Introduction
Cancer is caused by an imbalance between cell cycle
progression and apoptosis. Therefore, the majority of anticancer
drugs exert their antiproliferative and cytotoxic activity via cell
cycle arrest and induction of apoptosis, a controlled form of cell
death that is dysregulated in cancer (1). Cytotoxic nucleoside analogues were the
first chemotherapeutic agents used for the treatment of cancer
(2). To date, the most widely
researched cytotoxic nucleoside analogues are predominantly
isolated from Cordyceps sinensis and C.
militaris.
C. sinensis has been more extensively used
and investigated compared with C. militaris, however, both
species contain similar bioactive ingredients and exhibit medicinal
activity, and are widely used traditional Chinese medicines. A
number of bioactive ingredients have been isolated from C.
militaris, including adenosine, cordycepin, D-mannitol, and
exopolysaccharides. C. militaris has been widely used in
traditional Chinese medicine (3), and
is claimed to have extensive pharmacological properties, including
anti-inflammatory properties, cell cycle disruption, enhancement of
immune function, nucleic acid-containing (DNA/RNA) and
apoptosis-inducing activities; anti-fatigue, anti-bacterial and
anti-diabetic properties; improving lung, liver and kidney
functions; and aiding in the treatment of cancer as well as male
and female sexual dysfunctions (3).
C. militaris is therefore receiving much attention due to
these potential health benefits, and it is often used as a
substitute for C. sinensis in certain traditional Chinese
medicine prescriptions as the two species contain similar bioactive
ingredients (4). In view of the
growing popularity of C. militaris, cultivation of the C.
militaris stroma, a spore of C. militaris, is also
conducted. A wide diversity of compounds have been isolated from
different strains of Cordyceps as well as artificially
cultivated C. militaris (4).
Cordycepin, also known as 3-deoxyadenosine, is a
specific polyadenylation inhibitor and is the main functional
component in C. militaris (5).
Cordycepin is an active small molecule and is implicated in
modulating multiple physiological functions, including
immune-activation (anti-virus, anti-infection) (6), anti-inflammatory, anti-aging (adjustment
of the physical condition, an anticancer effect and enhancement of
sexual performance) (7) and
anti-tumor effects (8).
Throughout the last 60 years, the antitumor
mechanism of Cordycepin has become extensively studied. In 1961,
Jagger et al (9) investigated
the inhibition of Ehrlich mouse ascites tumor growth by cordycepin
and Klenow (10) also investigated
the effect of cordycepin on the incorporation of P32-orthophosphate
into the nucleic acid of ascites tumor cells in vitro. These
studies prompted further research into the association between
cordycepin and cancer. In the subsequent decades, numerous studies
with regard to cordycepin and cancer were reported. For example,
cordycepin was shown to induce antitumor effects or apoptosis in
human head and neck squamous cell carcinoma (11), bladder cancer (12), thyroid carcinoma (13), breast cancer (14), multiple myeloma (15), leukemia (5,16,17), lymphoma (18) and mouse leydig tumor cells (19). Additionally, the inhibitory effect of
cordycepin was observed in hematogenic metastasis of mouse melanoma
(20,21) and lung carcinoma cells (22). Cell cycle arrest was observed to be
promoted by cordycepin via the regulation of c-Jun N-terminal
kinase (JNK) in human bladder and colon cancer cells (23,24). In
hematological malignancies, cordycepin exhibited cytotoxic and
apoptogenic effects via the inactivation of polyadenylate
polymerase and the resulting inhibition of mRNA polyadenylation
(18). In terminal deoxynucleotidyl
transferase-positive leukemic cells, these effects are more
prominent (18).
