Introduction
Ovarian cancer is one of the most common
malignancies of the female reproductive organs, with an incidence
rate second only to cervical cancer. Epithelial tumors account for
50–70% of primary ovarian tumors, and 85–90% of ovarian
malignancies (1). In clinical
treatment, although surgery combined with chemotherapy is an
established treatment, the prognosis is poor when ovarian cancer is
detected late, with a recurrence rate of up to 85%, and a 5-year
survival rate of <30% (2). The
pathogenesis of ovarian cancer and the development of new
therapeutic strategies, such as endocrine treatment and targeted
therapies, are areas of current research (3).
Focal adhesion kinase (FAK) is a 125-kDa
non-receptor cytoplasmic tyrosine kinase. Over the last few years,
FAK, known as the first intracellular messenger, was found to be
involved in cell adhesion, spreading, proliferation, migration and
apoptosis (4). Further studies
demonstrated that FAK is highly expressed in a number of tumors and
is potentially correlated with tumor development and biological
behavior (5). Adrenomedullin (ADM) is
a 52-amino acid member of the calcitonin family of peptides. ADM is
present in a variety of human tissues and participates in a number
of pathophysiological processes. Previous studies have indicated
that ADM may stimulate tumor development and progression via
different pathways (6). To date,
little is known regarding the role of FAK and ADM in ovarian
cancer. The aim of the present study was to investigate the
expression and clinical significance of FAK and ADM in epithelial
ovarian cancer.
Materials and methods
Samples
A total of 60 ovarian tumor samples and 10 normal
ovarian tissue samples from patients with other diseases were
collected between March, 2008 and May, 2012 at the Department of
Gynecology and Obstetrics, Renmin Hospital of Wuhan University
(Wuhan, China). The samples were embedded in paraffin and cut in
4–5-µM sections. The ovarian tumors comprised 10 benign lesions and
50 epithelial ovarian cancers (20 serous, 17 mucous, 8 endometrioid
and 5 other types). The mean patient age was 50.5 years. The
malignant tumors were classified according to the International
Federation of Gynecology and Obstetrics (1988) criteria as
early-stage [n=15 (7, stage I; and 8, stage II)] and late-stage
[n=35 (28, stage III; and 7, stage IV)]. Following pathological
grading, 32 cases were classified as moderately or highly
differentiated and 18 cases as poorly differentiated or
undifferentiated.
The 10 cases of benign epithelial ovarian tumors
included 7 serous and 3 mucous tumors. The mean patient age was 42
years. The normal ovarian tissue samples were confirmed by
pathological examination and the mean age of the patients was 47.3
years. None of the patients had been administered chemotherapy,
radiotherapy, hormone therapy or immunotherapy prior to surgery.
The patients were followed up until May, 2013.
Written informed consent was obtained from all
patients, and the study was approved by the ethics committee of
Renmin Hospital of Wuhan University.
Reagents and methods
The rabbit anti-human FAK polyclonal antibody and
the streptavidin-peroxidase (SP) kit were purchased from Zhongshan
Biotechnology Co. (Beijing China). The rabbit anti-human ADM
polyclonal antibody and diaminobenzidine substrate were obtained
from Boster Bioengineering Co., Ltd. (Wuhan China). The
immunohistochemical SP method was applied according to the
manufacturer's instructions. Tissues with known FAK or ADM
expression were used as positive control and phosphate-buffered
saline instead of primary antibody was used as negative
control.
Result evaluation
The tissue sections were evaluated under a
microscope (MP5.0-RTV-CLR-10-C, MicroPublisher 5.0; Olympus Inc.,
Richmond Hill, ON, Canada) and cells with brown granules in the
cytoplasm and/or membrane were considered as FAK- or ADM-positive.
Semi-quantitative numerical integration was used to determine the
positivity level of each section based on the percentage of
positive cells and staining intensity. Scores were assigned
according to the percentage of positive cells (0, ≤5%; 1, 6–25%; 2,
26–50%; and 3, ≥51%) and intensity of staining (0, no staining; 1,
weak; 2, medium; and 3, strong) in 10 high-magnification fields. In
each sample, the scores for positive percentage and staining
intensity were added, reflecting different expression levels as
follows: -, ≤1; +, 2–3; ++, 4–5; and +++, >5.
