Introduction
Endometriosis is a common disease, which is
associated with severe symptoms, including pelvic pain and
infertility, and affects up to 10% of women of reproductive age
(1,2).
Endometriosis also possesses a malignant transformation potential
to ovarian carcinoma. Recently, several studies have indicated that
heme and iron, present in endometriotic cysts, damage the
endometriotic cells by inducing inflammation and oxidative stress
(3–7).
Simultaneously, several genes associated with acute antioxidant
response, DNA damage repair and cell cycle modulation are
activated. An increased understanding of the molecular biology
involved in endometriosis-associated ovarian carcinogenesis may
facilitate the identification of novel therapeutic targets.
The transcription factor hepatocyte nuclear factor
(HNF)-1β is overexpressed in endometriosis and clear cell carcinoma
of the ovary. HNF-1β may alleviate damage and promote the survival
of cells experiencing stress, by upregulating antioxidant protein
expression (8) and stimulating cell
cycle checkpoint machinery (9).
However, functional or clinical significance of this transcription
factor is unclear. The present review aimed to summarize the
potential association between HNF-1β and cell cycle checkpoint
machinery.
The present review also outlines future directions
for study, with respect to targeted therapy for ovarian clear cell
carcinoma.
Materials and methods
A computerized literature search was conducted to
identify relevant studies reported in the English language. MEDLINE
electronic databases (http://www.ncbi.nlm.nih.gov/sites/entrez) published
between 2000 and 2014 were searched, combining the keywords
‘ovarian clear cell carcinoma’, ‘carcinogenesis, ‘HNF-1β’ and ‘DNA
damage repair’. A variety of combinations of these terms were used,
depending on which database was searched. Each gene was also linked
to the relevant NCBI Entrez Gene pages (http://www.ncbi.nlm.nih.gov/sites/entrez).
Furthermore, the references in each article were searched to
identify potentially excluded studies.
Iron-induced DNA damage in endometriosis and
endometriosis-associated ovarian cancer
Endometriosis is associated with hemorrhage or
hemolysis. Repeated episodes of hemorrhage are a major cause of
reproductive disability amongst patients with peritoneal
endometriosis and endometriotic cysts (10). Erythrocytes existing outside the
vascular system rapidly lyse and release free hemoglobin, and the
blood scavenging system is required for effective hemoglobin
clearance (11). Hemoglobin slowly
undergoes spontaneous oxidation, also known as autoxidation
(3), and is oxidized easily from the
ferrous (Fe2+) oxygenated form (oxyhemoglobin,
HbO2) to the ferric (Fe3+) met-form
(methemoglobin, metHb) with generation of the superoxide anion
(O2−) as follows (4):
HbO2− → metHb +
O2−
Heme is also catabolized by heme oxygenases into
iron, biliverdin and carbon monoxide. Free ferrous iron, in its
catalytically active form, induces oxidative stress, if not
chelated. This is due to the fact that Fe2+ catalyzes
the non-enzymatic Fenton reaction (12), which produces highly reactive hydroxyl
radicals as follows:
Fe2+ +
H2O2− → Fe3+ +
HO− + •OH
Fe2+ is involved in the formation of
reactive oxygen species (ROS) and reactive nitrogen species (RNS),
which are damaging to the majority of cell components, including
DNA, membranes and proteins, and induce cell death. The hydroxyl
radical (•OH) and superoxide anion (O2−) are
toxic to living organisms. 8-hydroxy-2′-deoxyguanosine (8-OxodG) is
a major form of oxidative DNA, generated following exposure to free
radicals, and is released from cells following DNA repair (13). A study indicated that the levels of
8-OxodG in peritoneal fluid were higher in women with endometriosis
compared with those of the control groups (5). The repeated hemorrhage within the
endometriotic lesions, hemoglobin breakdown and subsequent heme and
iron accumulation mediate secondary tissue injury and result in
cell death (14). Therefore, dead
cells are unable to induce ovarian carcinogenesis.
Paradoxically, it is generally accepted that
persistent oxidative stress may be involved in chronic
inflammation, which in turn, may mediate chronic diseases including
cancer (6). Several studies reported
that continued oxidative stress leads not only to increased cell
survival, but also increased tumorigenic potential of cancer cells
(6,7).
Oxidative stress contributes to the expansion of genomic
instability throughout the genome, and subsequent gene mutations
involved in the initiation of tumorigenesis (15). In an oxidative stress situation, iron
overload-associated diseases, including hemochromatosis, chronic
viral hepatitis, asbestosis and endometriosis, may result in the
development of neoplasia, including hepatocellular carcinoma,
malignant mesothelioma and ovarian cancer (16). Such microenvironments share common
mechanistic foundations or provide a biological interface between
cell death and carcinogenesis induced by persistent oxidative
stress.