Cell proliferation inhibition induced by
cordycepin
It is important to understand how cordycepin
inhibits cell proliferation in tumor cells in order to develop it
as a new agent for the prevention and treatment of cancer. The A3
adenosine receptor (A3AR) is a member of the AR family;
it is overexpressed in cancer and inflammatory cells, however, low
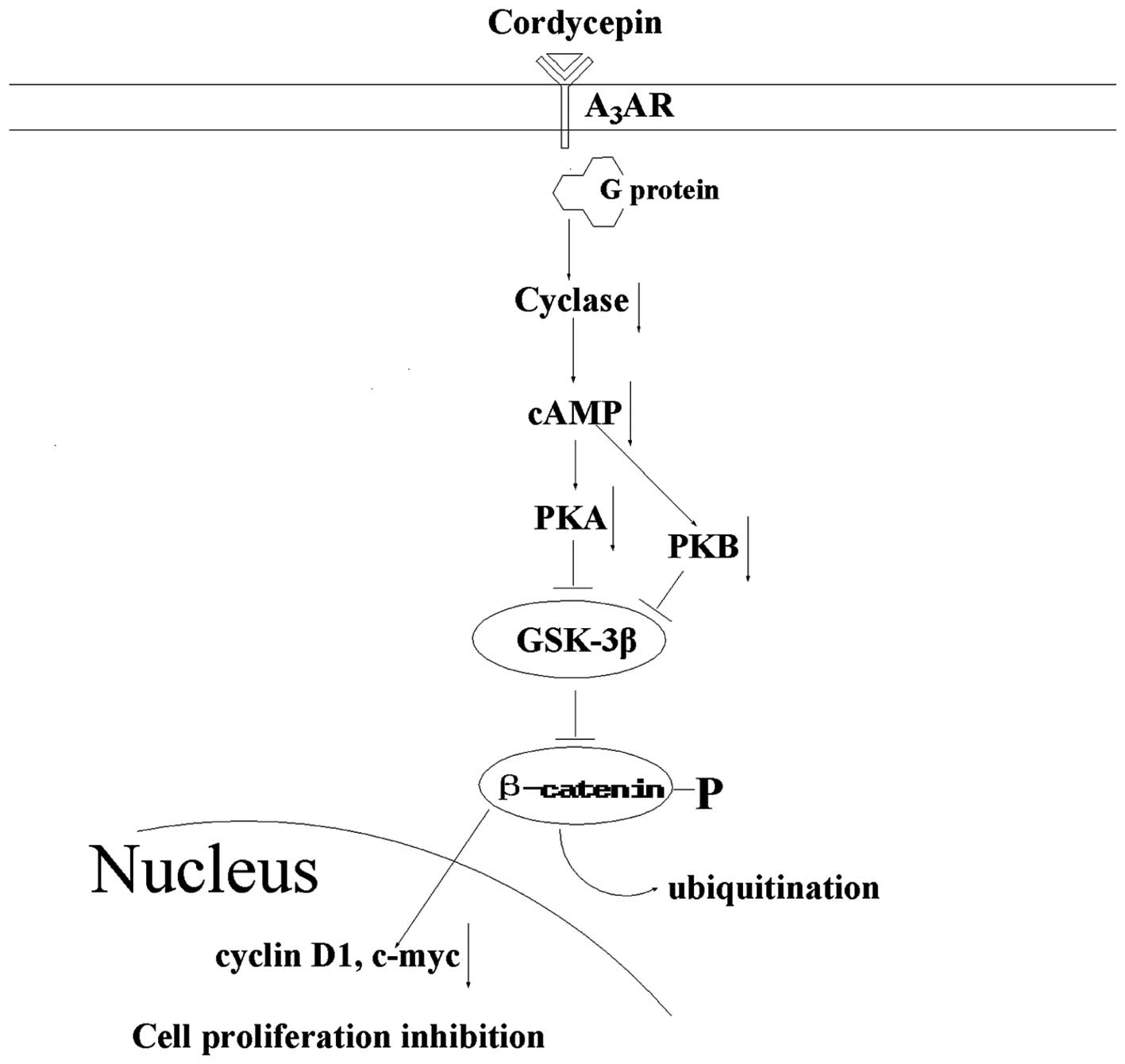
expression is exhibited in normal cells (25). Following cordycepin binding to
A3AR, G protein is activated and subsequently inhibits
the formation of cAMP and indirectly decreases phosphorylation of
the serine/threonine kinase glycogen synthase kinase (GSK)-3β. The
resulting increased phosphorylation of β-catenin causes it to be
removed from the cytoplasm by ubiquitination, thereby preventing
its nuclear import. A net suppression of cyclin D1 and c-myc is the
result of this, which leads to the inhibition of cell growth
(Fig. 1) (26). Thus, as cordycepin binds
A3AR, which inactivates the GSK-3β/β-catenin signaling
pathway and subsequently suppresses cell division, cordycepin may
present a potential therapeutic agent for the inhibition of tumor
cell proliferation.