Statistical analysis
Data were analyzed with the χ2 or
Fisher's exact tests using SPSS 13.0 software (SPSS, Inc., Chicago,
IL, USA) and the groups were compared with the Spearman's rank
correlation analysis. Survival data were analyzed with the
Kaplan-Meier method, tested with the log-rank method and further
analyzed for multiple factors using the Cox regression model.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of FAK and ADM in normal,
benign and malignant ovarian tissues
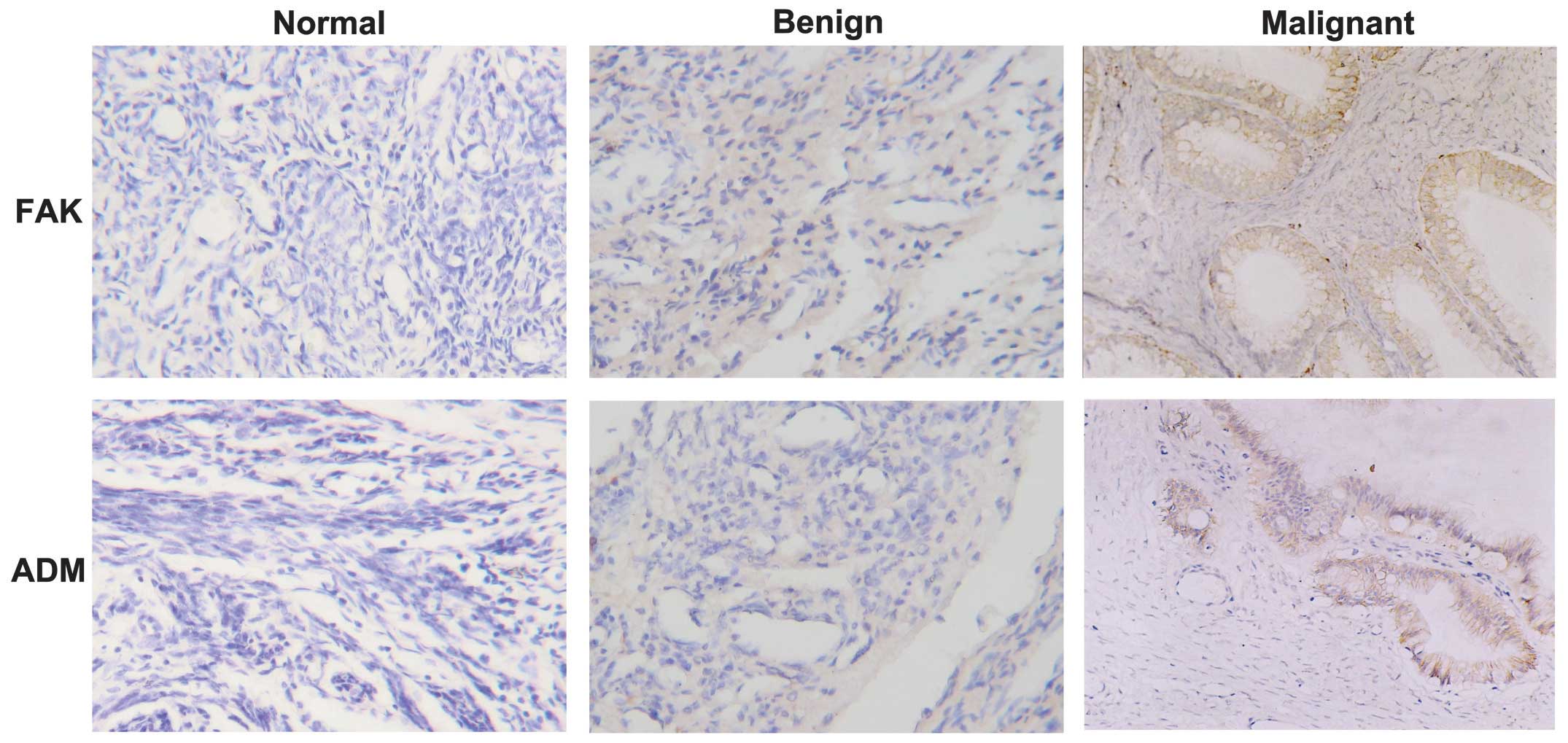
(Fig. 1) The FAK
protein was primarily expressed in the cytoplasm of cancer cells in
72.0% (36/50) of ovarian cancer cases and was weakly detected in a
limited number of normal and benign cases. The ADM protein was
mainly detected in the cytoplasm and/or membrane of cancer cells,
whereas different levels of ADM staining were observed in the
stroma and the center of the tumor. The ADM protein was positive in
78.0% (39/50) of ovarian cancers, 1 normal tissue sample (+) and 2
benign tumors (+ and ++). The statistical analysis demonstrated
that FAK and ADM were more highly expressed in ovarian cancer
compared with normal tissues and benign tumors (P<0.05), whereas
there was no significant difference between normal and benign
ovarian tissues (P>0.05) (Table
I).
