Introduction
Nasal mucosal malignant melanoma is clinically rare,
accounting for ~1% of all systemic malignant melanomas and 4% of
all malignant nasal tumors (1).
Malignant melanoma originates from melanoma cells, which are
derived from the neuroectoderm of the ectodermal mucosa (2). Melanoma of the mucosal membrane of the
respiratory tract and skin melanoma exhibit different clinical and
histopathological characteristics, however, the two have a similar
prognosis (2). Nasal mucosal
malignant melanoma has high local recurrence and metastasis rates,
a poor prognosis and a 5-year survival rate of 20–35%. Therefore,
it can be challenging to achieve a satisfactory curative effect
with simple surgical procedures, radiotherapy or chemotherapy
(1). The clinical data of 29 patients
with nasal mucosal malignant melanoma was obtained from the
Department of Otolaryngology Head and Neck Surgery, Tianjin Huanhu
Hospital (Tianjin, China) between October 1999 and June 2013, and
subsequently analyzed with regard to the clinical features of the
disease.
Materials and methods
Clinical data
In total, 29 patients with nasal mucosa malignant
melanoma who had received treatment at the Department of
Otolaryngology Head and Neck Surgery, Tianjin Huanhu Hospital
between October 1999 and June 2013 were included in the present
study. All cases were confirmed for the presence of nasal mucosal
malignant melanoma by pathological and immunohistochemical
analyses. Patient age, gender, location of tumor, clinical staging,
surgical therapy and adjuvant therapy were retrospectively
analyzed. The clinical characteristics are shown in Table I. This study was approved by the
ethics committee of Tianjin Huanhu Hospital and written informed
consent was obtained from all patients.
 | Table I.Effect of different clinical factors
on the survival rate of patients with nasal mucosal malignant
melanoma. |
Table I.
Effect of different clinical factors
on the survival rate of patients with nasal mucosal malignant
melanoma.
| Factors | n | 5-year survival rate,
% | P-value |
|---|
| Gender |
|
|
|
| Male | 18 | 27.8 | 1.000 |
|
Female | 11 | 27.3 |
|
| Age, years |
|
|
|
| ≤60 | 10 | 30.0 | 0.694 |
|
>60 | 19 | 26.3 |
|
| Tumor location |
|
|
|
| Nasal
septum | 7 | 71.4 | 0.008 |
| Lateral
wall of nasal cavity/paranasal sinus | 22 | 13.6 |
|
| Clinical stage |
|
|
|
|
T1+T2 | 18 | 44.4 | 0.012 |
|
T3+T4 | 11 | 0.0 |
|
| Surgical
treatment |
|
|
|
| Yes | 22 | 36.4 | 0.021 |
| No | 7 | 0.0 |
|
| Radiotherapy dose,
Gy |
|
|
|
| ≤54 | 11 | 27.3 | 1.000 |
|
>54 | 17 | 29.4 |
|
| Chemotherapy |
|
|
|
| Yes | 17 | 29.4 | 0.694 |
| No | 12 | 25.0 |
|
| Presence of black
pigmentation |
|
|
|
| Yes | 10 | 0.0 | 0.027 |
| No | 19 | 42.1 |
|
| >10 mitoses per
high-power field |
|
|
|
| Yes | 15 | 26.7 | 1.000 |
| No | 14 | 28.6 |
| Evidence of bone
invasion |
|
|
|
| Yes | 7 | 28.6 | 0.612 |
| No | 22 | 27.3 |
|
| Evidence of
necrosis |
|
|
|
| Yes | 8 | 25.0 | 1.000 |
| No | 21 | 28.6 |
|
Histopathological features
All slides were analyzed by three senior
pathologists. The pathological features that were investigated
included the presence of black pigmentation, the number of cells
undergoing mitosis per high-power field, and the presence of bone
invasion and necrosis.
Follow-up
Follow-up, with respect to survival and tumor
progression, was conducted by phone, letters and visits to the
patients.
