Introduction
The expected five-year survival rate for early-stage
cervical cancer is 91%. However, the five-year survival rate for
cervical cancer in an advanced stage is dramatically reduced to
12%, despite advances in surgical and radiation treatments
(1). Thus, a deeper understanding of
the mechanism underlying the progression of cervical cancer is
essential for improving the prognosis of advanced tumors.
Epithelial-mesenchymal transition (EMT) induced by transforming
growth factor-β (TGF-β) is well-established as a critical mechanism
of tumor progression (2). Epithelial
cells transdifferentiate into fibroblast-like cells, which results
in a lack of adhesion and actin cytoskeleton reorganization,
enhancing the migratory and invasive properties of cells (3). In addition, EMT is characterized by
alterations in the expression of a series of biomarkers, in
particular, reduced E-cadherin and increased Vimentin expression
levels. Ras homolog gene family, member C guanosine triphosphatase
(RhoC GTPase) is an important member of the Rho-GTPase family,
which regulates actin cytoskeleton reorganization during cellular
motility, and actively participates in the EMT process (4–6). However,
the role of RhoC in the process of EMT has remained to be
elucidated. Since the majority of cervical cancer cases are
squamous cell carcinomas and associated with high risk HPV
infection, the present study used the SiHa cell line which is HPV16
positive for the investigation. In the present study, a cellular
model of TGF-β1-induced EMT was established in the SiHa human
cervical cancer cell line and the alterations in RhoC activity and
expression were investigated, in addition to the effect of RhoC
inhibition on EMT.
Materials and methods
Cell culture
SiHa cells derived from human cervical squamous
carcinoma were obtained from the American Type Culture Collection
(Manassas, VA, USA). The cells were cultured in Dulbecco's modified
Eagle's medium (Gibco Life Technologies, Carlsbad, CA, USA)
containing 10% fetal calf serum (Gibco Life Technologies) and
incubated at 37°C in 5% CO2. TGF-β1 (PeproTech, Inc.,
Rocky Hill, NJ, USA) was added to the culture medium at 10 ng/ml,
and the culture medium containing TGF-β1 was changed every other
day. The cells were cultured for 7 days and observed daily; cells
cultured without TGF-β1 were used as control (7).
Immunofluorescence microscopy
The cells were cultured on coverslips until they
reached 60% confluence and were subsequently fixed in 4%
paraformaldehyde (Sigma-Aldrich, Santa Clara, CA, USA) for 15 min,
permeabilized with 0.5% Triton X-100/phosphate-buffered saline
(Sigma-Aldrich) for 10 min and then blocked with confining liquid
(0.1% Triton X-100/PBS, 2% BSA, 0.1% Sodum Azide; Sigma-Aldrich)
for 10 min. The cells were then stained with Alexa FITC-phalloidin
(dilution 1:20, Alexion Pharmaceuticals, Lausen, Switzerland) for
10 min and observed under a FV500 laser confocal microscope
(Olympus Corporation, Tokyo, Japan). The cells were incubated with
rabbit polyclonal anti-E-cadherin antibody (dilution 1:50; Boster
Biologics, Pleasanton, CA, USA) or mouse monoclonal anti-Vimentin
(dilution 1:20; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
antibody at 4°C overnight and stained with specific FITC-conjugated
secondary antibodies (mouse anti-rabbit IgG-FITC, dilution 1:300,
Santa Cruz Biotechnology, Dallas, TX, USA, sc-2359; or goat
anti-mouse IgM-FITC, dilution 1:400, Santa Cruz biotechnology,
Dallas, TX, USA, sc-2010) for 60 min at room temperature and viewed
under a IX71 fluorescence microscope (Olympus Corporation).