Antioxidants are key regulators in the maintenance
of cellular redox balance and inhibition of the susceptibility to
tumorigenesis. Animal experiments revealed that loss of
ROS-scavenging enzymes, for example peroxiredoxin, was associated
with an increase in cancer susceptibility (17). By contrast, the overexpression of
antioxidants, including NAD(P)H: quinone oxidoreductase 1 (NQO1),
has been correlated with numerous human malignancies, suggesting a
role in carcinogenesis (18). The
endometriosis-specific transcription factor, HNF-1β, functions to
alleviate endometriotic cell damage and also promotes survival of
cells under oxidative stress by upregulating antioxidant protein
expression (8). It was hypothesized
that this increase in antioxidants may reduce the susceptibility of
endometriotic cells to lethal injury from ROS, following exposure
to heme and iron. Therefore, overexpression of antioxidant enzymes
may, in part, be responsible for the increased risk of cancer
amongst women with endometriosis. Excess ROS induce significant
oxidative stress, resulting in cell death, whereas sublethal ROS
levels may adapt to prolong cell survival and increase the
tumorigenic potential of endometriotic cells. Each endometriotic
lesion may display differences with regards to the level of
responsiveness to ROS. Carcinogenesis requires a delicate balance
between oxidants and antioxidants.
Molecular changes implicated in clear cell
carcinoma
Endometriosis was suggested to confer an elevated
risk of epithelial ovarian cancer, particularly the clear cell and
endometrioid adenocarcinoma subtypes, also known as
endometriosis-associated ovarian cancer (EAOC) (1,2). Clear
cell carcinoma and endometrioid adenocarcinoma exhibit distinct
clinicopathological features and molecular phenotypes (19,20).
Yamada et al (21)
hypothesized that heme and iron-induced signals contribute to
carcinogenesis via three major mechanisms: i) By enhancing
oxidative stress, which promotes DNA mutagenesis and contributes to
tumor initiation; ii) via activation of detoxification pathways,
thereby contributing to tumor promotion; iii) by sustaining growth
of cancer cells via estrogen-dependent (endometrioid) or
-independent (clear cell) mechanisms. These studies provide novel
insights into the pathophysiology and significance of genetic
functional categories in EAOC. Several molecular changes that have
been implicated in the clear cell carcinoma pathway include: i)
Chemoresistance (22–26); ii) cell cycle regulation; iii)
detoxification; iv) hormone independency; v) chromosomal
instability; and vi) glycogen synthesis (24).
Clear cell carcinoma is more likely to exhibit
chemoresistance than high-grade serous carcinoma, demonstrating
that clear cell histology is highly resistant to conventional
platinum-based chemotherapy (23).
Potential doubling time for clear cell carcinoma cells was longer
than that of serous adenocarcinoma cells (61 vs. 30 h), suggesting
that the intrinsic chemoresistance may be associated with prolonged
doubling time (22). Decreased
proliferation rates of cancer cells promotes resistance to a number
of chemotherapeutics; a behavior observed in clear cell carcinoma.
This chemoresistance may also be associated with an increase in
cell cycle arrest during the DNA damage response, or enhanced drug
detoxification within the cell (27).
The cell cycle remains arrested to repair DNA damage induced by
heme and iron-induced oxidative stress (see the ‘Role of
transcription factor HNF-1β’ section for details). Genetic and
epigenetic dysregulation of chemoresistance-associated genes may
induce cell cycle arrest and suppress cell proliferation.
The pathogenesis of endometriosis-associated ovarian
carcinogenesis may be closely associated with heme and iron
overload and the associated overexpression of antioxidants
(27). Redox-sensitive antioxidant
genes may be associated with the downstream targets of HNF-1β
(27,28). HNF-1β mainly remains epigenetically
hypomethylated and enhances ROS detoxification, resulting in
typically low ROS levels in clear cell carcinoma (8,29).
Therefore, it is conceivable that excess ROS induces cell death,
while sublethal doses of oxidative stress may accelerate the
development and progression of clear cell carcinogenesis.
Clear cell and endometrioid carcinoma are
genetically distinct cancers, and whilst clear cell carcinoma
presents rare expression of the estrogen receptor (ER) and
progesterone receptor (PR), the ER and PR are often overexpressed
in endometrioid carcinoma. Endometrioid adenocarcinomas are more
likely to co-express ER and PR compared with the other histological
subtypes (30). By contrast, clear
cell carcinomas are characterized by their negative expression of
hormone receptors (30).