Cell apoptosis induced by cordycepin
Protein kinase A (PKA)/terminal
deoxynucleotidyl transferase (TdT) signaling pathway
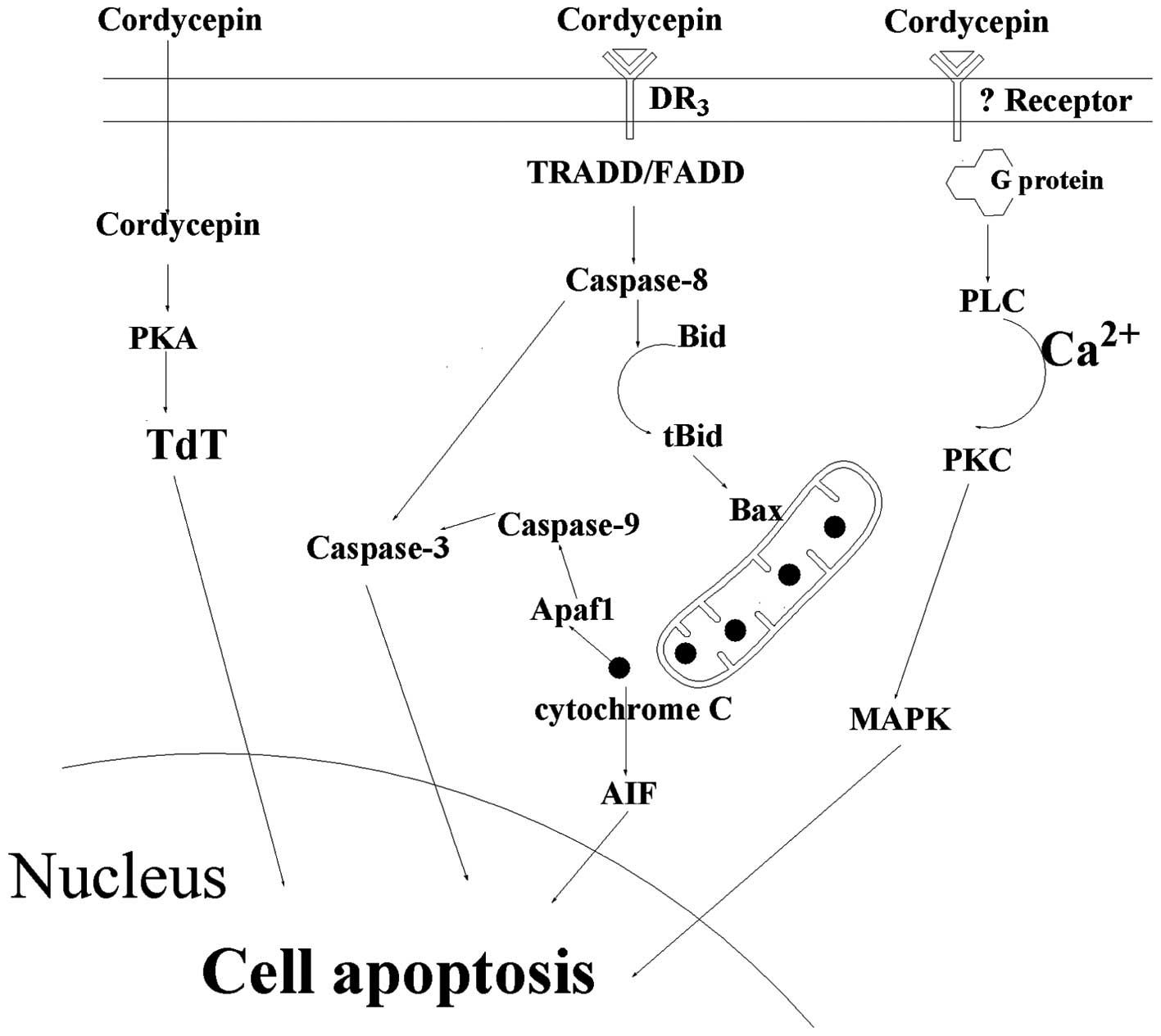
In 1996, Koc et al (27) speculated that cordycepin monophosphate
in TdT-positive cells may be able to activate PKA in place of cAMP,
and that PKA may phosphorylate TdT, augmenting its activity as an
endonuclease. In cell-free experiments, the activity of recombinant
TdT as an endonuclease digesting supercoiled plasmid DNA into
linear fragments was significantly increased following
phosphorylation of TdT by PKA. Therefore, the potential role of TdT
as an apoptotic endonuclease in TdT-positive leukemia cells
following cordycepin exposure requires investigation in the
future.
Caspase signaling pathway
Cordycepin is also involved in apoptosis through the
caspase pathway. In 2011, Jen et al (19) confirmed that cordycepin was able to
induce MA-10 mouse Leydig tumor cell apoptosis via the caspase-9
pathway (Fig. 2), where cordycepin
administration was found to increase the expression of caspase-9,
-3 and -7 proteins. The same year, Jeong et al (5) proposed a mechanism by which cordycepin
induces the apoptosis of human leukemia cells through a signaling
cascade involving a reactive oxygen species-mediated caspase
pathway, which was indicated by the generation of reactive oxygen
species, mitochondrial dysfunction, activation of capsases and
cleavage of poly (ADP ribose) polymerase 1 (PARP) in the study.
Imesch et al (28)
demonstrated activated caspase-dependent, intrinsic apoptosis,
which was indicated by the proteolytic cleavage of caspase-9,
caspase-3 and PARP. Choi et al (29) showed that cordycepin-induced cell