Statistical analysis
Statistical analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The survival rate was
analyzed by the Kaplan-Meier method and log-rank test. Fisher's
exact probability was used to analyze the balance between the two
groups. P<0.05 was used to indicate a statistically significant
difference.
Results
Patients
The study group included 18 males and 11 females,
with a gender ratio of 1.6:1. The median age at diagnosis was 61.5
years (range, 49–73 years). The most common symptoms were nasal
hemorrhage (21/29) and nasal obstruction (15/29). The initial
average duration of symptoms was 33 months. The overall 3- and
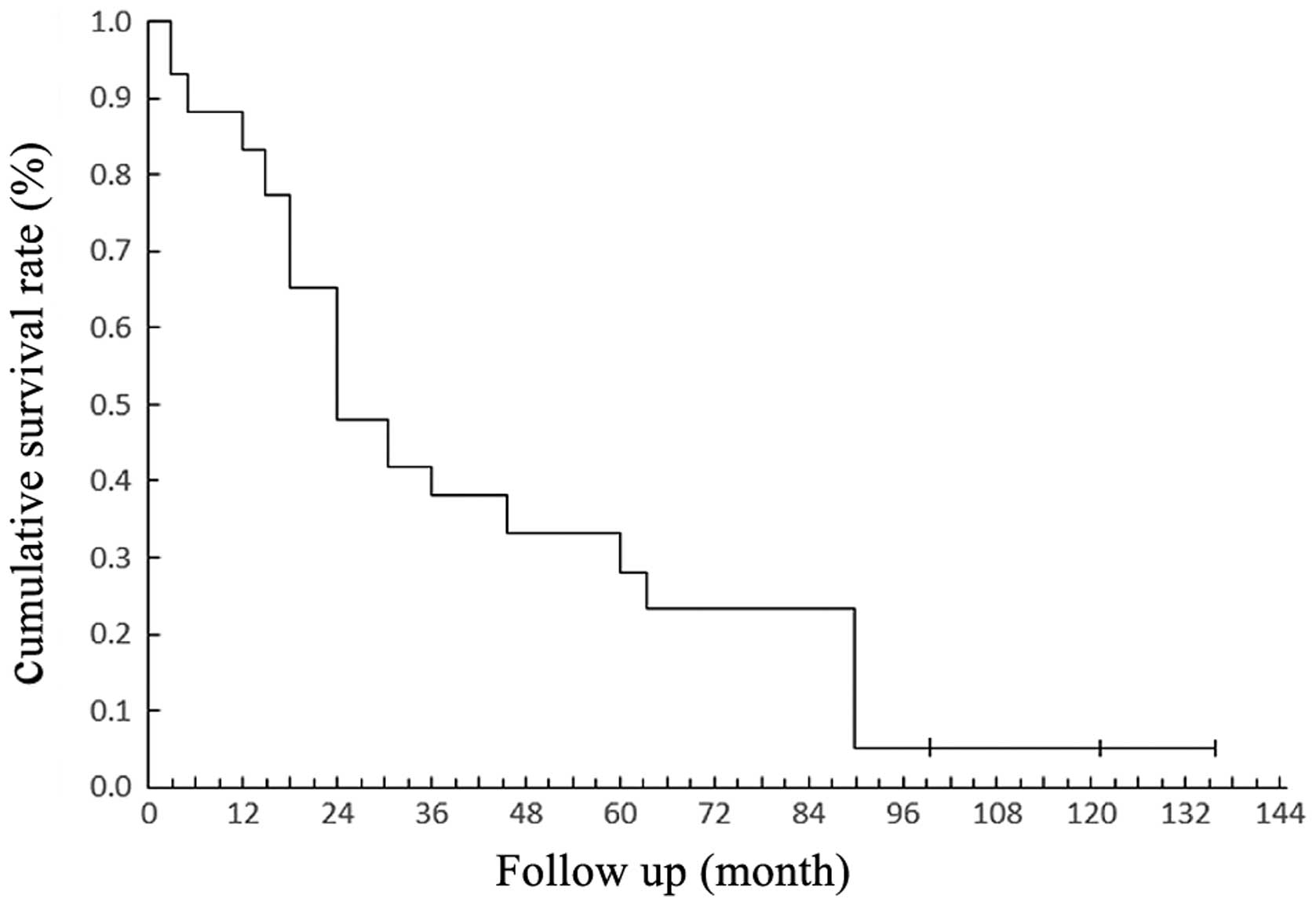
5-year survival rates were 48.3% and 27.6%, respectively (Fig. 1).