Western blot analysis
The cells were lysed in a lysis buffer and the
proteins were separated on 12% SDS-polyacrylamide gels and
transferred to nitrocellulose membranes (Sigma-Aldrich). The
membrane was blocked with a 5% milk solution in TBST
(Tris-HCl-buffered saline supplemented with 0.5% Tween-20;
Sigma-Aldrich), and incubated with a primary antibodies (goat
polyclonal IgG anti-RhoC, rabbit polyclonal IgG anti-E cadherin and
goat polyclonal IgG anti-β actin, 1:200, Santa Cruz Biotechnology,
sc-12116, sc-7870 and sc-1616; mouse monoclonal IgM anti-vimentin,
1:200, Themo Fisher Scienfic, Walthan, MA, USA, MA3-745) in 5%
milk/TBST overnight, followed by incubation with the corresponding
horseradish peroxidase-linked secondary antibodies (1:5000, Santa
Cruz Biotechnology; sc-2922, sc-2357 and sc-2005). Following
extensive washing, the signals were detected using an enhanced
chemiluminscence-detecting reagent (• GE Healthcare Life Sciences,
Chalfont, UK). For each western blotting result, the experiment was
performed ≥3 times, and representative images are presented in the
results.
RhoC activity assay
RhoC activity was assessed using the Rho-binding
domain of Rhotekin (RBD; Upstate Biotechnology, Temecula, CA, USA)
according to the manufacturer's instructions. Briefly, the cells
were lysed in radioimmunoprecipitation assay buffer [50 mM Tris (pH
7.2), 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mM
NaCl, 10 µg/ml each of leupeptin and aprotinin and 1 mM
phenylmethanesulfonylfluoride (PMSF); Sigma-Aldrich]. The cell
lysates were clarified by centrifugation at 13,000 × g for 10 min
at 4°C and were subsequently incubated for 40 min with Rhotekin Rho
Binding Domain-agarose. The agarose beads were washed 3 times with
washing buffer comprised of Tris buffer containing 1% Triton X-100,
150 mM NaCl, 10 mM MgCl2, 10 µg/ml each of leupeptin and aprotinin
and 0.1 mM PMSF. The Rho that bound to the beads was the GTP-bound
form of the Rho protein and was detected by western blotting using
RhoC antibodies.
In vitro cell invasion assay
A modified cell migration assay was performed using
a Transwell apparatus (8-µm pore; Corning Life Sciences, Cambridge,
MA, USA) as previously described (8).
Briefly, the upper surface of the Transwell chambers were
pre-coated overnight with Matrigel (BD Biosciences, Franklin Lakes,
NJ, USA) diluted 1:4 in serum-free medium. The cell suspension
(2.5×105 cells in 100 µl serum-free medium) was transferred to a
polycarbonate filter (pore size, 8 µm) in the upper compartment of
the apparatus, and 400 µl complete culture medium was placed in the
lower compartment. The apparatus was then placed in a humidified
incubator containing 5% CO2 for 24 h at 37°C. Cells that
migrated through the membrane were exposed to hematoxylin and eosin
staining. The number of cells penetrating the membrane was
determined by evaluating 5 independent high-power fields in
triplicate at x400 magnification, using a under a CX41-72C02
microscope (Olympus Corporation).
RhoC short interfering (si)RNA
transfection
A specific siRNA directed against human RhoC
messenger RNA was designed and synthesized by Shanghai GenePharma
Co., Ltd. (Shanghai, China). The sequences selected for anti-RhoC
siRNA were: Sense, 5′-ACUGUCUUGAGAACUAUATT-3′ and antisense,
5′-UAUAGUUCUCAAAGACAGUAG-3′. The siRNA was introduced into SiHa
cells by RNAi-Mate transfection reagents (Shanghai GenePharma Co.,
Ltd.), according to the manufacturer's instructions. The cells were
cultured in 6-well plates in 2 ml serum-enriched medium. When 80%
confluence was reached, 5 µg siRNA and 15 µl RNAi-Mate mixture was
added to the cell cultures and incubation was continued for 48
h.