death in MDA-MB-231 cells was associated with the translocation of
Bax from the cytosol to the mitochondria, a feature of the
mitochondria-mediated apoptotic pathway; this was confirmed by DNA
fragmentation, terminal deoxynucleotidyl transferase dUTP nick end
labeling, and immunocytochemical analysis. Additionally, cordycepin
induced a dose-dependent increase in mitochondrial translocation of
Bax, causing the cytosolic release of cytochrome c and
activation of caspase-9 and -3. Notably, autophagy-associated cell
death was observed for MCF-7 cells, demonstrated by the detection
of an autophagosome-specific protein and large membranous vacuole
ultrastructure morphology in the cytoplasm.
Cell proliferation is inhibited by cordycepin via
binding to A3AR; however programmed cell death will
occur following the interaction of cordycepin and the death
receptor, DR3, which is a death-domain-containing tumor necrosis
factor family receptor (30). A
previous study demonstrated that the expression levels of certain
proteins related to apoptosis, including p53 and Bax, were
increased following treatment with cordycepin for 18 h, and also
confirmed that DR3, caspase-8, caspase-1, cleaved caspase-3 and
cleaved PARP expression was increased (31). After cordycepin binding to the DR3
receptor, DR3 recruits initiator caspase-8 via the adaptor protein
tumor necrosis factor receptor type 1-associated DEATH domain
protein/Fas-associated protein with death domain (FADD). Caspase-8
subsequently oligomerizes, and is activated via autocatalysis. This
indicates that activated caspase-8 stimulates apoptosis via two
parallel cascades: Direct activation of caspase-3 or the release of
cytochrome c.