Tumor location and staging
The tumors were located in the nasal septum of 7
patients, the lateral wall of the nasal cavity in 12 patients, the
maxillary sinus in 5 patients and the ethmoid sinus in 5 patients.
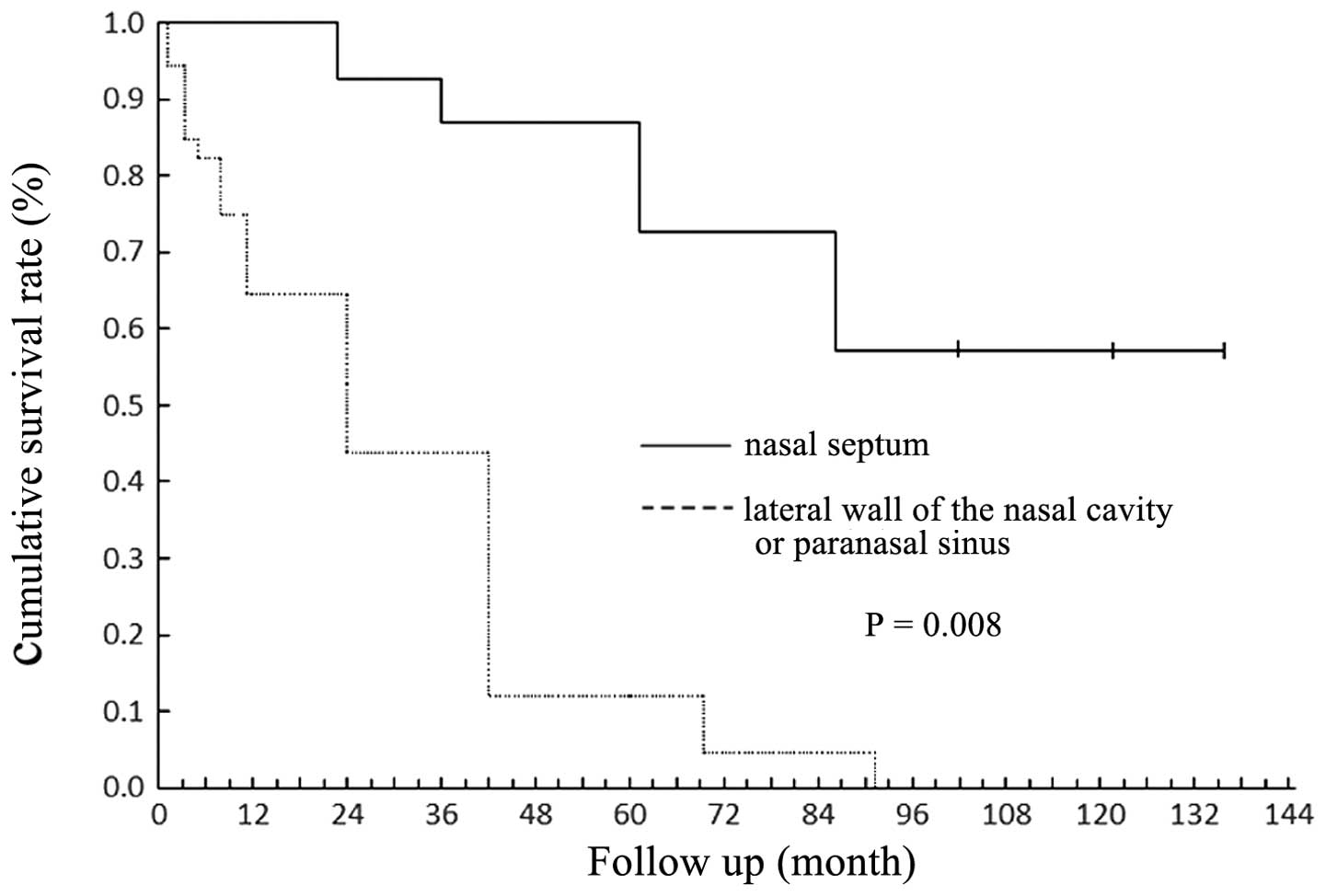
Melanomas in the nasal septum conferred a better prognosis than
those in other regions (P=0.008; Fig.