Statistical analysis
Results are expressed as the mean ± standard
deviation. A Student's t-test was used to perform comparisons using
SPSS software, version 12.1 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
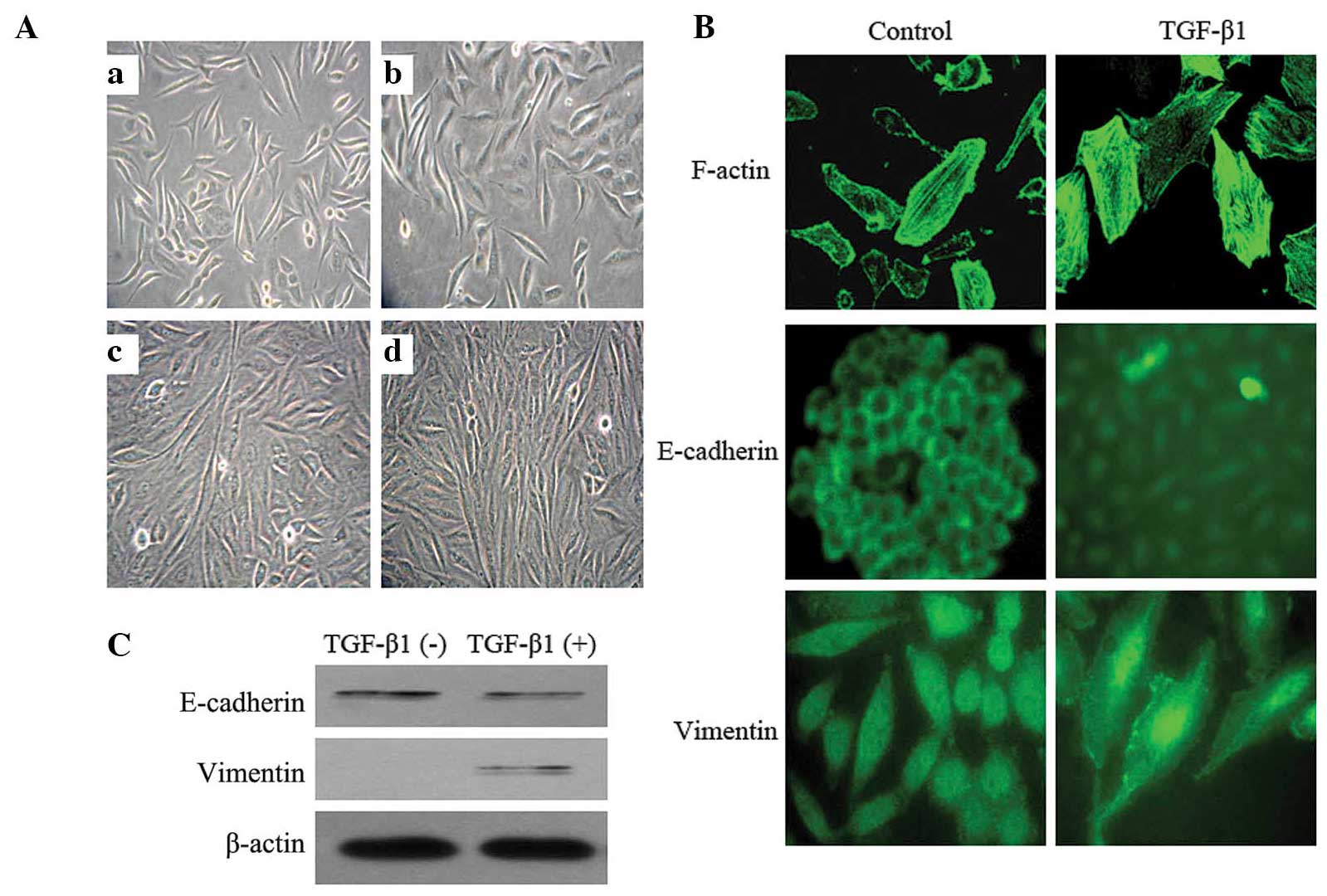
TGF-β1 induces EMT in SiHa cells
Following 2 days of 10 ng/ml TGF-β1 treatment, the
morphology of the SiHa cells began to change. The cells were
enlarged and lengthened, and exhibited a spindle-like shape. The
cells demonstrated a distinct spindle-like shape 4 days later.
Seven days later, the morphological alterations were marked: The
cells were arranged in a paliform shape, and the length was
increased by ~7 fold compared with that of the normal control cells
cultured without TGF-β1 (Fig.
1A).
In addition, TGF-β1 stimulation resulted in
reorganization of the actin cytoskeleton, from a regular
arrangement of circumferential ring-like structures into actin
stress fibers, which were aggregated, broken and arranged in a
disordered manner. Immunofluorescent staining demonstrated an
E-cadherin fluorescent signal in the SiHa cell membrane, which was
reduced or absent following TGF-β1 treatment. Simultaneously,
Vimentin was expressed de novo, which manifested as the
aggregation of radiating filaments around the peripheral region of
the nuclei (Fig. 1B). The alterations
in expression levels of E-cadherin and Vimentin were confirmed by
western blotting (Fig. 1C). The
results of the present study provided morphological and molecular
evidence that EMT occurred in the SiHa cells subjected to TGF-β1
treatment.
