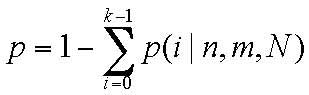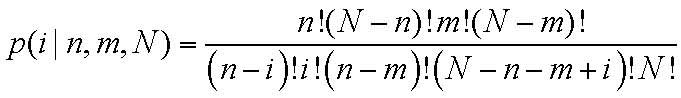Introduction
Gastric cancer undergoes genetic and epigenetic
alterations during its progression, and is the fourth most common
malignancy and the second leading cause of cancer mortality
worldwide (1,2). Surgical intervention remains as the
preferred treatment for gastric cancer; however, even with
intervention, the 5-year survival rate is only ~40% (3). Cisplatin-based chemotherapy is currently
one of the most frequently used therapies. However, numerous
patients do not respond to this chemotherapy and must tolerate the
associated toxic and adverse effects. Therefore, it is clinically
important to distinguish the mechanisms that underlie
chemoresistance and the malignant phenotypes of gastric cancer
(3). The identification of novel and
reliable diagnostic biomarkers and therapeutic strategies is also
of the utmost importance (2).
Previous studies that sought to identify convincing candidate genes
that characterize the heterogeneity of gastric cancer, although far
from complete or conclusive, may provide the foundation for
systematic analyses of their genetic contributions to this type of
tumor, and their regulatory pathways and networks may offer insight
into the molecular basis of the pathological or clinical
characteristics (1–3).
microRNAs (miRNA/miR) are a class of naturally
occurring, small regulatory RNAs that function as negative gene
regulators and modulate numerous biological processes, including
cellular differentiation, proliferation, apoptosis and metabolism,
by targeting varying genes (4).
miRNAs have become a major focus in the field of cancer research
(5) and the theory that miRNA
profiles may reflect the developmental lineage and differentiated
state of tumors has been extensively studied in a number of
different types of cancer, including gastric cancer (6–8). Notably,
miR-34a, which possesses anti-oncogenic activity in certain types
of cancer, is downregulated in gastric cancer and
cisplatin-resistant cell lines (9,10). A
previous study has demonstrated that miR-34a is involved in the
sensitivity of gastric cancer to chemotherapies (9). However, the exact molecular mechanism of
miR-34a downregulation and its role in gastric cancer development
and progression has not been established. Furthermore, it is
predicted that a series of factors are involved in the
cancer-associated molecular signatures of miR-34a (11). Thus, a comprehensive and systematic
analysis of miR-34a-target genes in gastric cancer is of great
significance and may provide an opportunity to identify a critical
regulatory network for diagnosing and predicting prognosis in
gastric cancer.
The present study aimed to systematically analyze
the expression of miR-34a predicted target genes associated with
tumorigenesis, chemoresistance to cisplatin-based chemotherapy and
prognosis in gastric cancer.
Materials and methods
Natural language processing (NLP)
analysis of gastric cancer
Data selection, extraction and filtering was
conducted as previously described (12). The search was performed using the
PubMed database (Medline; www.ncbi.nlm.nih.gov/pubmed), attempting to cover all
papers published between January 1980 and March 2012, with a
combination of the following keywords: ‘gastric cancer’ AND
‘cisplatin’ OR ‘resistance’ OR ‘carcinogenesis’ OR ‘tumorigenesis’
OR ‘prognosis’; and ‘1980/01/01’ [program delivery assessment tool
(PDAT)]: ‘2012/03/20’ (PDAT) (12).
All the associated genes and proteins reported in each of the
studies were compiled into a list, followed by gene mention tagging
using a biomedical named entity recognizer (ABNER; http://pages.cs.wisc.edu/~bsettles/abner/). In
addition, the gene symbol in the Entrez gene database of NCBI was
considered to be the most common and was therefore used for the
study (13). The flow of the NLP
analysis was as follows: i) Document searching and formatting; ii)
gene mention tagging using ABNER; iii) conjunction resolution; iv)
gene name normalization based on the Entrez database; and v)
statistical analysis.
Statistical analysis, gene ontology (GO) analysis,
pathway analysis and network analysis were also performed as
previously described (12).
Statistical analysis
The frequency of the occurrence of each gene was
calculated. The higher the frequency of the gene, the greater the
likelihood of the association between gastric cancer and that
specific gene. The following formulae were used:
N represents the total number of studies in the
literature from the PubMed database; m and n represent the
frequency of genes and gastric cancer, respectively, in the
literature from the PubMed database; and k represents the
assumption of the actual concomitant occurrence of a gene and a
disease. All statistical analyses were performed using GraphPad
Prism version 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.01 was considered to indicate a statistically
significant difference.
Gene ontology
The analysis was conducted using the GSEABase
package from the R Project for Statistical Computing platform
(www.r-project.org/), and the genes were
classified according to biological processes, cellular components
and molecular functions.
Pathway analysis
Genes were mapped to the Kyoto Encyclopedia of Genes
and Genomes (KEGG) pathway database using GenMAPP software version
2.1 (www.genmapp.org/), and the enrichment
P-value was calculated for each pathway (14).
Network analysis
A total of 3 different interaction associations were
integrated as previously described (12). Briefly, the pathway data were
downloaded from the KEGG database and were then used to analyze the
genomic interaction between genes with the KEGGSOAP package from
The R Project for Statistical Computing platform (www.bioconductor.org/packages/2.4/bioc/html/KEGGSOAP.html),
including 3 types of associations: Enzyme-enzyme interactions,
protein-protein interactions and gene expression interactions
(15). The protein-protein
interaction data were downloaded from the The MIPS Mammalian
Protein-Protein Interaction Database
(mips.helmholtz-muenchen.de/proj/ppi/) (16). For interactions that had been
previously reported, the co-citation algorithm in the PubMed
abstracts was used: The study analyzed whether a gene term and all
its term variants co-occurred within the sentences, calculated the
frequency of the co-citation gene, and performed a statistical
analysis using the same method as described in the NLP analysis.
The resulting network was displayed by using the Medusa software
(17).
Prediction of miR-34a target
genes
The analysis of the miR-34a predicted targets was
subsequently determined using a combination of 3 independent
software packages as described previously (12,18): i)
PicTar2005 (pictar.mdc-berlin.de/cgi-bin/PicTar_vertebrate.cgi);
ii) miRandaV5 (www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/);
and iii) TargetScan 5.1 (www.targetscan.org/); GO, pathway and network analyses
of miR-34a targets were performed as described in the NLP
analysis.