2). According to the American Joint Committee on Cancer (AJCC)
staging system (3), 28% of patients
presented with tumor stage (T)1, 34% with T2, 21% with T3 and 17%
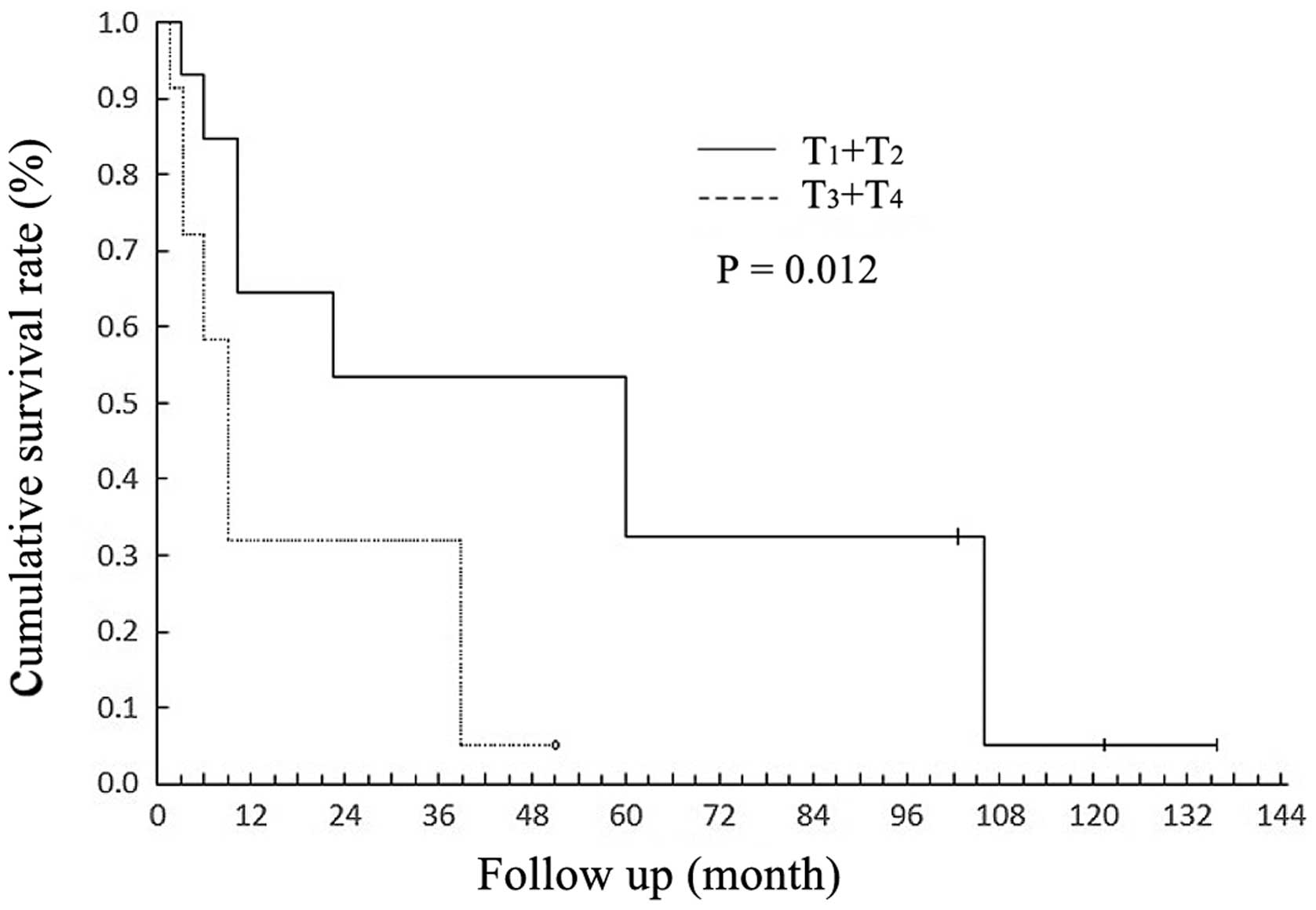
with T4. Significant differences were observed in the survival
rates of patients with T3 + T4 vs. T1 + T2 (P=0.012; Fig. 3). In total, 5 patients exhibited
distant metastasis; 2 presented with brain metastasis, 1 with bone
metastasis, 1 with lung metastasis and 1 with liver metastasis.