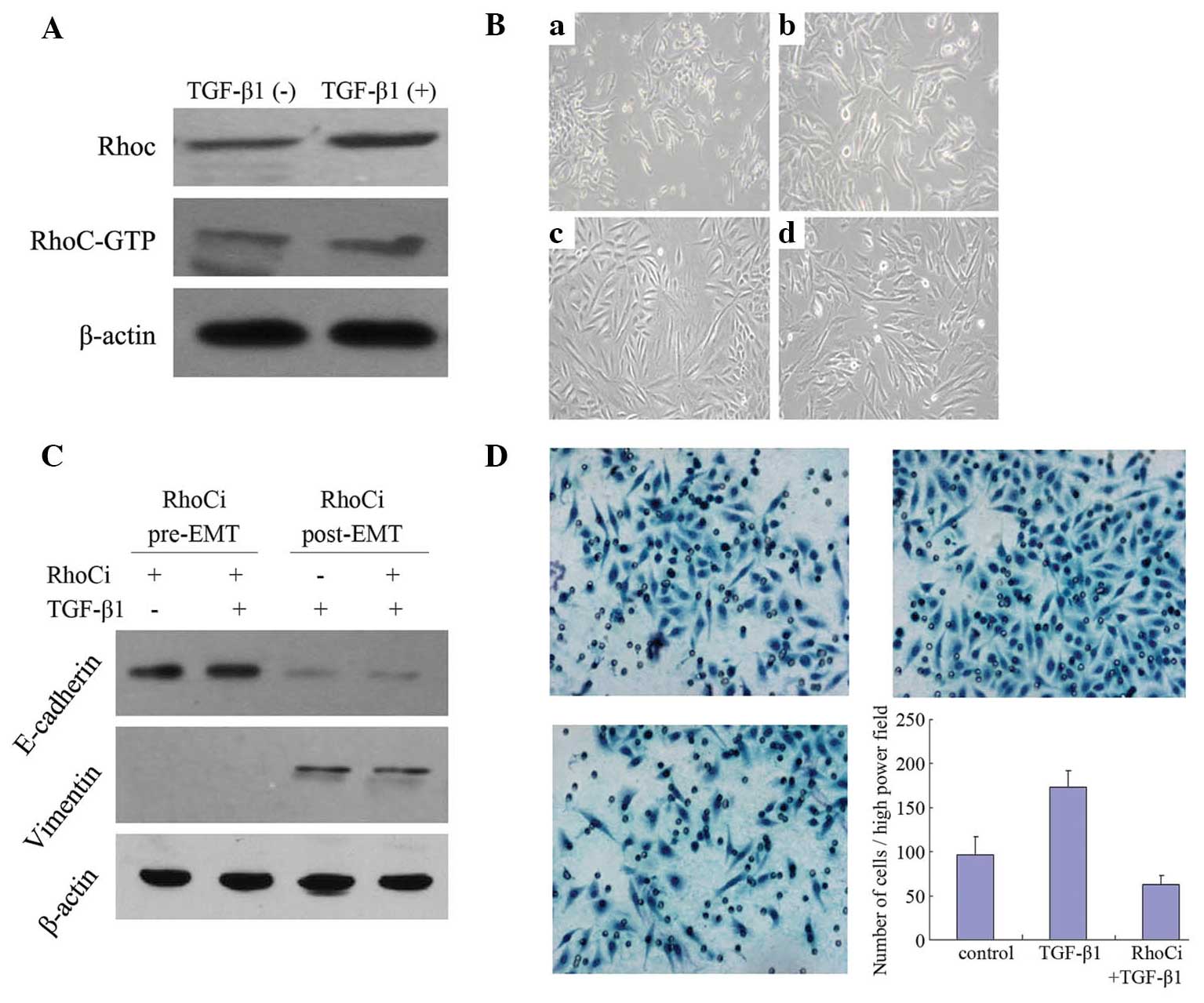
RhoC is essential for TGF-β1-induced
EMT
Western blot analysis demonstrated that the RhoC and
RhoC-GTP levels were increased following 4 days of 10 ng/ml TGF-β1
treatment (Fig. 2A), indicating that
TGF-β1 may increase RhoC protein expression and activity.