Direct activation of caspase-3
Caspase-8 may directly cleave and activate caspase-3
(Fig. 2). In particular, caspase-3
(also known as CPP32/Yama/apopain) (32–34)
comprises a 32 kDa zymogen that is cleaved into 17 kDa and 12 kDa
subunits when it interacts with caspase-8/9. When the procaspase is
cleaved at a particular residue, the active heterotetramer may then
form via hydrophobic interactions, resulting in four anti-parallel
β-sheets from p17 and two from p12 to combine to form a
heterodimer. The zymogen feature of caspase-3 is necessary as, if
unregulated, caspase activity would kill cells indiscriminately.
Caspase 3 is an executioner caspase, and therefore its zymogen has
almost no activity until it is cleaved by an initiator caspase
after apoptotic signaling events have occurred (35). Western blot analysis demonstrated the
induction of active caspase-3 and PARP cleavage by cordycepin
treatment, indicating that cordycepin is able to activate the
caspase-3 pathway (36).
Release of cytochrome c
When caspase-3 is activated, concurrently, caspase-8
also cleaves the pro-apoptotic Bcl-2 family protein, Bid. Following
this, truncated Bid (tBid) translocates to the mitochondria and
induces cytochrome c release, which results in an increase
of the Bax/Bcl-xL ratio (37).
Cytochrome c sequentially binds apoptotic protease
activating factor-1 (Apaf1), forms an activation complex with
caspase-9 and activates caspase-3 (3). Furthermore, cytochrome c has been
demonstrated to activate apoptosis-inducing factor, which migrates
to the nucleus and induces cell apoptosis; however, the associated
signaling pathway is yet to be confirmed experimentally (3).
p38/JNK signaling pathway
Based on the increased expression of PKC,
extracellular signal-regulated kinase 1/2 (ERK1/2) and c-JNK, as
determined by Western blot analysis, Pao et al (7) reported that cordycepin stimulated
intracellular phospholipase C/PKC and mitogen-activated protein
kinase (MAPK) signal transduction pathways to induce cell death in
MA-10 mouse Leydig tumor cells. This result indicated that
cordycepin induces tumor cell death through PKC/MAPK signaling
pathways. Lee et al (38)
performed Western blot analysis, which demonstrated that
JNK-inactivating phosphatase was upregulated in response to
treatment with cordycepin in human hepatocellular carcinoma Hep3B
cells. Thus, according to the results of Pao et al (7) and Lee et al (38), we hypothesize that cordycepin induces
cell apoptosis via the activation of PLC/PKC and subsequent
inactivation of JNK, which is an important component of the MAPK
and downstream PLC/PKC signaling pathways. Western blot analysis
performed by Chen et al (39,40) also
found that 24 h treatment with 10 or 100 µmol/l cordycepin
exhibited a synergistically apoptotic effect through the activation
of JNK/caspase-7/PARP pathway in human OC3 oral cancer cells. These
analyses indicate that cordycepin may also induce cancer cell
apoptosis/death by PKC, MAPK, JNK, caspase-7, and PARP
pathways.
Conclusions and perspectives
Collectively, the findings that are discussed in the
present review provide a novel insight into the effect of
cordycepin on cell apoptosis, which supports new concepts for the
treatment of cancer. Cell proliferation and apoptosis involve the
functional cooperation of many signaling molecules. As summarized
in Fig. 1, cordycepin inhibits cell
proliferation via binding A3AR, activating G protein,
inhibiting cAMP formation, decreasing GSK-3β/β-catenin activation
and suppressing cyclin D1 and c-myc expression.