Treatment
Of the 29 patients, 22 underwent surgical treatment.
In total, 15 were treated by endoscopic surgery, 7 were treated by
an endoscopic-assisted approach, 5 underwent lateral rhinotomy and
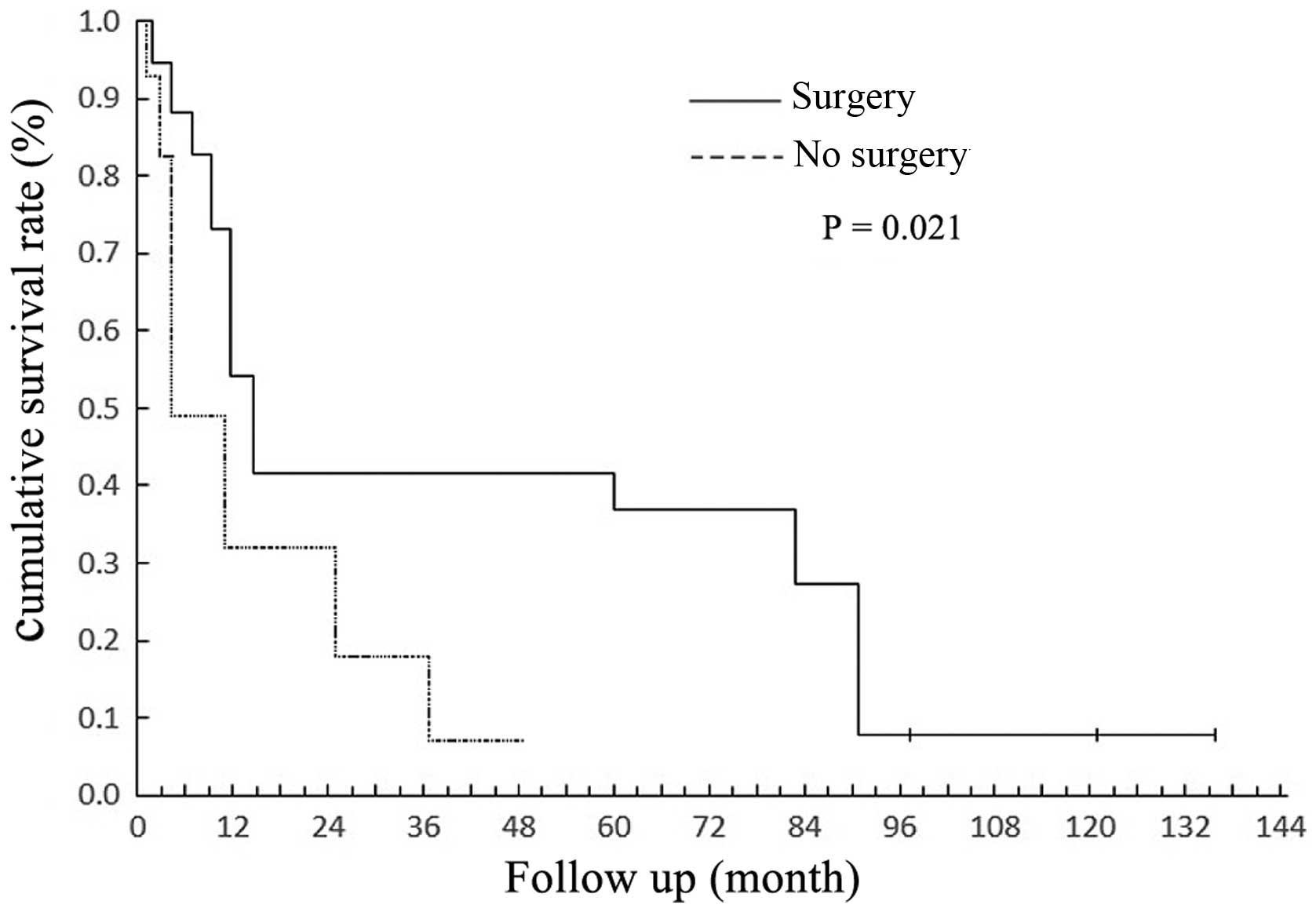
2 underwent resection of the maxilla. The 5-year survival rate of
the patients who had undergone surgery was higher than that of
those who had not received surgical intervention (P=0.021; Fig. 4).
In total, 28 patients were treated with radiation
therapy at a dose of 30–70 Gy (average, 50 Gy). Overall, 17
patients (58.6%) received a total radiation dose of >54 Gy. The
5-year survival rate of patients administered a radiation dose of
>54 Gy was not significantly improved (P=1.000). Chemotherapy
was administered to 17 of the patients. However, the results
revealed that chemotherapy treatment had no effect on the 5-year
survival rate (P=0.694; Table I).
Histopathology and follow-up
The histopathological examination revealed that
melanin pigmentation was an important indicator of prognosis
(P=0.027). However, the number of cells undergoing mitosis in each
high-power field, and the presence of bone invasion or necrosis,
had no statistical significance (P>0.05; Table I).
The patients were followed up for
5–135 months
The median follow-up period was 70.8 months. During
follow-up, 15 patients experienced local recurrence, with a
recurrence time of 0.8–63 months (average, 18.6 months). Distant
metastases occurred in 19 patients, with a transfer time of 2–60
months (average, 12.3 months).
Discussion
Malignant melanoma is a malignant tumor of
epithelial tissue origin. It has a low incidence rate, often
occuring in the skin, but rarely in the mucosa. Primary nasal
