rosen JE, Hamid MA, Haas S and
Moreno-Ruiz N: Management of a presacral teratoma in an adult. J
Clin Oncol. 26:2586–2589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szyllo K and Lesnik N: Sacrococcygeal
teratoma - case report and review of the literature. Am J Case Rep.
14:1–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsutsui A, Nakamura T, Mitomi H, et al:
Successful laparoscopic resection of a sacrococcygeal teratoma in
an adult: Report of a case. Surg Today. 41:572–575. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roka YB, Koirala R, Bajracharya A, Shah S
and Khaniya S: Giant sacrococcygeal teratoma in an adult: Case
report. Br J Neurosurg. 23:628–629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hunter CJ, Ford HR, Estrada JJ and Stein
JE: Alpha-fetoprotein levels correlate with the pathologic grade
and surgical outcomes of pediatric retroperitoneal teratomas.
Pediatr Surg Int. 25:331–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lewis WT and Nyguen D: Radiological case
submission: Mature presacral teratoma. Mil Med. 174:214–216. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pendlimari R, Leonard D and Dozois EJ:
Rare malignant neuroendocrine transformation of a presacral
teratoma in patient with Currarino syndrome. Int J Colorectal Dis.
25:1383–1384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Xu H, Li Y, et al: Laparoscopic
resection of presacral teratomas. J Minim Invasive Gynecol.
15:649–651. 2008. View Article : Google Scholar : PubMed/NCBI
|