The most conspicuous feature of clear cell carcinoma
is the abundance of intracellular glycogen (31). The HNF-1β signature is associated with
glycogen metabolism, including that of glucose-6-phosphatase
(32). Hypoxia induces a switch from
oxidative phosphorylation to glycolysis, increasing glycogen
synthesis (31). Hypoxia inducible
factor (HIF)-1α overexpression in clear cell carcinoma resulted in
an increase in the production of glycogen synthase 1 (GYS1),
indicating that a hypoxic environment promoted glycogen synthesis.
Taken together, these results indicated that HNF-1β overexpression
in clear cell carcinoma has a role in glycogen synthesis, cell
cycle regulation and detoxification.
DNA damage response in clear cell
carcinoma
The genome is constantly damaged by endogenous
compounds, for example ROS resulting from metabolic processes, or
exogenous compounds, including ionizing radiation, ultraviolet and
environmental toxins. Oxidative stress, chronic injury and
inflammation promote DNA damage and chromosomal aberrations, which
are processed by series of pathways known as the ‘DNA damage
response (DDR)’. The DDR pathways regulate cell fate decisions
regarding DNA repair, cell cycle arrest and ultimately cell death,
senescence or survival. The DDR represents a complex network of
signaling pathways, which halt the cell cycle to allow time for DNA
repair. DDR pathways coordinate multiple repair processes,
including nucleotide excision repair (NER), base excision repair
(BER), mismatch repair (MMR), DNA double strand break (DSB) repair
and post replication repair (PRR), facilitating the maintenance of
genomic integrity following exposure to various stressors (33).
Individual DNA base damage and DNA intrastrand
crosslinks are removed by the processes of BER and NER,
respectively. The BER process constitutes the primary defense
mechanism exerted by DNA glycosylase enzymes, 8-oxoguanine DNA
glycosylase (OGG1) and X-ray repair complementing defective repair
in Chinese hamster cells 1. The NER pathway repairs DNA damage
induced by UV light and oxidative stress (34). Furthermore, cellular sensing machinery
for DNA damage includes: i) Poly(ADP-ribose) polymerase 1; ii)
members of the phosphatidylinositol 3-kinase protein family known
as ataxia-telangiectasia mutated (ATM), ataxia-telangiectasia and
Rad3-related (ATR); iii) checkpoint kinases 1 and 2 (Chk1 and
Chk2); iv) the dual-specificity protein phosphatases CDC25A-C; and
v) cyclin-dependent kinases (CDK2/4) (35). Generally, single-strand DNA initiates
ATR-Chk1 pathway activation, whereas DSBs promote ATM-Chk2
activation. DSBs are repaired by non-homologous end joining or by
homologous recombination. Chk1, an important signal transducer in
the cell cycle checkpoint pathway, represents a core component,
which contributes to all cell cycle checkpoints (36).
Recent studies have demonstrated the chemoresistance
mechanism in clear cell carcinoma (37). Among ovarian cancer, excision repair
cross-complementation group 1 (ERCC1) and ERCC3 are specifically
overexpressed in clear cell carcinoma (38). The AT rich interactive domain 1A,
SWI-like (ARID1A) is a chromatin remodeling gene frequently mutated
in a variety of female reproductive organ-derived cancers,
including clear cell carcinoma (39).
Thus, the cell cycle checkpoint machinery has become a focus of
research in the field of anticancer drug resistance. Genome-wide
and proteome-based approaches provide crucial information regarding
the target molecules that modulate the cell cycle checkpoint
pathways. The HNF-1β gene is one of the key molecules associated
with the ATR-Chk1 pathway.
Role of transcription factor HNF-1β
Hypomethylation and increased protein levels of
HNF-1β are specific features of endometriosis and its malignant
transformation into clear cell carcinoma of the ovary. The DNA
repair capacity of a cell contributes to its genomic integrity and
is vital to the normal functioning of an organism. A recent study
reported that a molecular link between ATR and Chk1 was significant
in the cell cycle regulatory pathway in clear cell carcinoma
(9). Transient phosphorylation of
Chk1 is critical for successful recovery of the cell cycle
following stalled DNA replication (9). This section summarizes recent progress
in the elucidation of transcription factor HNF-1β-mediated
regulation of DNA damage-induced cell cycle arrest.
As shown in Fig. 1,
HNF-1β is critical to the anti-oxidative effects observed in
endometriosis and clear cell carcinoma (27). Furthermore, this transcription factor
inhibits apoptosis of cancer cells. However, the precise mechanism
of HNF-1β action is yet to be fully elucidated. Shigetomi et
al (9) confirmed that HNF-1β
induced G2/M phase arrest and anti-apoptosis in clear cell
carcinoma cells via an enhancement of the oxidative DNA
damage-induced ATR-Chk1-associated pathway. Blockade of this event
by HNF-1β short interfering (si)RNA induced apoptosis and an
impaired G2/M checkpoint following exposure to oxidative stress
(9,40). Notably, HNF-1β performs a critical
role in DDR by controlling cell cycle regulation via activation of
the ATR-Chk1 pathway. These results provide novel insights into the
critical roles of HNF-1β in the regulation of oxidative
stress-induced G2/M cell cycle arrest and anti-apoptosis in clear
cell carcinoma cells.