To investigate the role of RhoC in EMT in cervical
cancer cells, RhoC was silenced in SiHa cells by siRNA transfection
prior to TGF-β1 treatment or four days following TGF-β1 treatment.
The cells in which RhoC was downregulated did not exhibit a
mesenchymal morphology (Fig. 2B) or
alterations in the expression of EMT markers, E-cadherin and
Vimentin (Fig. 2C), following TGF-β1
treatment. This result indicated that RhoC is required for the
process of EMT. However, silencing of RhoC following pretreatment
with TGF-β1 did not alter the incidence of EMT (Figs. 2B and C). In addition, following
TGF-β1 stimulation, the invasiveness of SiHa cells was elevated
~2-fold (P<0.01), while this phenomenon was not observed in
cells in which RhoC was silenced (P>0.05; Fig. 2D). These results indicate that
inhibition of RhoC expression may block the process of
TGF-β1-induced EMT, however it is insufficient to reverse EMT.
Discussion
EMT has been observed in several epithelial tumors,
including oral squamous cell carcinoma (9), breast cancer (10), endometrial cancer (11) and pancreatic carcinoma (12). TGF-β1 has been demonstrated to be able
to induce EMT, and is important in tumor development (13–16).
In the present study, SiHa cells underwent an EMT
process following TGF-β1 treatment: The cells were clearly enlarged
and spindle-shaped and E-Cadherin levels were significantly
reduced, while Vimentin levels were increased. A reduction in the
expression of E-cadherin initiates a series of reactions which
finally result in breakdown of the intercellular tight junctions
between cells, allowing cells to gain movement capability (4). A previous study demonstrated that the
expression level of E-cadherin was reduced in EMT (17).
TGF-β1-induced cytoskeletal remodeling indicates
that RhoC, which serves an important function in regulating stress
fiber formation, may participate in the TGF-β1-induced EMT process.
In order to examine this hypothesis, RhoC expression was analyzed
in TGF-β1-treated cells. Following TGF-β1 stimulation, the RhoC
protein expression levels in SiHa cells were significantly
increased compared with those of the blank control group, and RhoC
GTPase activity was also significantly increased. Similarly, Cho
and Yoo (4) demonstrated that TGF-β1
serves a dual role in inducing Rho activity, instantly and
temporarily increasing activity, followed by a slower increasing
stage. A similar dual activation phenomenon of Rho was also
observed in prostate adenocarcinoma (18). The mechanism of this dual activation
remains to be elucidated. However, in the present study, due to the
various observation time-points that were selected, this phenomenon
was not observed.
The present study also demonstrated that when the
RhoC expression levels increased, the invasive capability of the
SiHa cells was also increased. When the RhoC expression levels were
pre-inhibited, the invasive capability of the SiHa cells was
significantly reduced, even with continuous TGF-β1 stimulation.
These data indicated that the enhancement of TGF-β1-induced
cellular invasive capability is dependent on upregulation of RhoC
expression. Simpson et al (19) reported that when RhoA was inhibited,
RhoC promoted lysophosphatidic acid-induced MCF-7 breast cancer
cell invasive capability. The authors also observed that inhibition
of RhoA gene expression increased the invasive capacity of the
MCF-7 cells. Analogous effects were observed in colon cancer
(6). Taken together, the findings of
previous studies and the present study, indicate that knockdown of
the RhoC gene may inhibit the TGF-β1-induced invasiveness of SiHa
cells.
In addition, the association between RhoC and EMT
was further investigated. A RhoC-specific siRNA was transfected
into SiHa cells, prior to TGF-β1 treatment and following EMT
occurrence, respectively. Inhibition of RhoC expression blocked the
EMT process, however, if EMT had already occurred in the cells,
then silencing of RhoC using the siRNA was not sufficient to
reverse the EMT process and the associated morphological changes.
Cho and Yoo (4) demonstrated that the
Rho-Rock inhibitor, Y27632, was able to completely block
TGF-β1-induced EMT. Another previous study demonstrated that a
RhoA-specific inhibitor did not delay or block TGF-β1-induced EMT
in SiHa cells (7). Therefore, a
comparison of RhoA and RhoC expression levels and the alterations
in their activity may be critical in further understanding the
TGF-β1-induced EMT process.