 | Table I.Expression of FAK and ADM in different
ovarian tissues. |
Table I.
Expression of FAK and ADM in different
ovarian tissues.
| Tissue type | Total (n=70) | FAK+, no. (%)
(n=41) | ADM+, no. (%)
(n=42) |
|---|
| Normal | 10 | 2
(20.0) | 1
(10.0) |
| Benign | 10 | 3
(30.0) | 2
(20.0) |
| Cancer | 50 | 36 (72.0) | 39 (78.0) |
Expression of FAK and ADM in
epithelial ovarian cancer and correlation with clinicopathological
parameters
The expression of FAK was significantly correlated
with clinical stage, histological grade and lymph node metastasis
(P<0.05); however, there was no significant association with age
or histological type (P>0.05). The expression of ADM was
positively correlated with pathological grade and lymph node
metastasis (P<0.05), but not with age, histological type or
clinical stage (P>0.05) (Table
II).
 | Table II.Correlation between FAK or ADM
expression and clinicopathological parameters. |
Table II.
Correlation between FAK or ADM
expression and clinicopathological parameters.
| Total
Clinicopathological parameters | Total (n=50) | FAK+, no. (%)
(n=36) | P-value | ADM+, no. (%)
(n=39) | P-value |
|---|
| Age (years) |
|
| 1.000 |
| 1.000 |
| ≤50 | 18 | 13 (72.22) |
| 14 (77.78) |
|
|
>50 | 32 | 23 (71.88) |
| 25 (78.13) |
|
| FIGO stage |
|
| 0.017 |
| 1.000 |
| I/II | 19 | 10 (52.6) |
| 15 (78.9) |
|
|
III/IV | 31 | 26 (83.9) |
| 24 (77.4) |
|
| Histological
type |
|
| 0.688 |
| 0.646 |
|
Mucous | 17 | 11 (64.70) |
| 14 (82.35) |
|
|
Serous | 20 | 16 (80.00) |
| 15 (75.00) |
|
|
Endometrioid | 8 | 6
(75.00) |
| 7
(87.50) |
|
|
Others | 5 | 3
(60.00) |
| 3
(60.00) |
| Lymph node
metastasis |
|
| 0.011 |
| 0.000 |
| No | 28 | 16 (57.1) |
| 17 (60.71) |
|
| Yes | 22 | 20 (90.9) |
| 22 (100.0) |
|
| Pathological grading
(differentiation) |
|
| 0.000 |
| 0.033 |
|
Moderate/high | 30 | 16 (75.00) |
| 20 (66.7) |
|
|
Poor/undifferentiated | 20 | 20 (100.00) |
| 19 (95.0) |
|
Correlation between the expressions of
FAK and ADM
The Spearman's rank correlation analysis
demonstrated that, among all 50 cases of epithelial ovarian cancer,
31 were positive and 6 were negative for both proteins, suggesting
a rank positive correlation between the two (r=0.314, P=0.026)
(Table III).
 | Table III.Rank correlation between FAK and ADM
expression. |
Table III.
Rank correlation between FAK and ADM
expression.