mucosal malignant melanoma originates from the dendritic
melanocytes of the nasal mucosa that develop from the embryonic
neural crest, which belongs to the neuroendocrine cell system.
Early-stage nasal mucosal malignant melanoma does not exhibit any
specific clinical manifestations, which makes early diagnosis
challenging and often results in misdiagnosis. The local recurrence
and metastasis rates are high, and the prognosis is poor. In recent
years, the diagnosis and treatment of this disease has improved due
to the widespread use of imaging techniques and endoscopy (1).
A previous study reported that 70% of malignant
melanoma patients were >60 years of age, with a male to female
ratio of 1.5:1 (4). In the present
study, 19 patients were aged 60 years, accounting for 65.5% of the
cohort, and the gender ratio was 1.6:1. The median age at diagnosis
was 61.5 years. The results of the present study indicate that
gender and age are not important factors affecting nasal mucosal
malignant melanoma patients (P=1.000 and P=0.694,
respectively).
The lateral wall of the nasal cavity and the nasal
septum are common sites for primary nasal mucosal malignant
melanoma, accounting for 41.4 and 24.1% of cases, respectively. The
study found that the prognosis of patients with melanoma of the
nasal septum was better than that of patients with melanoma of the
sinuses or nasal lateral wall (P=0.008), which was similar to the
findings of Patel et al (5).
This study suggests that the symptoms of melanoma in the nasal
septum manifest at an earlier clinical stage than that of melanoma
located at other locations, including the sinuses and nasal lateral
wall, which subsequently results in earlier diagnosis and
treatment.