Integrative analysis of miR-34a target
genes and NLP results
The overlap of the miR-34a target genes and gastric
cancer-associated genes and gene network analysis was subsequently
performed.
Results
NLP analysis of gastric cancer
The initial computerized search identified 22,885
primary studies and a total of 1,183 gastric cancer-associated
genes, using the aforementioned search strategies. The 20 most
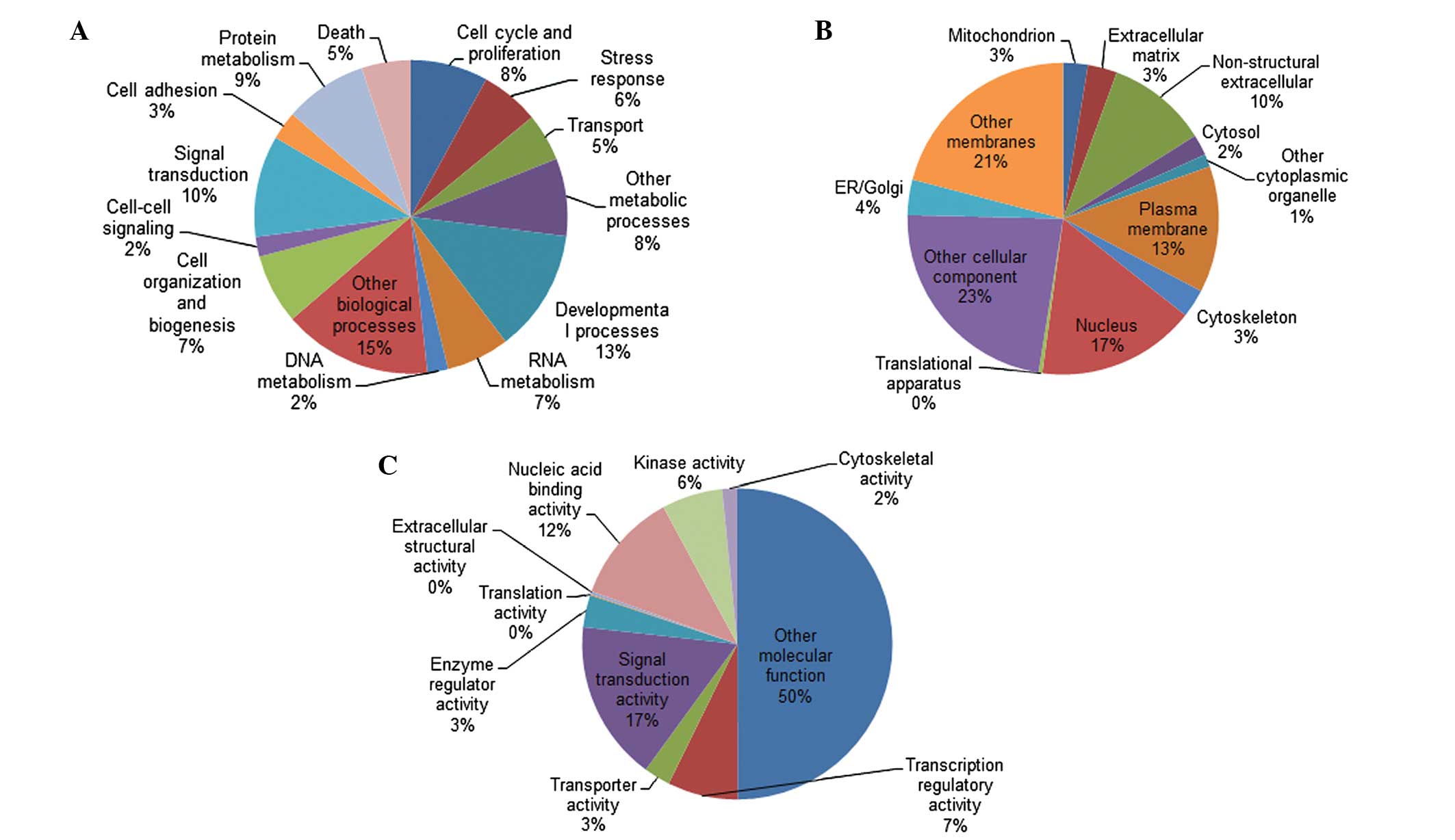
frequently cited genes are listed in Table I. The 1,183 genes were categorized in