| Expression | ADM+ | ADM- | Total |
|---|
| FAK+ | 31 | 5 | 36 |
| FAK- | 8 | 6 | 14 |
| Total | 39 | 11 | 50 |
Correlation between FAK or ADM
expression and prognosis in epithelial ovarian cancer
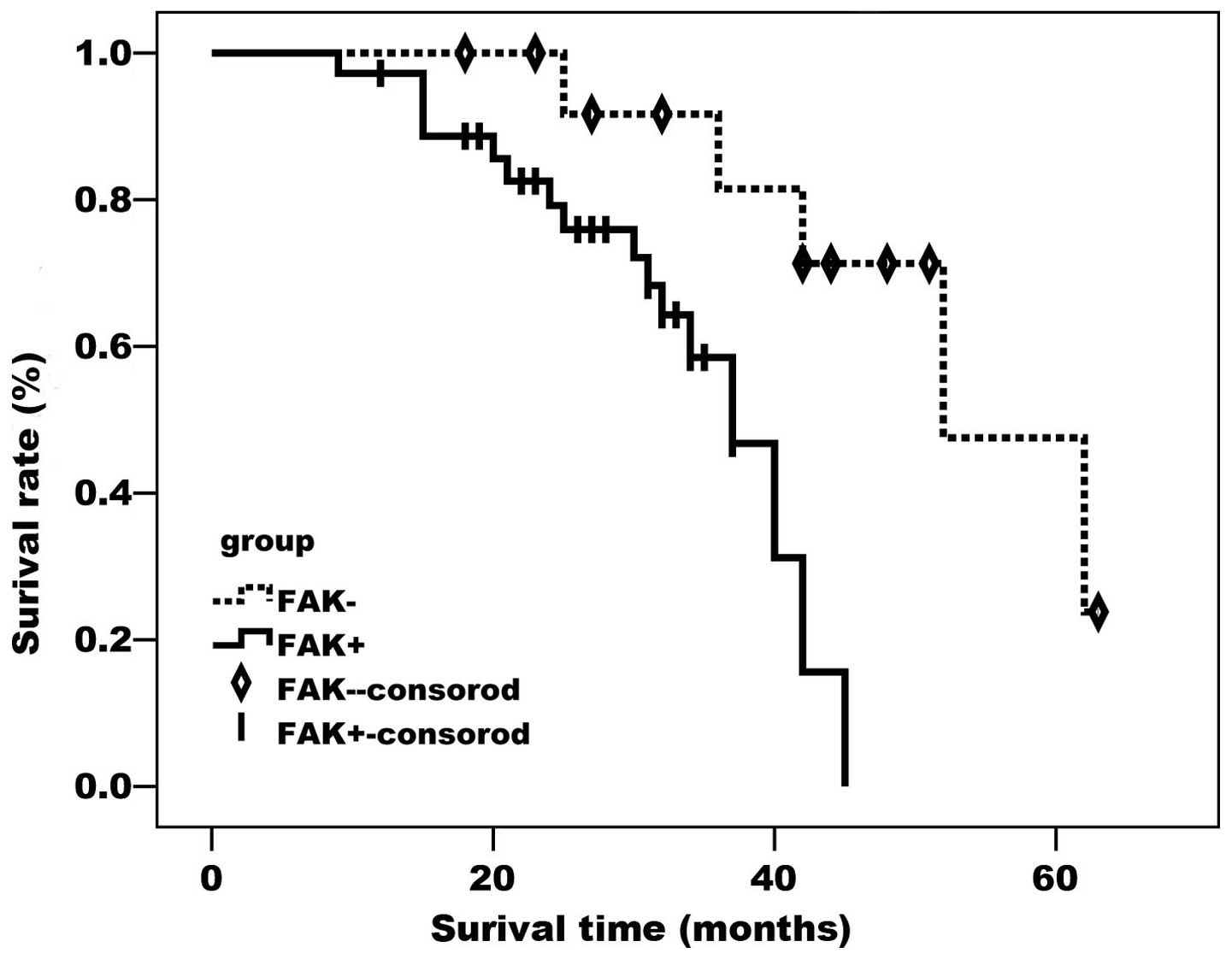
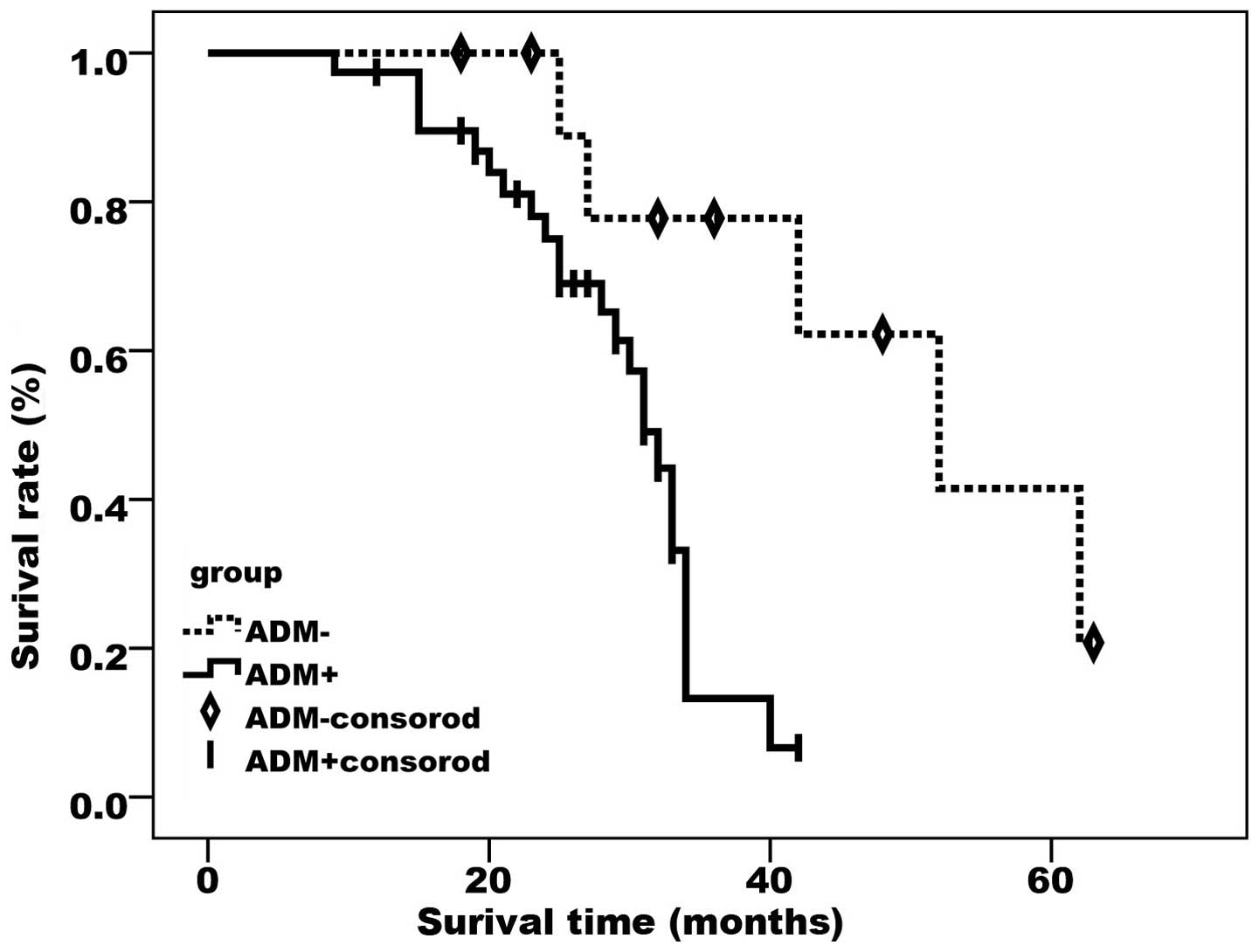
As demonstrated by the Kaplan-Meier curves (Figs. 2 and 3),
FAK- and ADM-positive cases had a significantly lower survival rate
compared with cases negative for the two proteins (P<0.05).
Discussion
Cell signaling is currently being extensively
investigated worldwide. FAK is a critical factor in cell signal
transduction pathways and is involved in tumorigenesis, invasion
and migration (5). Although ADM is
expressed in numerous normal tissues, including the lung, colon,
heart, uterus and ovary, it is overexpressed in tumor tissues,
including lung cancer, neuroblastoma, gastrointestinal tumors,
ovarian cancer and cervical squamous cell carcinoma (6). The present study investigated the effect
of FAK and ADM on the invasion and migration of ovarian cancer and
analyzed the correlation between their expression and
clinicopathological parameters.
FAK is mostly expressed in the cytoplasm of ovarian
cancer cells. Although cancer as well as paracancerous tissues were
positive for FAK expression, the former exhibited a significantly
higher level, with the latter exhibiting weak expression in the
majority of cases, similar to that observed in normal tissues.
Compared with normal and benign ovarian tissues, FAK is
overexpressed in malignant ovarian tissues (P<0.05), exhibiting
higher levels in cases with lymph node metastasis or poor
differentiation (P<0.05), whereas its expression was also
associated with clinical stage (P<0.05). Based on these
findings, the upregulation of FAK expression may be associated with
tumorigenesis, cancer cell proliferation, invasion and migration.