In 1970, Ballantyne (6) introduced the clinical staging system for
melanoma of the skin of the head and neck. This system includes 3
stages: Stage I for localized disease, stage II for regional
disease, and stage III for distant metastasis. The prognostic value
of this system has been recognized in the literature. According to
Ballantyne's clinical staging system, primary nasal mucosal
melanoma accounted for 75.9% of the present study population.
Although Ballanyne's clinical stage can reflect the overall
survival, the majority of patients in the present study were stage
I, whereas the number of stage II and III patients was small
(24.1%), and thus, Ballanyne's clinical stage was not considered an
independent prognostic factor. The AJCC staging system provides an
even distribution of tumor stages and accurately reflects
stage-specific prognostic information, effectively reflecting the 5
year survival rate (P=0.012) in the present study. Thus, we
recommend that the current AJCC sinonasal staging system should be
used as the standard staging system for patients with nasal mucosal
malignant melanoma. This is consistent with conclusions made by the
American Anderson cancer research center study (7). Therefore, we propose that the modern
AJCC staging system may be the best melanoma staging system for
patients with nasal sinus mucosa.
The surgical treatment for nasal mucosal malignant
melanoma is complete resection of the tumor. This traditional
surgical approach involves a lateral nasal incision or resection of
the maxilla, however, due to the particular anatomical structure of
the nasal passage, complete resection of the tumor can be
challenging. Surgery is convenient for exposure and resection, but
has the disadvantages of a large amount of trauma, bleeding,
complications, post-operative scarring and a longer duration of
hospitalization (8). With the wide
application of nasal endoscopy, endoscopic resection of sinonasal
tumors has gradually developed in recent years (9). In the present study, 29 patients
underwent surgical treatment. Of those patients, 22 (75.9%)
underwent simple nasal endoscopic surgery or endoscopic-assisted
surgery. It was revealed that patients who had received surgical
treatment exhibited an improved 5-year survival rate (P=0.021).
The treatment of nasal mucosa malignant melanoma
remains controversial. However, comprehensive treatment is widely
accepted, in particular, surgery combined with radiotherapy is
considered to be the optimum treatment in order to gain local
control (10). Nasal mucosal
malignant melanoma patients are sensitive to radiation therapy, but
the radiation dose remains a point of controversy. Previous data
has revealed that adjuvant radiotherapy can reduce the local
recurrence rate of tumors, but is unable to improve the overall
survival rate for patients. Furthermore, the optimal total dose of
radiation and the distribution plan are yet to be determined
(11). A previous study found that a
high dose (>54 Gy) of irradiation improved the local control
rate of tumors and led to a lower recurrence rate (12). However, Krengli et al (13) indicated that large doses of
radiotherapy had no clear effect on tumor control or the 5-year
survival rate of patients. In the present study, 17 (58.6%)
patients received a total dose of >54 Gy. Despite this, the
5-year survival rate of these patients was not effectively improved
(P=1.000). Analysis showed that, following surgery and/or
radiotherapy local tumor control was achieved, however, due to the
high rates of local recurrence and metastasis and the poor
prognosis, radiation therapy may not improve the overall 5-year
survival rate. The present study found that patients receiving
chemotherapy did not demonstrate an improved 5-year survival rate
(P=0.694). This may be due to an insensitivity of the malignant
melanoma to the chemotherapy drugs.
In cases of head and neck skin malignant melanoma, a
risk level can be accurately determined, and the pathological
tissue can provide a theoretical basis for the histopathological
features (8). However, in cases of
nasal mucosal malignant melanoma, there are no clear factors
affecting the prognosis pathology. Previous data has established
that hyperpigmentation, mitosis, sinus bone invasion and necrosis
may affect the prognosis of tumors (14,15). The
present study found that the presence of black pigmentation was the
only pathological prognostic factor (P=0.027). By contrast, the
number of cells undergoing mitosis, and the absence of sinus bone
invasion and necrosis were not associated with patient
prognosis.