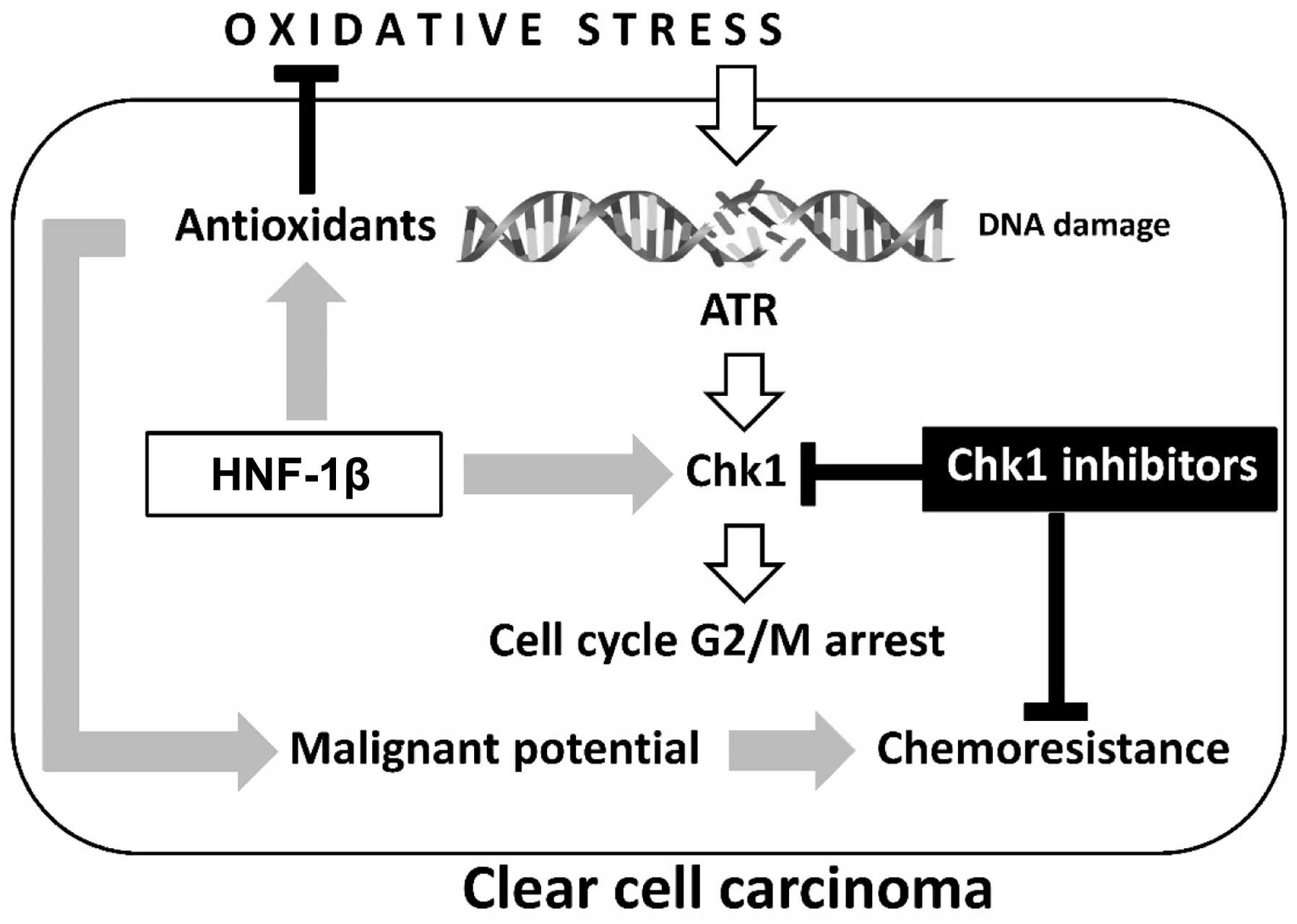 | Figure 1.Two major hallmarks, oxidative stress
and antioxidants, are crucial in the development of ovarian clear
cell carcinoma: Oxidative stress contributes to immediate
phosphorylation of Chk1 by ATR in the presence of DNA damage.