As reported by Halder et al (7), the inhibition of FAK phosphorylation may
reduce the invasion, migration and spreading ability of cancer
cells; in addition, suppression of FAK expression may restore the
sensitivity of ovarian cancer cells to drug therapy. FAK is a
critical regulator of cellular signaling pathways essential to cell
survival and division and its overexpression in epithelial and
mesenchymal tumors suggests that FAK may play a role in cell
invasion and mobility (8). In normal
cells, FAK may serve as a sensor of cell adhesion, which may
inhibit anchorage-dependent cell growth (9); however, the overexpression of FAK in
transformed or cancer cells may relieve this inhibition, resulting
in cell overgrowth without adhesion or anchorage. It was reported
that FAK expression was increased in the majority of malignant
tumors, including colon, thyroid, prostate and brain cancers, but
weakly expressed in benign tumors (10,11). Sood
et al (12) found that the
protein level of FAK in ovarian adenocarcinoma is 4-fold higher
compared with that in normal ovarian tissues; their results were
consistent with those of other previous studies (13). However, no differences in FAK
expression have yet been described in tumor cells from different
patients. Our findings indicate that significantly high levels of
FAK in ovarian cancer may be an independent indicator of poor
prognosis.
The present study also demonstrated a significant
difference in ADM expression between ovarian cancer and
normal/benign ovarian tissues (P<0.05). In ovarian cancer cases,
no staining for ADM was observed in non-tumor areas, suggesting
that ADM was produced specifically by ovarian cancer cells. As
regards clinicopathological parameters, the ADM expression was
correlated with differentiation level and lymph node metastasis,
but not with age, histological type or clinical stage, which was
consistent with the findings of Frede et al (14). The intensity and localization of ADM
staining were associated with tumor differentiation. In poorly
differentiated or undifferentiated samples, ADM staining was strong
and evenly distributed; in samples exhibiting moderate or high
differentiation, ADM staining was weak and mainly localized at the
periphery or along the border of cancer foci. These results suggest
that poorly differentiated ovarian cancer may grow rapidly and
invade actively and aggressively, whereas moderately or highly
differentiated ovarian cancer exhibits a lower malignant potential.
ADM is involved in cell proliferation, angiogenesis and
immunosuppression (15). Increased
expression of ADM in cancer cells may result in aggressive tumor
growth, in turn producing more ADM (16). Ovarian cancers exhibiting moderate or
high differentiation are relatively less malignant and the high ADM
levels may help the cancer cells to spread, which is consistent
with the localization of ADM staining at the periphery or along the
border of cancer foci (17). This
morphological characteristic suggests an association between tumor
behavior and ADM expression: Increased malignancy correlates with
higher ADM levels. Therefore, ADM may be an important determinant
and marker of aggressive tumor behavior.
The Spearman's rank correlation analysis
demonstrated that there is a positive correlation between the
expressions of FAK and ADM. As a key component of the signaling
pathways triggered by integrins, FAK is a kinase involved in cell
carcinogenesis, differentiation and metastasis and is also involved
in normal cell transformation and development, adhesion,
proliferation and apoptosis (18).
Overexpression of FAK may result in the dysregulation of ADM
expression. It was reported that ADM is a mediator in the crosstalk
between mast and tumor cells, a stimulator of angiogenesis and
mitosis of tumor cells and an apoptosis inhibitor of vascular
endothelial cells (19,20). Therefore, ADM may have a critical
function in tumor development and metastasis.
In conclusion, FAK and ADM are involved in cell
invasion and migration, which are characteristic features of
malignant tumors and the main causes of cancer-related mortality.
The present study demonstrated the potential value of FAK and ADM
as biomarkers in epithelial ovarian cancer. FAK and ADM may be
useful for the early diagnosis of high-risk patients, determination
of malignant potential, assessment of prognosis and treatment
design. FAK and ADM may also be investigated as potential
therapeutic targets for the inhibition of tumor invasion and
metastasis.
References
|
1
|
Morgan RJ Jr, Alvarez RD, Armstrong DK,
Boston B, Burger RA, Chen LM, Copeland L, Crispens MA, Gershenson
D, Gray HJ, et al: National Comprehensive Cancer Network:
Epithelial ovarian cancer. J Natl Compr Canc Netw. 9:82–113.