Cordycepin induces cell apoptosis through three
signaling pathways: PKA/TdT signaling pathway, caspase signaling
pathway and p38/JNK signaling pathway. The caspase signaling
pathway is the most prominent signal pathway by which cell
apoptosis is induced by cordycepin. Following cordycepin binding to
the DR3 receptor, DR3 activates caspase-8 via FADD. Subsequently,
caspase-8 may directly activate caspase-3 or induce mitochondria to
release cytochrome c, which also activates caspase-3 with
the help of Apaf1 and caspase-9.
These results indicate that a cocktail therapy with
cordycepin may greatly reduce the risk of cancer cell metastasis of
all types. The significance of cordycepin as a natural medicine is
that may be used to treat or prevent cancer progression in the
future (41). Further studies are
required to determine which of the signaling pathways is most
important for the treatment of cancer with cordycepin. In addition,
many of the previous studies were focused on the activity of
cordycepin at a cellular level. Future in vivo studies
investigating the inhibition of tumor progression by cordycepin may
provide further insight into the mechanisms behind its
activity.
Acknowledgements
This work was supported by grants from the Natural
Scientific Foundation of Chinese Shandong Province (grant no.
ZR2010CQ031 and ZR2014CM046) and Shanghai Key Lab of Human
Performance, Shanghai University of Sport (grant no.
11DZ2261100).
References
|
1
|
Thomadaki H, Tsiapalis CM and Scorilas A:
Polyadenylate polymerase modulations in human epithelioid cervix
and breast cancer cell lines, treated with etoposide or cordycepin,
follow cell cycle rather than apoptosis induction. Biol Chem.
386:471–480. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tuli HS, Sharma AK, Sandhu SS and Kashyap
D: Cordycepin: A bioactive metabolite with therapeutic potential.
Life Sci. 93:863–869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee HH, Park C, Jeong JW, et al: Apoptosis
induction of human prostate carcinoma cells by cordycepin through
reactive oxygen species-mediated mitochondrial death pathway. Int J
Oncol. 42:1036–1044. 2013.PubMed/NCBI
|
|
4
|
Lim L, Lee C and Chang E: Optimization of
solid state culture conditions for the production of adenosine,
cordycepin and D-mannitol in fruiting bodies of medicinal
caterpillar fungus cordyceps militaris (L: Fr.) Link (Ascomycetes).
Int J Med Mushrooms. 14:181–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeong JW, Jin CY, Park C, et al: Induction
of apoptosis by cordycepin via reactive oxygen species generation
in human leukemia cells. Toxicol In vitro. 25:817–824. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim HG, Shrestha B, Lim SY, et al:
Cordycepin inhibits lipopolysaccharide-induced inflammation by the
suppression of NF-kappaB through Akt and p38 inhibition in RAW
264.7 macrophage cells. Eur J Pharmacol. 545:192–199. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pao HY, Pan BS, Leu SF and Huang BM:
Cordycepin stimulated steroidogenesis in MA-10 mouse leydig tumor
cells through the protein kinase C pathway. J Agric Food Chem.
60:4905–4913. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao WL, Ko BS, Liu TA, et al: Cordycepin
suppresses integrin/FAK signaling and epithelial-mesenchymal
transition in hepatocellular carcinoma. Anticancer Agents Med Chem.
14:29–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jagger Dv, Kredich Nm and Guarino AJ:
Inhibition of Ehrlich mouse ascites tumor growth by cordycepin.
Cancer Res. 21:216–220. 1961.PubMed/NCBI
|
|
10
|
Klenow H: Effect of cordycepin on the
incorporation of P32-orthophosphate into the nucleic acids of
ascites tumor cells in vitro. Biochem Biophys Res Commun. 5:156–9.
1961. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu WC, Hsiao JR, Lian YY, Lin CY and Huang
BM: The apoptotic effect of cordycepin on human OEC-M1 oral cancer
cell line. Cancer Chemother Pharmacol. 60:103–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee EJ, Kim WJ and Moon SK: Cordycepin
suppresses TNF-alpha-induced invasion, migration and matrix
metalloproteinase-9 expression in human bladder cancer cells.