HNF-1β overexpression induces antioxidant expression and persistent
Chk1 activation, which are associated with detoxification and cell
cycle arrest, respectively. The chemoresistance of clear cell
carcinoma may be due to aberrant retention of the G2 checkpoint
through overexpression of HNF-1β. Oxidative stress-mediated excess
ROS induces cell death, while sublethal ROS may adapt to prolonged
cell survival and increase the tumorigenic potential of
endometriotic cells. Carcinogenesis requires a balance between
oxidants and antioxidants. Collectively, our data demonstrate the
importance of HNF-1β as a promising treatment strategy for
overcoming chemoresistance in clear cell carcinoma. HNF-1β,
hepatocyte nuclear factor-1β; ROS, reactive oxygen species; Chk1,
checkpoint 1; ATR, ataxia-telangiectasia and Rad3-related. |
On one hand, cell cycle arrest provides
pre-malignant endometriotic cells with the opportunity to
persistently repair DNA following oxidative damage. However, on the
other hand, HNF-1β induces an increase in G2/M accumulation and
decrease in the mitotic entry of DNA-damaged cells and apoptosis.
These cells fail to maintain genomic integrity and may enhance
malignant potential. Several reports have indicated that elevated
levels of HNF-1β are associated with chemoresistance, which may be
suppressed by HNF-1β siRNA expression (9,40).
Targeting Chk1 also blocks HNF-1β-induced Chk1 phosphorylation and
markedly increases lethality in vitro. These data led to the
hypothesis that combining checkpoint inhibitors with DNA damaging
agents may force cancer cells into premature and lethal mitosis,
via an accumulation of genomic instability by overriding
checkpoints. The role of HNF-1β in DDR through the ATR-Chk1
pathway, and potential combinatorial treatment strategies will be
discussed with a mechanistic rationale.
Chk1 inhibitors as molecular targeted
therapeutics
The potential implications of the aforementioned
factors in therapeutic intervention were subsequently evaluated.
Integration of gene expression profiling and proteomics analysis to
identify genomic alterations and aberrant molecular pathways in a
clinical setting is necessary to facilitate personalized targeted
therapy. If predictive biomarkers are identified, the use of
targeted agents may improve prognosis.
DNA damage activates DDR and the DNA repair pathway,
including Chk1. Chk1 is a serine-threonine checkpoint kinase
central to the DDR network, which represents one of the most
important targets for anti-cancer therapeutics. In preclinical
studies, Chk1 inhibition enhanced the cytotoxicity of several
chemotherapeutic agents (9). UCN-01
(7-hydroxystaurosporine), the first non-selective Chk1 inhibitor
introduced in a clinical study, combined with irinotecan
demonstrated activity in patients with advanced triple-negative
breast cancer (41). In the phase II
clinical study, however, this regimen induced limited activity in
triple-negative breast cancer (41).
Although Chk1 inhibitors, for example AZD7762 and PF-477736, also
failed to induce anti-cancer effects as significant as those
exerted by single agents, they have the potential, as
chemosensitizers, to sensitize cancer cells to a variety of
DNA-damaging agents. AZD7762 is an adenosine triphosphate
(ATP)-competitive Chk1/2 inhibitor. PF-477736 is a potent,
selective ATP-competitive Chk1 inhibitor. Phase I/II clinical
studies revealed that Chk1 inhibitors exhibited limited activity,
which often outweighed the marginal efficacy benefit in combination
with chemotherapy in certain types of cancer, including advanced
solid cancer and refractory acute leukemia (41,42).
Unfortunately, there have as yet been no reports in the literature
of profound enhancements in patient survival.
Two major factors, which have crucial roles in the
development of clear cell carcinoma, include HNF-1β overexpression
and persistent Chk1 activation, associated with detoxification and
cell cycle arrest, respectively (Fig.
1). Chemoresistance may be regulated by HNF-1β-dependent
continued activation of Chk1 (9).
Accurate assessment of the overexpression of HNF-1β and continued
activation of Chk1 in clear cell carcinoma tissues aid the
identification of personalized Chk1 therapies to molecularly
selected patients. Therefore, Chk1 inhibitors will be approved for
selected patients whose HNF-1β and Chk1 status has been defined by
immunohistochemical analysis. Such future personalized therapeutic
approaches warrant further research in vivo, via animal
studies and clinical trials.
Conclusion
The present study reviews current knowledge
regarding the epigenetic and genetic alterations, and aberrant
molecular pathways underlying clear cell carcinoma, as well as
discussing the advancement of personalized therapeutic approaches.
Furthermore, the association of HNF-1β with cell cycle checkpoint
machinery was also evaluated as a mechanism for
chemoresistance.