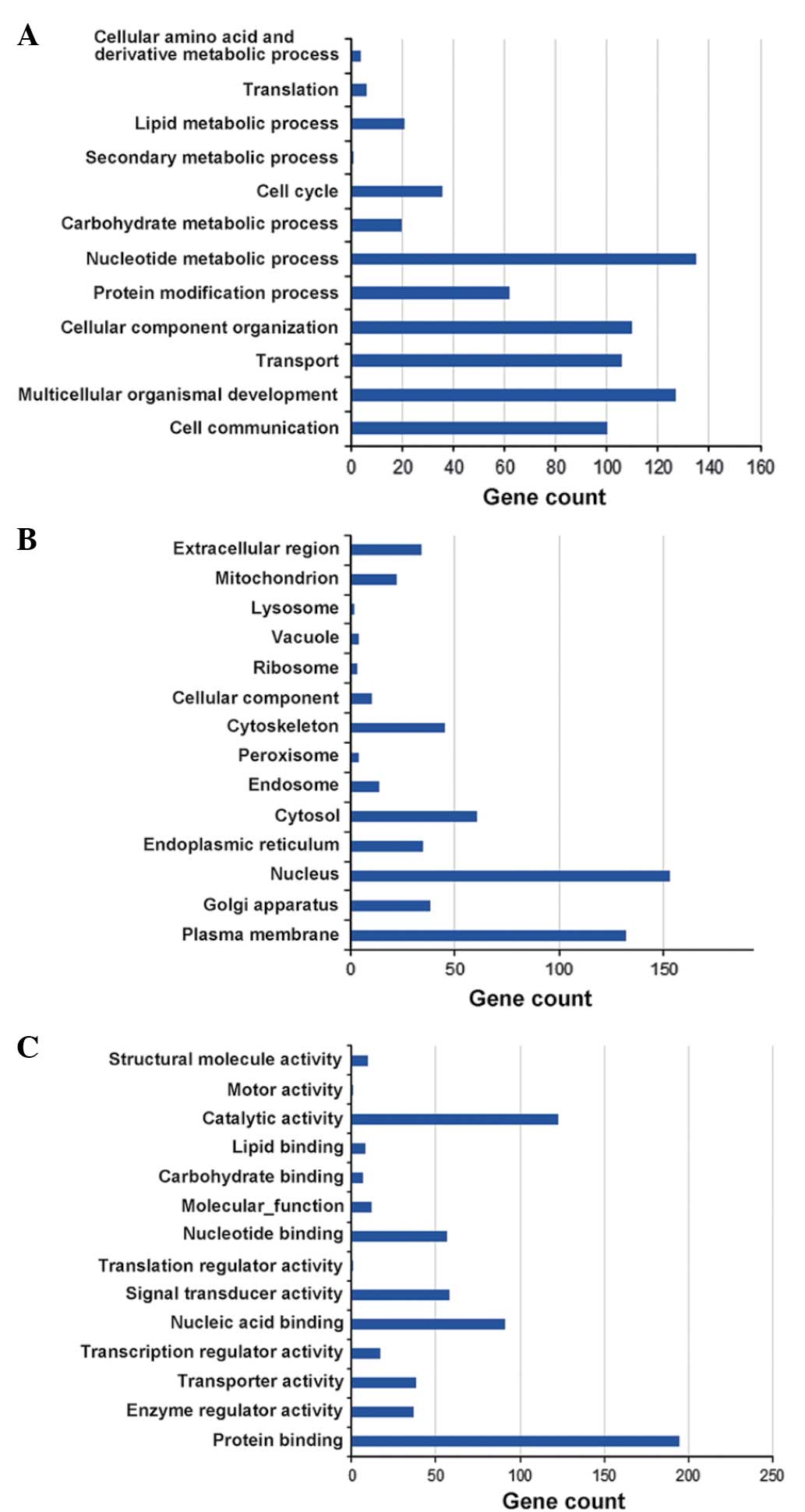
GO according to biological process, cellular component and
molecular function (Fig. 1). Pathway
analysis was then performed and indicated that there were 148
pathways available. Among these pathways, the representation in 33
signaling pathways was statistically significant (P<0.01;
Table II). It has previously been
hypothesized that gene networks reflect the physiological situation
as a whole, in addition to the stability of gene regulatory