Phytother Res. 24:1755–1761. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Chen YC, Lin YT, Huang SH and Wang
SM: Cordycepin induces apoptosis of CGTH W-2 thyroid carcinoma
cells through the calcium-calpain-caspase 7-PARP pathway. J Agric
Food Chem. 58:11645–11652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HJ, Burger P, Vogel M, et al: The
nucleoside antagonist cordycepin causes DNA double strand breaks in
breast cancer cells. Invest New Drugs. 30:1917–1925. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen LS, Stellrecht CM and Gandhi V:
RNA-directed agent, cordycepin, induces cell death in multiple
myeloma cells. Br J Haematol. 140:682–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuda H, Akaki J, Nakamura S, et al:
Apoptosis-inducing effects of sterols from the dried powder of
cultured mycelium of Cordyceps sinensis. Chem Pharm Bull (Tokyo).
57:411–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kodama EN, McCaffrey RP, Yusa K and
Mitsuya H: Antileukemic activity and mechanism of action of
cordycepin against terminal deoxynucleotidyl transferase-positive
(TdT+) leukemic cells. Biochem Pharmacol. 59:273–281. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thomadaki H, Tsiapalis CM and Scorilas A:
The effect of the polyadenylation inhibitor cordycepin on human
Molt-4 and Daudi leukaemia and lymphoma cell lines. Cancer
Chemother Pharmacol. 61:703–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jen CY, Lin CY, Huang BM and Leu SF:
Cordycepin induced MA-10 mouse leydig tumor cell apoptosis through
Caspase-9 pathway. Evid Based Complement Alternat Med.
2011:9845372011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshikawa N, Kunitomo M, Kagota S,
Shinozuka K and Nakamura K: Inhibitory effect of cordycepin on
hematogenic metastasis of B16-F1 mouse melanoma cells accelerated
by adenosine-5′-diphosphate. Anticancer Res. 29:3857–3860.
2009.PubMed/NCBI
|
|
21
|
Yoshikawa N, Yamada S, Takeuchi C, et al:
Cordycepin (3′-deoxyadenosine) inhibits the growth of B16-BL6 mouse
melanoma cells through the stimulation of adenosine A3 receptor
followed by glycogen synthase kinase-3beta activation and cyclin D1
suppression. Naunyn Schmiedebergs Arch Pharmacol. 377:591–595.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura K, Yoshikawa N, Yamaguchi Y, et
al: Antitumor effect of cordycepin (3′-deoxyadenosine) on mouse
melanoma and lung carcinoma cells involves adenosine A3 receptor
stimulation. Anticancer Res. 26:43–47. 2006.PubMed/NCBI
|
|
23
|
Lee SJ, Kim SK, Choi WS, Kim WJ and Moon
SK: Cordycepin causes p21WAF1-mediated G2/M cell-cycle arrest by
regulating c-Jun N-terminal kinase activation in human bladder
cancer cells. Arch Biochem Biophys. 490:103–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee SJ, Moon GS, Jung KH, Kim WJ and Moon
SK: c-Jun N-terminal kinase 1 is required for cordycepin-mediated
induction of G2/M cell-cycle arrest via p21WAF1 expression in human
colon cancer cells. Food Chem Toxicol. 48:277–283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Madi L, Ochaion A, Rath-Wolfson L, et al:
The A3 adenosine receptor is highly expressed in tumor versus
normal cells: potential target for tumor growth inhibition. Clin
Cancer Res. 10:4472–4479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fishman P, Bar-Yehuda S, Liang BT and
Jacobson KA: Pharmacological and therapeutic effects of A3
adenosine receptor agonists. Drug Discov Today. 17:359–366. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koc Y, Urbano AG, Sweeney EB and McCaffrey
R: Induction of apoptosis by cordycepin in ADA-inhibited
TdT-positive leukemia cells. Leukemia. 10:1019–1024.