Clear cell carcinoma develops high intrinsic
resistance to conventional platinum-based chemotherapy (43). Genome-wide profiling analyses and
proteomic-based studies revealed that genes associated with
oxidative stress, detoxification and chemoresistance were enriched
in clear cell carcinoma (21,24–27). These
analyses identified the molecular basis of diverse biological
phenomena mediated by transcription factor HNF-1β, and suggest that
HNF-1β contributes to the enhancement of cell cycle checkpoint
machinery against oxidative stress-induced DNA damage. Clear cell
carcinoma-specific HNF-1β is vital for proper cell cycle G2/M phase
arrest and induces chemoresistance. HNF-1β siRNA treatment
abrogates cell cycle arrest and results in apoptosis in
HNF-1β-overexpressing clear cell carcinoma cells (40). The loss of HNF-1β may also enhance
chemosensitivity (9). Therefore,
pharmacological inactivation of HNF-1β is an emerging concept
underlying the development of novel anti-cancer agents. However,
since the HNF-1β gene is expressed in important organs, including
the liver and kidney, this targeted therapy may induce significant
adverse reactions.
The progression of clear cell carcinoma is driven by
specific genomic alterations, including hypomethylation of the
HNF-1β gene, leading to the generation of abnormal proteins
(continued Chk1 phosphorylation) that can be targeted. Based on
recent observations, targeting HNF-1β-associated downstream
pathways, for example ATR-Chk1 signaling, may mediate tumoricidal
effects (9). The ATR kinase serves as
a transducer of the oxidative stress-dependent damage signal,
phosphorylating and activating the downstream effector kinase Chk1.
Chk1 inhibitors inhibit the growth of sensitive
HNF-1β-overexpressing cells by inducing significant levels of cell
apoptosis, in addition to contributing to therapeutic efficacy of
HNF-1β-based treatments (41,42). The effectiveness of Chk1 inhibitors
may be dependent on HNF-1β overexpression in clear cell carcinoma.
Therefore, target-based agents are active only in molecularly
selected populations of patients. The combination of conventional
chemotherapy and targeted Chk1-inhibitor therapy may be explored as
a personalized treatment modality for selected patients presenting
tumors with HNF-1β overexpression. HNF-1β and Chk1 may also
represent candidates for novel therapeutic targets in clear cell
carcinoma.
In conclusion, the present review provides a
possible explanation for why clear cell carcinoma still possesses
the cell cycle arrest response, and discusses current knowledge
regarding how this type of cancer affects the ATR-Chk1 network.
Predictive biomarkers of ATR-Chk1 network activity may indicate the
specific subgroup of patients able to benefit from Chk1
inhibitors.
Acknowledgements
The present review was supported by a grant-in-aid
for Scientific Research from the Ministry of Education, Science and
Culture of Japan (no. 26293361) to the Department of Obstetrics and
Gynecology, Nara Medical University (to Professor Hiroshi
Kobayashi).
References
|
1
|
Bulun SE: Endometriosis. N Engl J Med.
360:268–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dzatic-Smiljkovic O, Vasiljevic M, Djukic
M, Vugdelic R and Vugdelic J: Frequency of ovarian endometriosis in
epithelial ovarian cancer patients. Clin Exp Obstet Gynecol.
38:394–398. 2011.PubMed/NCBI
|
|
3
|
Abugo OO and Rifkind JM: Oxidation of
hemoglobin and the enhancement produced by nitroblue tetrazolium. J
Biol Chem. 269:24845–24853. 1994.PubMed/NCBI
|
|
4
|
Shikama K and Matsuoka A: Human
haemoglobin: A new paradigm for oxygen binding involving two types
of alphabeta contacts. Eur J Biochem. 270:4041–4051. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Polak G, Wertel I, Barczyński B,
Kwaśniewski W, Bednarek W and Kotarski J: Increased levels of
oxidative stress markers in the peritoneal fluid of women with
endometriosis. Eur J Obstet Gynecol Reprod Biol. 168:187–190. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mahalingaiah PK and Singh KP: Chronic
oxidative stress increases growth and tumorigenic potential of
MCF-7 breast cancer cells. PLoS One. 9:e873712014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akasaka J, Uekuri C, Shigetomi H, Koike M
and Kobayashi H: Hepatocyte nuclear factor (HNF)-1β and its
physiological importance in endometriosis. Biomed Rep. 1:13–17.
2013.PubMed/NCBI
|
|
9
|
Shigetomi H, Sudo T, Shimada K, Uekuri C,
Tsuji Y, Kanayama S, Naruse K, Yamada Y, Konishi N and Kobayashi H:
Inhibition of cell death and induction of G2 arrest accumulation in
human ovarian clear cells by HNF-1β transcription factor:
Chemosensitivity is regulated by checkpoint kinase CHK1. Int J
Gynecol Cancer. 24:838–843. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi H, Kajihara H, Yamada Y, Tanase
Y, Kanayama S, Furukawa N, Noguchi T, Haruta S, Yoshida S, Naruse
K, et al: Risk of carcinoma in women with ovarian endometrioma.