networks and the highly connected hub genes, which are crucial to
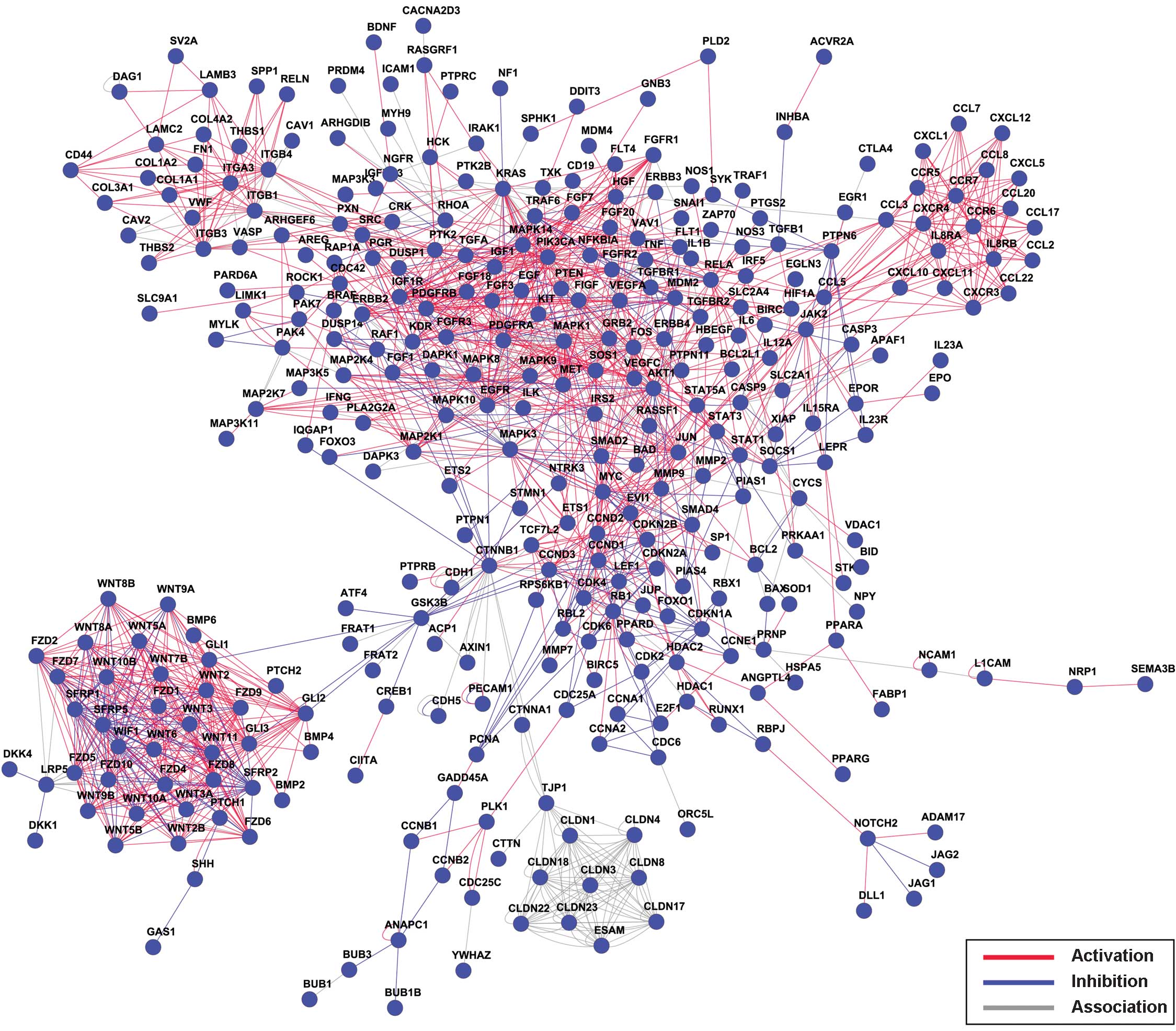
the stability of the network. Thus, the gene network analysis of
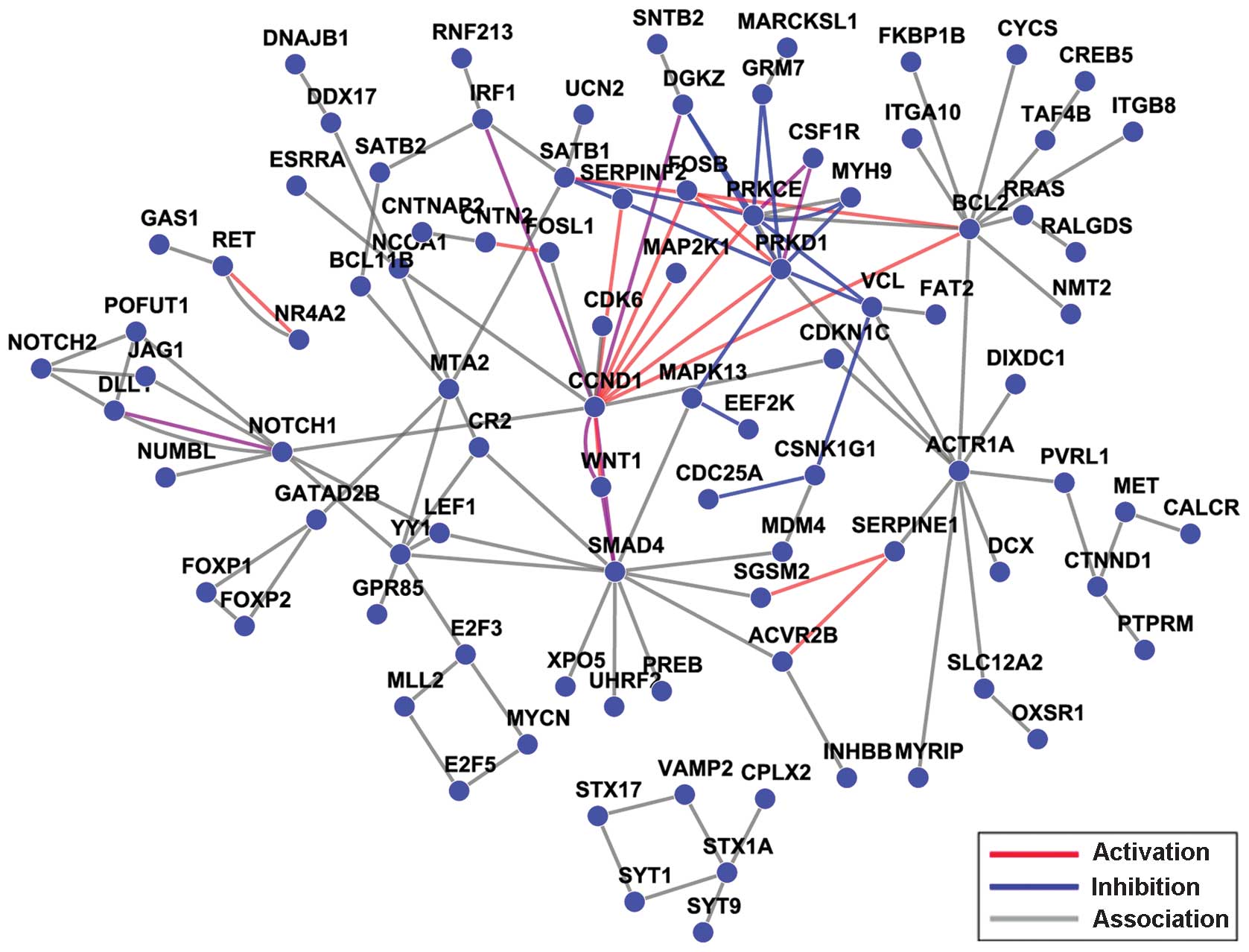
the 1,183 identified genes was conducted and is shown in Fig. 2, which presents the relationships
between the genes as a whole. A connectivity analysis was also
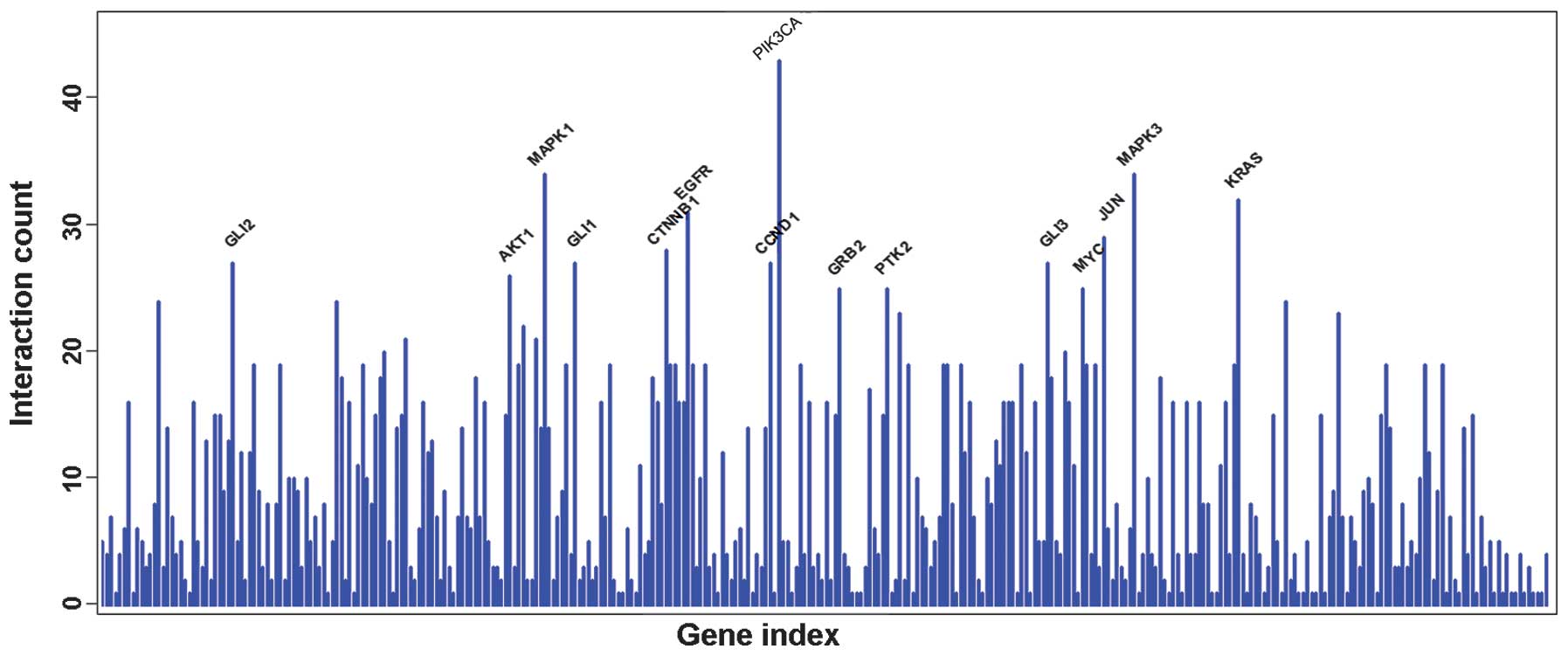
performed. As demonstrated in Fig. 3,
the PIK3CA gene has the most interaction gene counts.