1996.PubMed/NCBI
|
|
28
|
Imesch P, Hornung R, Fink D and Fedier A:
Cordycepin (3′-deoxyadenosine), an inhibitor of mRNA
polyadenylation, suppresses proliferation and activates apoptosis
in human epithelial endometriotic cells in vitro. Gynecol Obstet
Invest. 72:43–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi S, Lim MH, Kim KM, Jeon BH, Song WO
and Kim TW: Cordycepin-induced apoptosis and autophagy in breast
cancer cells are independent of the estrogen receptor. Toxicol Appl
Pharmacol. 257:165–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chinnaiyan AM, O'Rourke K, Yu GL, Lyons
RH, Garg M, Duan DR, Xing L, Gentz R, Ni J and Dixit VM: Signal
transduction by DR3, a death domain-containing receptor related to
TNFR-1 and CD95. Science. 274:990–992. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SY, Debnath T, Kim SK and Lim BO:
Anti-cancer effect and apoptosis induction of cordycepin through
DR3 pathway in the human colonic cancer cell HT-29. Food Chem
Toxicol. 60:439–447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nicholson DW, Ali A, Thornberry NA, et al:
Identification and inhibition of the ICE/CED-3 protease necessary
for mammalian apoptosis. Nature. 376:37–43. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tewari M, Quan LT, O'Rourke K, et al:
Yama/CPP32 beta, a mammalian homolog of CED-3, is a
CrmA-inhibitable protease that cleaves the death substrate poly
(ADP-ribose) polymerase. Cell. 81:801–809. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fernandes-Alnemri T, Litwack G and Alnemri
ES: CPP32, a novel human apoptotic protein with homology to
Caenorhabditis elegans cell death protein Ced-3 and mammalian
interleukin-1 beta-converting enzyme. J Biol Chem. 269:30761–30764.
1994.PubMed/NCBI
|
|
35
|
Walters J, Pop C, Scott FL, et al: A
constitutively active and uninhibitable caspase-3 zymogen
efficiently induces apoptosis. Biochem J. 424:335–345. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baik JS, Kwon HY, Kim KS, Jeong YK, Cho YS
and Lee YC: Cordycepin induces apoptosis in human neuroblastoma
SK-N-BE (2)-C and melanoma SK-MEL-2 cells. Indian J Biochem
Biophys. 49:86–91. 2012.PubMed/NCBI
|
|
37
|
Shimizu S and Tsujimoto Y: Proapoptotic
BH3-only Bcl-2 family members induce cytochrome c release, but not
mitochondrial membrane potential loss, and do not directly modulate
voltage-dependent anion channel activity. Proc Natl Acad Sci USA.
97:577–582. 2000.PubMed/NCBI
|
|
38
|
Lee HH, Jeong JW, Lee JH, et al:
Cordycepin increases sensitivity of Hep3B human hepatocellular
carcinoma cells to TRAIL-mediated apoptosis by inactivating the JNK
signaling pathway. Oncol Rep. 30:1257–1264. 2013.PubMed/NCBI
|
|
39
|
Chen YH, Hao LJ, Hung CP, Chen JW, Leu SF
and Huang BM: Apoptotic effect of cisplatin and cordycepin on OC3
human oral cancer cells. Chin J Integr Med. 20:624–632. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen YH, Wang JY, Pan BS, et al:
Cordycepin enhances cisplatin apoptotic effect through caspase/MAPK
pathways in human head and neck tumor cells. Onco Targets Ther.
6:983–998. 2013.PubMed/NCBI
|
|
41
|
Tian X, Zhao X, Yin K, Mao D, Yang J and
Wang Q: Immunoregulation ability comparison of cordycepin and
Flammulina velutipes polysaccharide in rat body after exhaustive
exercise. Int J Biol Biol Sci. 2:136–142. 2013.
|