In summary, although the incidence of nasal mucosal
malignant melanoma is relatively low, the prognosis is poor. The
present study confirmed that the AJCC sinus grading system can
provide an accurate analysis of the prognosis of patients with
malignant melanoma of the nasal mucosa. Surgery is the main
treatment for such patients, and although the optimal dose of
irradiation and its distribution scheme are yet to be determined,
auxiliary radiotherapy remains the main or palliative treatment.
The presence of black pigmentation may be a pathological prognostic
factor. However, due to the small number of cases in the present
study, future clinical studies with large sample sizes, randomized
grouping and long-term follow-up periods are required in order to
validate these findings.
References
|
1
|
Manolidis S and Donald PJ: Malignant
mucosal melanoma of the head and neck: review of the literature and
report of 14 patients. Cancer. 80:1373–1386. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prasad ML, Busam KJ, Patel SG,
Hoshaw-Woodard S, Shah JP and Huvos AG: Clinicopathologic
differences in malignant melanoma arising in oral squamous and
sinonasal respiratory mucosa of the upper aerodigestive tract. Arch
Pathol Lab Med. 127:997–1002. 2003.PubMed/NCBI
|
|
3
|
Greene FL, Page DL, Fleming ID, Fritz A,
Balch CM and Haller DG: AJCC Cancer Staging Manual. 6th.
Springer-Verlag; New York, NY: pp. 59–67. 2002
|
|
4
|
Dauer EH, Lewis JE, Rohlinger AL, Weaver
AL and Olsen KD: Sinonasal melanoma: a clinicopathologic review of
61 cases. Otolaryngol Head Neck Surg. 138:347–352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel SG, Prasad ML, Escrig M, et al:
Primary mucosal malignant melanoma of the head and neck. Head Neck.
24:247–257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ballantyne AJ: Malignant melanoma of the
skin of the head and neck. An analysis of 405 cases. Am J Surg.
120:425–431. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moreno MA, Roberts DB, Kupferman ME, et
al: Mucosal melanoma of the nose and paranasal sinuses, a
contemporary experience from the M. D. Anderson Cancer Center.
Cancer. 116:2215–2223. 2010.PubMed/NCBI
|
|
8
|
Bachar G, Loh KS, O'Sullivan B, et al:
Mucosal melanomas of the head and neck: experience of the Princess
Margaret Hospital. Head Neck. 30:1325–1331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Penel N, Mallet Y, Mirabel X, Van JT and
Lefebvre JL: Primary mucosal melanoma of head and neck: prognostic
value of clear margins. Laryngoscope. 116:993–995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Owens JM, Roberts DB and Myers JN: The
role of postoperative adjuvant radiation therapy in the treatment
of mucosal melanomas of the head and neck region. Arch Otolaryngol
Head Neck Surg. 129:864–868. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Temam S, Mamelle G, Marandas P, et al:
Postoperative radiotherapy for primary mucosal melanoma of the head
and neck. Cancer. 103:313–319. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wada H, Nemoto K, Ogawa Y, et al: A
multi-institutional retrospective analysis of external radiotherapy
for mucosal melanoma of the head and neck in Northern Japan. Int J
Radiat Oncol Biol Phys. 59:495–500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krengli M, Masini L, Kaanders JH, et al:
Radiotherapy in the treatment of mucosal melanoma of the upper
aerodigestive tract: analysis of 74 cases. A Rare Cancer Network
study. Int J Radiat Oncol Biol Phys. 65:751–759. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McLean N, Tighiouart M and Muller S:
Primary mucosal melanoma of the head and neck. Comparison of
clinical presentation and histopathologic features of oral and
sinonasal melanoma. Oral Oncol. 44:1039–1046. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thompson LD, Wieneke JA and Miettinen M:
Sinonasal tract and nasopharyngeal melanomas: a clinicopathologic
study of 115 cases with a proposed staging system. Am J Surg
Pathol. 27:594–611. 2003. View Article : Google Scholar : PubMed/NCBI
|