 | Table I.List of the 20 most frequently cited
genes in studies reporting on gastric cancer. |
Table I.
List of the 20 most frequently cited
genes in studies reporting on gastric cancer.
| Gene | Count | P-value | Description |
|---|
| TP53 | 189 |
1.00×10−14 | Tumor protein
p53 |
| ERBB2 | 133 |
1.00×10−14 | v-erb-b2
erythroblastic leukemia viral oncogene |
| VEGFA | 112 |
1.00×10−13 | Vascular endothelial
growth factor A |
| BCL2 | 106 |
1.00×10−13 | B-cell CLL/lymphoma
2 |
| PTGS2 | 96 |
1.00×10−12 |
Prostaglandin-endoperoxide synthase 2
(COX-2) |
| EGFR | 94 |
1.00×10−12 | Epidermal growth
factor receptor |
| JAG1 | 57 |
7.54×10−9 | Jagged 1 (Alagille
syndrome) |
| CCND1 | 54 |
1.02×10−8 | Cyclin D1 |
| TCEAL1 | 53 |
1.00×10−11 | Transcription
elongation factor A (SII)-like 1 |
| MMP9 | 47 |
3.44×10−9 | Matrix
metallopeptidase 9 |
| IL10 | 45 |
1.00×10−11 | Interleukin 10 |
| MAPK8 | 43 |
1.00×10−11 | Mitogen-activated
protein kinase 8 |
| DPYD | 40 |
1.00×10−11 | Dihydropyrimidine
dehydrogenase |
| IL6 | 40 |
1.00×10−11 | Interleukin 6
(interferon, β2) |
| CDKN2A | 39 |
1.00×10−11 | Cyclin-dependent
kinase inhibitor 2A (p16) |
| TNF | 39 |
1.00×10−11 | Tumor necrosis
factor (TNF superfamily, member 2) |
| CD44 | 38 |
1.00×10−10 | CD44 molecule
(Indian blood group) |
| MLH1 | 35 |
1.00×10−10 | MutL homolog 1,
colon cancer, nonpolyposis type 2 |
| MAPK3 | 35 |
1.00×10−10 | Mitogen-activated
protein kinase 3 |
| STAT3 | 28 |
1.00×10−9 | Signal transducer
and activator of transcription 3 |
 | Table II.Signaling pathways represented by
gastric cancer-associated genes (P<0.01). |
Table II.
Signaling pathways represented by
gastric cancer-associated genes (P<0.01).
| Title | Count | P-value |
|---|
| p53 signaling
pathway | 41 |
2.41×10−12 |
| Wnt signaling
pathway | 56 |
3.25×10−12 |
| Focal adhesion | 72 |
2.55×10−11 |
| Cytokine-cytokine
receptor interaction | 82 |
3.17×10−11 |
| ErbB signaling
pathway | 35 |
3.02×10−10 |
| Hedgehog signaling
pathway | 27 |
4.02×10−10 |
| Cell cycle | 40 |
2.04×10−12 |
| Melanogenesis | 37 |
2.92×10−9 |
| Neurotrophin
signaling pathway | 42 |
6.21×10−9 |
| T-cell receptor
signaling pathway | 37 |
1.75×10−8 |
| Toll-like receptor
signaling pathway | 34 |
1.10×10−7 |
| Adherens
junction | 27 |
5.00×10−7 |
| Cell adhesion
molecules | 39 |
7.98×10−7 |
| Chemokine signaling
pathway | 50 |
1.24×10−6 |
| Leukocyte
transendothelial migration | 34 |
5.12×10−6 |
| MAPK signaling
pathway | 62 |
6.68×10−6 |
| Apoptosis | 27 |
1.63×10−5 |
| Hematopoietic cell
lineage | 26 |
3.90×10−5 |
| Jak-STAT signaling
pathway | 38 |
1.01×10−4 |
| Dorso-ventral axis
formation | 11 |
1.07×10−4 |
| Natural killer cell
mediated cytotoxicity | 34 |
1.82×10−4 |
| B-cell receptor
signaling pathway | 22 |
2.06×10−4 |
| TGF-beta signaling
pathway | 24 |
2.50×10−4 |
| FcεRI signaling
pathway | 22 |
3.81×10−4 |
| Regulation of actin
cytoskeleton | 46 |
4.84×10−4 |
| VEGF signaling
pathway | 21 |
5.73×10−4 |
| Base excision
repair | 12 |
9.71×10−4 |
| ECM-receptor
interaction | 22 |
1.15×10−3 |
| Adipocytokine
signaling pathway | 18 |
2.32×10−3 |
| mTOR signaling
pathway | 15 |
2.48×10−3 |
| GnRH signaling
pathway | 24 |
2.95×10−3 |
| Antigen processing
and presentation | 21 |
4.98×10−3 |
| Axon guidance | 28 |
5.54×10−3 |
Analysis of miR-34a predicted
targets
Considering that miRNAs exert biological effects via
their numerous targets, the predicted target genes of miR-34a were
analyzed using 3 commonly used computational algorithms:
TargetScan4.0, PicTar and miRanda. A total of 460 potential unique
gene symbols targeted by miR-34a were obtained, and all these genes
were categorized by GO analysis (Fig.