Front Biosci (Elite Ed). 3:529–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Liu X, Janes J, Stapley R, Patel
RP, Gladwin MT and Kim-Shapiro DB: Mechanism of faster NO
scavenging by older stored red blood cells. Redox Biol. 2:211–219.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cadet J, Delatour T, Douki T, Gasparutto
D, Pouget JP, Ravanat JL and Sauvaigo S: Hydroxyl radicals and DNA
base damage. Mutat Res. 424:9–21. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei H, Cai Q and Rahn RO: Inhibition of UV
light- and Fenton reaction-induced oxidative DNA damage by the
soybean isoflavone genistein. Carcinogenesis. 17:73–77. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobayashi H, Kajiwara H, Kanayama S,
Yamada Y, Furukawa N, Noguchi T, Haruta S, Yoshida S, Sakata M,
Sado T and Oi H: Molecular pathogenesis of endometriosis-associated
clear cell carcinoma of the ovary (review). Oncol Rep. 22:233–240.
2009.PubMed/NCBI
|
|
15
|
Xue X and Shah YM: Intestinal iron
homeostasis and colon tumorigenesis. Nutrients. 5:2333–2351. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toyokuni S: Mysterious link between iron
overload and CDKN2A/2B. J Clin Biochem Nutr. 48:46–49. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rolfs F, Huber M, Gruber F, Böhm F,
Pfister HJ, Bochkov VN, Tschachler E, Dummer R, Hohl D, Schäfer M,
et al: Dual role of the antioxidant enzyme peroxiredoxin 6 in skin
carcinogenesis. Cancer Res. 73:3460–3469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Zhang Y, Wu Q, Cui X, Lin Z, Liu S
and Chen L: Clinical implications of high NQO1 expression in breast
cancers. J Exp Clin Cancer Res. 33:142014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanase Y, Yamada Y, Shigetomi H, Kajihara
H, Oonogi A, Yoshizawa Y, Furukawa N, Haruta S, Yoshida S, Sado T,
et al: Modulation of estrogenic action in clear cell carcinoma of
the ovary (Review). Exp Ther Med. 3:18–24. 2012.PubMed/NCBI
|
|
20
|
Kajihara H, Yamada Y, Shigetomi H,
Higashiura Y and Kobayashi H: The dichotomy in the histogenesis of
endometriosis-associated ovarian cancer: Clear cell-type versus
endometrioid-type adenocarcinoma. Int J Gynecol Pathol. 31:304–312.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamada Y, Shigetomi H, Onogi A, Haruta S,
Kawaguchi R, Yoshida S, Furukawa N, Nagai A, Tanase Y, Tsunemi T,
et al: Redox-active iron-induced oxidative stress in the
pathogenesis of clear cell carcinoma of the ovary. Int J Gynecol
Cancer. 21:1200–1207. 2011.PubMed/NCBI
|
|
22
|
Itamochi H, Kigawa J, Akeshima R, Sato S,
Kamazawa S, Takahashi M, Kanamori Y, Suzuki M, Ohwada M and
Terakawa N: Mechanisms of cisplatin resistance in clear cell
carcinoma of the ovary. Oncology. 62:349–353. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pectasides D, Pectasides E, Psyrri A and
Economopoulos T: Treatment issues in clear cell carcinoma of the
ovary: A different entity? Oncologist. 11:1089–1094. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshida S, Furukawa N, Haruta S, Tanase Y,
Kanayama S, Noguchi T, Sakata M, Yamada Y, Oi H and Kobayashi H:
Theoretical model of treatment strategies for clear cell carcinoma
of the ovary: Focus on perspectives. Cancer Treat Rev. 35:608–615.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mogami T, Yokota N, Asai-Sato M, Yamada R,
Koizume S, Sakuma Y, Yoshihara M, Nakamura Y, Takano Y, Hirahara F,
et al: Annexin A4 is involved in proliferation, chemo-resistance
and migration and invasion in ovarian clear cell adenocarcinoma
cells. PLoS One. 8:e803592013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsuda H, Ito YM, Ohashi Y, Wong KK,
Hashiguchi Y, Welch WR, Berkowitz RS, Birrer MJ and Mok SC:
Identification of overexpression and amplification of ABCF2 in
clear cell ovarian adenocarcinomas by cDNA microarray analyses.