2011.PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colombo N, Peiretti M, Parma G, et al:
Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 21:v23–v30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baron V, Calléja V, Ferrari P, Alengrin F
and Van Obberghen E: p125Fak focal adhesion kinase is a substrate
for the insulin and insulin-like growth factor-I tyrosine kinase
receptors. J Biol Chem. 273:7162–7168. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer - a new therapeutic opportunity. Nat Rev Cancer.
5:505–515. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nikitenko LL, Leek R, Henderson S, et al:
The G-protein-coupled receptor CLR is upregulated in an autocrine
loop with adrenomedullin in clear cell renal cell carcinoma and
associated with poor prognosis. Clin Cancer Res. 19:5740–5748.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Halder J, Landen CN, Lutgendorf SK, et al:
Focal adhesion kinase silencing augments docetaxel-mediated
apoptosis in ovarian cancer cells. Clin Cancer Res. 11:8829–8836.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang YT, Lee LT, Lee PP, Lin YS and Lee
MT: Targeting of focal adhesion kinase by flavonoids and
small-interfering RNAs reduces tumor cell migration ability.
Anticancer Res. 25:2017–2025. 2005.PubMed/NCBI
|
|
9
|
Gao M, Liu X, Sun H, Ren H, Wang L and
Shen Y: Study on FAK regulation of migration of vascular
endothelial cells depending upon focal adhesion proteins. Sheng Wu
Yi Xue Gong Cheng Xue Za Zhi. 30:567–571. 2013.(In Chinese).
PubMed/NCBI
|
|
10
|
Grisaru-Granovsky S, Salah Z, Maoz M,
Pruss D, Beller U and Bar-Shavit R: Differential expression of
protease activated receptor 1 (Par1) and pY397FAK in benign and
malignant human ovarian tissue samples. Int J Cancer. 113:372–378.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saldanha SN and Tollefsbol TO: Pathway
modulations and epigenetic alterations in ovarian tumorbiogenesis.
J Cell Physiol. 229:393–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sood AK, Coffin JE, Schneider GB, Fletcher
MS, DeYoung BR, Gruman LM, Gershenson DM, Schaller MD and Hendrix
MJ: Biological significance of focal adhesion kinase in ovarian
cancer: role in migration and invasion. Am J Pathol. 165:1087–1095.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Judson PL, He X, Cance WG and Van Le L:
Overexpression of focal adhesion kinase, a protein tyrosine kinase,
in ovarian carcinoma. Cancer. 15:1551–1556. 1999. View Article : Google Scholar
|
|
14
|
Frede S, Freitag P, Otto T, Heilmaier C
and Fandrey J: The proinflammatory cytokine interleukin 1beta and
hypoxia cooperatively induce the expression of adrenomedullin in
ovarian carcinoma cells through hypoxia inducible factor 1
activation. Cancer Res. 65:4690–4697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cuttitta F, Pío R, Garayoa M, Zudaire E,
Julián M, Elsasser TH, Montuenga LM and Martínez A: Adrenomedullin
functions as an important tumor survival factor in human
carcinogenesis. Microsc Res Tech. 57:110–119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zudaire E, Martinez A and Cuttitta F:
Adrenomedullin and cancer. Regul Pept. 112:175–183. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pang X, Shang H, Deng B, Wen F and Zhang
Y: The Interaction of Adrenomedullin and Macrophages Induces
Ovarian Cancer Cell Migration via Activation of RhoA Signaling
Pathway. Int J Mol Sci. 14:2774–2787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Provenzano PP and Keely PJ: The role of
focal adhesion kinase in tumor initiation and progression. Cell Adh
Migr. 3:347–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karpinich NO, Hoopes SL, Kechele DO,
Lenhart PM and Caron KM: Adrenomedullin function in vascular
endothelial cells: Insights from genetic mouse models. Curr
Hypertens Rev. 7:228–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zudaire E, Martínez A, Garayoa M, Pío R,
Kaur G, Woolhiser MR, Metcalfe DD, Hook WA, Siraganian RP, Guise
TA, et al: Adrenomedullin is a cross-talk molecule that regulates
tumor and mast cell function during human carcinogenesis. Am J
Pathol. 168:280–291. 2006. View Article : Google Scholar : PubMed/NCBI
|