4). The gene ontology analysis results for the biological
process catergory revealed that miR-34a-target genes were
predominantly associated with nucleotide metabolic processes,
multicellular organism development and cellular component
organization. In the pathway analysis, 98 pathways were obtained in
the miR-34a targets-pathway. Specific pathways that were identified
by the analysis included the PI3K-Akt signaling pathway, the p53
signaling pathway, the notch signaling pathway, adherens junctions,
the cell cycle, galactose metabolism and the HIF-1 signaling
pathway. These pathways have already been demonstrated to be
involved in the development, progression and chemosensitivity of
gastric cancer. Additionally, in the network analysis of the
miR-34a predicted targets (Fig. 5),
the connectivity of the CCND1 gene was the highest among the
110 hub genes that were obtained.
Integrative analysis of miR-34a target
genes and the NLP results
The overlap between the 460 miR-34a target genes and
the 1,183 prognosis-associated genes in gastric cancer obtained
from the NLP analysis was calculated. A total of 30 overlap genes
that were associated with the development and progression of
gastric cancer and that were also potential miR-34a target genes
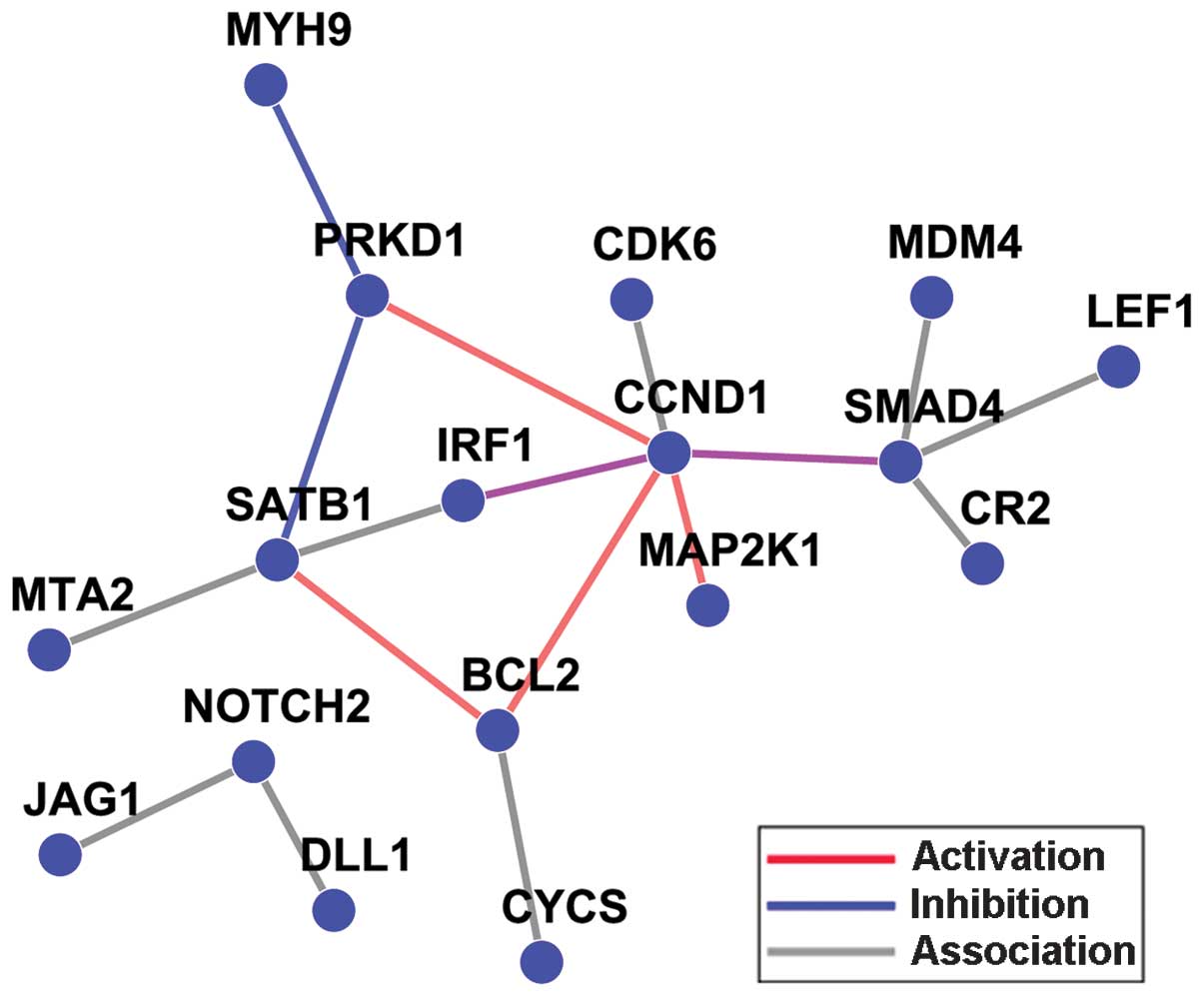
were obtained using this integrative analysis (Table III). A network analysis was also
conducted to map the overlapped genes (Fig. 6). From the current results, it appears
reasonable to conclude that the SMAD4, CCND1,
MAP2K1, BCL2 and NOTCH2 hub genes are
potential miR-34a target genes and are also essential in the
molecular mechanism of gastric cancer. The SMAD4,
CCND1, MAP2K1, BCL2 and NOTCH2 genes
represent the Smad signaling pathway, the cell cycle, MAPK
signaling pathway, apoptosis pathway and the Notch signaling
pathway, respectively.
 | Table III.Integrative analysis of miR-34a
target genes and the NLP results. |
Table III.
Integrative analysis of miR-34a
target genes and the NLP results.