EMT is a complex biological process, involving
multiple signaling pathways associated with cancer formation and
development. In addition, these intracellular pathways may
cross-talk with one another (20). A
number of proteins that are involved in the EMT process are also
tumor malignant process marker proteins (21). Therefore, EMT may be a critical
process in recognizing the relevant molecular expression patterns
in invasive disease, and may also represent a potential target in
tumor therapy (21,22). In conclusion, targeted inhibition of
RhoC activity may be potentially useful in the treatment of
cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81072134 and
81202036).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rees JR, Onwuegbusi BA, Save VE, et al: In
vivo and in vitro evidence for transforming growth
factor-beta1-mediated epithelial to mesenchymal transition in
esophageal adenocarcinoma. Cancer Res. 66:9583–9590. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho HJ and Yoo J: Rho activation is
required for transforming growth factor-β-induced
epithelial-mesenchymal transition in lens epithelial cells. Cell
Biol Int. 31:1225–1230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mukai M, Endo H, Iwasaki T, et al: RhoC is
essential for TGF-beta1-induced invasive capacity of rat ascites
hepatoma cells. Biochem Biophys Res Commun. 346:74–82. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bellovin DI, Simpson KJ, Danilov T, et al:
Reciprocal regulation of RhoA and RhoC characterizes the EMT and
identifies RhoC as a prognostic marker of colon carcinoma.
Oncogene. 25:6959–6967. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi JY, Hur KC, Lee E, et al:
TGFbeta1-mediated epithelial to mesenchymal transition is
accompanied by invasion in the SiHa cell line. Eur J Cell Biol.
81:457–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu SD: Current Protocols for Molecular
Biology. Publish House of Advanced Education; Beijing: pp. 381–385.
1993
|
|
9
|
Chaw SY, Majeed AA, Dalley AJ, et al:
Epithelial to mesenchymal transition (EMT) biomarkers - E-cadherin,
beta-catenin, APC and Vimentin - in oral squamous cell
carcinogenesis and transformation. Oral Oncol. 48:997–1006. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y and Zhou BP: Epithelial-mesenchymal
transition - a hallmark of breast cancer metastasis. Cancer Hallm.
1:38–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mirantes C, Espinosa I, Ferrer I, Dolcet
X, Prat J and Matias-Guiu X: Epithelial-to-mesenchymal transition
and stem cells in endometrial cancer. Hum Pathol. 44:1973–1981.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karamitopoulou E: Role of
epithelial-mesenchymal transition in pancreatic ductal
adenocarcinoma: Is tumor budding the missing link? Front Oncol.
3:2212013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wendt MK, Smith JA and Schiemann WP:
Transforming growth factor-β-induced epithelial-mesenchymal
transition facilitates epidermal growth factor-dependent breast
cancer progression. Oncogene. 29:6485–6498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shirakihara T, Horiguchi K, Miyazawa K, et
al: TGF-β regulates isoform switching of FGF receptors and
epithelial-mesenchymal transition. EMBO J. 30:783–795. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heldin CH, Vanlandewijck M and Moustakas
A: Regulation of EMT by TGFβ in cancer. FEBS Lett. 586:1959–1970.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reinacher-Schick A, Baldus SE, Romdhana B,
et al: Loss of Smad4 correlates with loss of the invasion
suppressor E-cadherin in advanced colorectal carcinomas. J Pathol.
202:412–420. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edlund S, Landström M, Heldin CH and
Aspenström P: Transforming growth factor-beta-induced mobilization
of actin cytoskeleton requires signaling by small GTPases Cdc42 and
RhoA. Mol Biol Cell. 13:902–914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simpson KJ, Dugan AS and Mercurio AM:
Functional analysis of the contribution of RhoA and RhoC GTPases to
invasive breast carcinoma. Cancer Res. 64:8694–8701. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spaderna S, Schmalhofer O, Hlubek F, et
al: A transient, EMT-linked loss of basement membranes indicates
metastasis and poor survival in colorectal cancer.
Gastroenterology. 131:830–840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guarino M: Epithelial-mesenchymal
transition and tumour invasion. Int J Biochem Cell Biol.
39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|