Clin Cancer Res. 11:6880–6888. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kajihara H, Yamada Y, Kanayama S, Furukawa
N, Noguchi T, Haruta S, Yoshida S, Sado T, Oi H and Kobayashi H:
Clear cell carcinoma of the ovary: Potential pathogenic mechanisms
(Review). Oncol Rep. 23:1193–1203. 2010.PubMed/NCBI
|
|
28
|
Mandai M, Matsumura N, Baba T, Yamaguchi
K, Hamanishi J and Konishi I: Ovarian clear cell carcinoma as a
stress-responsive cancer: Influence of the microenvironment on the
carcinogenesis and cancer phenotype. Cancer Lett. 310:129–133.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamaguchi K, Huang Z, Matsumura N, Mandai
M, Okamoto T, Baba T, Konishi I, Berchuck A and Murphy SK:
Epigenetic determinants of ovarian clear cell carcinoma biology.
Int J Cancer. 135:585–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hecht JL, Kotsopoulos J, Hankinson SE and
Tworoger SS: Relationship between epidemiologic risk factors and
hormone receptor expression in ovarian cancer: Results from the
Nurses' Health Study. Cancer Epidemiol Biomarkers Prev.
18:1624–1630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iida Y, Aoki K, Asakura T, Ueda K,
Yanaihara N, Takakura S, Yamada K, Okamoto A, Tanaka T and Ohkawa
K: Hypoxia promotes glycogen synthesis and accumulation in human
ovarian clear cell carcinoma. Int J Oncol. 40:2122–2130.
2012.PubMed/NCBI
|
|
32
|
Cuff J, Salari K, Clarke N, Esheba GE,
Forster AD, Huang S, West RB, Higgins JP, Longacre TA and Pollack
JR: Integrative bioinformatics links HNF1B with clear cell
carcinoma and tumor-associated thrombosis. PLoS One. 8:e745622013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dai Y and Grant S: New insights into
checkpoint kinase 1 in the DNA damage response signaling network.
Clin Cancer Res. 16:376–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zharkov DO: Base excision DNA repair. Cell
Mol Life Sci. 65:1544–1565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smith J, Tho LM, Xu N and Gillespie DA:
The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and
cancer. Adv Cancer Res. 108:73–112. 2010.PubMed/NCBI
|
|
36
|
Reinhardt HC and Yaffe MB: Kinases that
control the cell cycle in response to DNA damage: Chk1, Chk2, and
MK2. Curr Opin Cell Biol. 21:245–255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sugiyama T, Kamura T, Kigawa J, Terakawa
N, Kikuchi Y, Kita T, Suzuki M, Sato I and Taguchi K: Clinical
characteristics of clear cell carcinoma of the ovary: A distinct
histologic type with poor prognosis and resistance to
platinum-based chemotherapy. Cancer. 88:2584–2589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reed E, Yu JJ, Davies A, Gannon J and
Armentrout SL: Clear cell tumors have higher mRNA levels of ERCC1
and XPB than other histological types of epithelial ovarian cancer.
Clin Cancer Res. 9:5299–5305. 2003.PubMed/NCBI
|
|
39
|
Mao TL and Shih IeM: The roles of ARID1A
in gynecologic cancer. J Gynecol Oncol. 24:376–381. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsuchiya A, Sakamoto M, Yasuda J, Chuma M,
Ohta T, Ohki M, Yasugi T, Taketani Y and Hirohashi S: Expression
profiling in ovarian clear cell carcinoma: Identification of
hepatocyte nuclear factor-1 beta as a molecular marker and a
possible molecular target for therapy of ovarian clear cell
carcinoma. Am J Pathol. 163:2503–2512. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma CX, Ellis MJ, Petroni GR, Guo Z, Cai
SR, Ryan CE, Lockhart AC, Naughton MJ, Pluard TJ, et al: A phase II
study of UCN-01 in combination with irinotecan in patients with
metastatic triple negative breast cancer. Breast Cancer Res Treat.
137:483–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaufmann SH, Karp JE, Litzow MR, Mesa RA,
Hogan W, Steensma DP, Flatten KS, Loegering DA, Schneider PA,
Peterson KL, et al: Phase I and pharmacological study of cytarabine
and tanespimycin in relapsed and refractory acute leukemia.
Haematologica. 96:1619–1626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takano M, Kikuchi Y, Yaegashi N, Kuzuya K,
Ueki M, Tsuda H, Suzuki M, Kigawa J, Takeuchi S, Tsuda H, et al:
Clear cell carcinoma of the ovary: A retrospective multicentre
experience of 254 patients with complete surgical staging. Br J
Cancer. 94:1369–1374. 2006. View Article : Google Scholar : PubMed/NCBI
|