| Targets | Count | P-value | Gene
description |
|---|
| BCL2 | 106 |
1.00×10−12 | B-cell CLL/lymphoma
2 |
| JAG1 | 57 |
7.54×10−9 | Jagged 1 |
| CCND1 | 54 |
1.02×10−8 | Cyclin D1 |
| MET | 18 |
1.00×10−12 | Met proto-oncogene
(hepatocyte growth factor receptor) |
| CYCS | 14 |
1.00×10−12 | Cytochrome
c, somatic |
|
SERPINE1 | 12 |
1.00×10−11 | Serpin peptidase
inhibitor, clade E, member 1 |
| SMAD4 | 8 |
4.95×10−8 | SMAD family member
4 |
| MAP2K1 | 6 |
1.19×10−6 | Mitogen-activated
protein kinase kinase 1 |
| AREG | 4 |
5.40×10−6 | Amphiregulin |
| SATB1 | 4 |
5.49×10−7 | SATB homeobox
1 |
| PDCD4 | 3 |
5.11×10−5 | Programmed cell
death 4 |
| CDK6 | 3 |
6.78×10−4 | Cyclin-dependent
kinase 6 |
| GAS1 | 2 |
4.45×10−4 | Growth
arrest-specific 1 |
| IGFBP3 | 2 |
1.27×10−1 | Insulin-like growth
factor binding protein 3 |
| IRF1 | 2 |
2.54×10−2 | Interferon
regulatory factor 1 |
| PTPRD | 2 |
7.21×10−4 | Protein tyrosine
phosphatase, receptor type, D |
| NR4A2 | 1 |
1.32×10−1 | Nuclear receptor
subfamily 4, group A, member 2 |
| NOTCH2 | 1 |
1.14×10−1 | Notch homolog
2 |
| PDGFRA | 1 |
2.60×10−1 | Platelet-derived
growth factor receptor, α polypeptide |
| CDC25A | 1 |
1.53×10−1 | Cell division cycle
25 homolog A |
| MTA2 | 1 |
5.66×10−2 | Metastasis
associated 1 family, member 2 |
| SOX4 | 1 |
5.66×10−2 | SRY (sex
determining region Y)-box 4 |
| MYH9 | 1 |
1.84×10−1 | Myosin, heavy chain
9, non-muscle |
| DLL1 | 1 |
8.18×10−2 | δ-like 1 |
| LEF1 | 1 |
1.46×10−1 | Lymphoid
enhancer-binding factor 1 |
| PRKD1 | 1 |
1.50×10−1 | Protein kinase
D1 |
| JMJD1C | 1 |
1.86×10−2 | Jumonji
domain-containing 1C |
| CR2 | 1 |
1.37×10−1 | Complement
component receptor 2 |
| KITLG | 1 |
1.88×10−1 | KIT ligand |
| MDM4 | 1 |
1.16×10−1 | Mdm4 p53-binding
protein homolog |
Discussion
The present study performed a systematic review of a
pooled collection of English language studies of gastric
cancer-associated molecules. Following classification of the genes
into 3 functional groups by GO analysis, gastric cancer-associated
networks and pathways were established to identify the key
molecules involved. Next, computational methods were used to
predict the miR-34a targets, followed by screening for matched gene
symbols in the NCBI human sequences and GO, and pathway and network
analysis. Finally, in the integrative analysis of gastric
cancer-associated miR-34a-targets, hub genes were identified by
overlap calculation and the network and pathways of the associated
hub genes were further analyzed.
The mechanisms involved in the pathogenesis of
gastric cancer are not yet fully clarified. At present, the genes
involved in the complex multi-step process of gastric cancer tumor
progression, metastasis, relapse and tolerance remain to be fully
elucidated. Systematic analysis on the deregulated gene expression,
epigenetic or genetic abnormalities may demonstrate their
diagnostic potential.
miRNAs have become a major research focus in the
field of cancer research (5). A
number of miRNAs serve as candidates for clinical biomarkers and
have been demonstrated to be useful in characterizing the tumor
tissues and reflecting the developmental lineage and differentiated
state of cancer (19,20). Previous studies have indicated that
miRNAs are involved in the molecular pathogenesis, clinical cancer
progression and prognosis of gastric cancer (8,9,11,21). One
specific miRNA, miR-34a, has been investigated extensively in
various types of tumor, including gastric cancer. Inactivation of
miR-34a is a common event during tumorigenesis, and the restoration
of miR-34a activity has been indicated to be useful in the
prevention of chemotherapy resistance (22). In addition, in gastric
mucosa-associated lymphoid tissue lymphoma and diffuse large B-cell
lymphoma, reduced expression of miR-34a and increased expression of
its target proteins of FOXP1, p53 and BCL2 predict a poor overall
survival (11). Moreover, the
molecular targets of miR-34a are not limited to those few examples.
Furthermore, the present study focused on miR-34a since previous
studies have reported malignant activity associated with the
downregulation of miR34a and it is often deleted in several
cancers, including gastric cancer (9,23). The
loss of miR-34a expression has been linked to the resistance to
apoptosis induced by the chemotherapeutic p53-activating agents
(11). Although the function of
miR-34a is relatively well documented, knowledge of miR-34a-targets
and miR-34a pathways associated with cancer would provide a more
comprehensive understanding of its significance in gastric cancer.
One proposed mechanism may be associated with multi-level
regulatory control, including tumor suppressor genes, oncogenes and
invasion-associated genes. Therefore, for gastric cancer,
systematic analysis of malignant behavior-associated
miR-34a-targets and their potential molecular mechanisms requires
investigation. In the present study, gastric cancer-associated
genes and miR-34a target genes were analyzed separately using
computational and bioinformatic methods, and then integrated in
order to identify the host gene signature of the miR-34a
targets.
In the NLP analysis, 1,183 genes that were
associated with the carcinogenesis, progression and chemoresistance
of gastric cancer were identified. The potentially functional
classification of the genes was obtained from the GO analysis. The
pathway analysis identified 148 pathways and 33 of these were
statistically significant, including the p53 signaling pathway, the
Wnt signaling pathway, the cell cycle, the MAPK signaling pathway,
apoptosis, and the TGF-β signaling pathway. A number of previous
studies have identified the same pathways to be involved in
tumorigenesis, metastasis and chemotherapy resistance (2,11,24,25). In
addition, the network and connectivity of those 1,183 genes was
constructed in the present study. The highly connected hub genes
are crucial to the stability of the network. PIK3CA, with the
highest connectivity, had a total of 43 gene connections. A
previous study demonstrated that PIK3CA is mutated frequently in a
range of human tumors and that its activation is associated with a
number of chemotherapeutic agents (26). The results of the present study are
consistent with a previous study that analyzed lung
cancer-associated genes with NLP and concluded that the gene with
the highest connectivity was PIK3CA (12).
In order to obtain the miR-34a target genes in
gastric cancer, 3 computational algorithms (miRanda, PicTar, and
TargetScan) were used to analyze the predicted targets. From this
analysis, 460 unique gene symbols targeted by miR-34a were
obtained. These genes were categorized using GO, followed by
pathway and network analysis in parallel with the NLP analysis. The
results demonstrated that the putative target genes of miR-34a
include the tumor-associated genes CCND1, SMAD4,
PRKD1, BCL2, NOTCH2 and SATB1, among
others. A total of 98 pathways were obtained in the miR-34a targets
pathway analysis, and the PI3K-Akt signaling pathway was identified
as the most significant pathway. The 3 genes with the highest
connectivity among all 110 hub gene obtained in the miR-34a
targets-network analysis were CCND1, SMAD4 and
BCL2. The CCND1 gene encodes Cyclin D1, a key protein
required for G1/S cell cycle transition. Mutations,
amplification and overexpression of Cyclin D1 are frequently
observed in a number of different types of cancer and may
contribute to tumorigenesis (27).
SMAD4, which is mutated in a variety of tumors and functions
as a tumor suppressor, belongs to the Darwin family of proteins
that modulate members of the TGF-β protein superfamily (28). BCL2 is considered to be an important
anti-apoptotic protein and is a member of the BCL2 family of
regulator proteins.
In the subsequent integrative analysis of NLP and
miR-34a targets, 30 hub genes were obtained. The results indicated
that miR-34a is essential in carcinogenesis, progression and the
response to chemotherapy in gastric cancer through the Smad
signaling pathway, the cell cycle, the MAPK signaling pathway, the
apoptosis pathway, the Notch signaling pathway and other pathways.
The overlapped targeting hub genes and their pathways may become
novel targets for controlling gastric cancer or reversing
chemoresistance. Notably, the PIK3CA gene and its pathway,
which had the highest connectivity in the NLP analysis, were not
involved in the final integrative analysis. This is in agreement
with the evidence that PIK3CA was the most significant hub
gene in the NLP analysis of lung cancer, but it was not involved in
the overlapped analysis with miR-21 (12). The possible explanation for these
discrepancies may be that the computational target gene prediction
methods have certain limitations in determining actual
multifactorial associations.
Collectively, the present study systematically
analyzed gastric cancer-associated genes and the putative targets
of miR-34a by using a computational and bioinformatics approach.
Although additional experiments are required to confirm these
results, the systematic integration of miR-34a-targets and their
potential modulators provides an efficient approach to discover
novel target genes and co-regulatory networks in gastric cancer.
Identification of these molecular pathways and networks controlled
by miR-34a may provide unique insights into the pathogenesis of
gastric cancer.
References
|
1
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Z, Miao L, Xin X, Zhang J, Yang S,
Miao M, Kong X and Jiao B: Underexpressed CNDP2 participates in
gastric cancer growth inhibition through activating the MAPK
signaling pathway. Mol Med. 20:17–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Correa P: Gastric cancer: Overview.
Gastroenterol Clin North Am. 42:211–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kong YW, Ferland-McCollough D, Jackson TJ
and Bushell M: microRNAs in cancer management. Lancet Oncol.
13:e249–e258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aushev VN, Zborovskaya IB, Laktionov KK,
et al: Comparisons of microRNA patterns in plasma before and after
tumor removal reveal new biomarkers of lung squamous cell
carcinoma. PLoS One. 8:e786492013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szabó DR, Luconi M, Szabó PM, et al:
Analysis of circulating microRNAs in adrenocortical tumors. Lab
Invest. 94:331–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao Y, Suo AL, Li ZF, et al: MicroRNA
profiling of human gastric cancer. Mol Med Rep. 2:963–970.
2009.PubMed/NCBI
|
|
9
|
Cao W, Yang W, Fan R, et al: miR-34a
regulates cisplatin-induce gastric cancer cell death by modulating
PI3K/AKT/survivin pathway. Tumour Biol. 35:1287–1295. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y and
Zheng X: miR-34a inhibits migration and invasion by down-regulation
of c-Met expression in human hepatocellular carcinoma cells. Cancer
Lett. 275:44–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He M, Gao L, Zhang S, Tao L, Wang J, Yang
J and Zhu M: Prognostic significance of miR-34a and its target
proteins of FOXP1, p53, and BCL2 in gastric MALT lymphoma and
DLBCL. Gastric Cancer. 17:431–441. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao W, Xu J, Liu L, Shen H, Zeng H and Shu
Y: A systematic-analysis of predicted miR-21 targets identifies a
signature for lung cancer. Biomed Pharmacother. 66:21–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith L, Tanabe LK, Ando RJ, et al:
Overview of BioCreative II gene mention recognition. Genome Biol. 9
(Suppl 2):S22008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dahlquist KD, Salomonis N, Vranizan K,
Lawlor SC and Conklin BR: GenMAPP, a new tool for viewing and
analyzing microarray data on biological pathways. Nat Genet.
31:19–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ogata H, Goto S, Fujibuchi W and Kanehisa
M: Computation with the KEGG pathway database. Biosystems.
47:119–128. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mewes HW, Albermann K, Heumann K, Liebl S
and Pfeiffer F: MIPS: A database for protein sequences, homology
data and yeast genome information. Nucleic Acids Res. 25:28–30.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hooper SD and Bork P: Medusa: A simple
tool for interaction graph analysis. Bioinformatics. 21:4432–4433.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Volinia S, Calin GA, Liu CG, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosenfeld N, Aharonov R, Meiri E, et al:
MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol.
26:462–469. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang F, Sun GP, Zou YF, Hao JQ, Zhong F
and Ren WJ: MicroRNAs as promising biomarkers for gastric cancer.
Cancer Biomark. 11:259–267. 2012.PubMed/NCBI
|
|
22
|
Hermeking H: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cha YH, Kim NH, Park C, Lee I, Kim HS and
Yook JI: MiRNA-34 intrinsically links p53 tumor suppressor and Wnt
signaling. Cell Cycle. 11:1273–1281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kolligs FT, Bommer G and Göke B:
Wnt/beta-catenin/tcf signaling: A critical pathway in
gastrointestinal tumorigenesis. Digestion. 66:131–144. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mishra L, Shetty K, Tang Y, Stuart A and
Byers SW: The role of TGF-beta and Wnt signaling in
gastrointestinal stem cells and cancer. Oncogene. 24:5775–5789.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Endoh H, Yatabe Y, Kosaka T, Kuwano H and
Mitsudomi T: PTEN and PIK3CA expression is associated with
prolonged survival after gefitinib treatment in EGFR-mutated lung
cancer patients. J Thorac Oncol. 1:629–634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 11:558–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Inman GJ: Linking Smads and
transcriptional activation. Biochem J. 386:e1–e3. 2005. View Article : Google Scholar : PubMed/NCBI
|