Introduction
Giant cell tumor of the tendon sheath (GCTS) was
initially described by Chassaignac in 1852 (1), and the lesion was defined as a malignant
tumor at that time (2). Subsequently,
Heurteux produced a detailed description of GCTS (3). It was found that the disease occurs more
frequently in middle-aged women compared with men (4). According to the position (large or facet
joint; intra- or extra-articular), characteristics (benign or
malignant) and growth pattern (diffuse or localized) of the tumor,
GCTS may be classified as one of four types: Localized-type
tenosynovial giant cell tumor (L-GCTS); diffuse-type tenosynovial
giant cell tumor (D-GCTS); pigmented villonodular synovitis (PVNS)
(5,6);
and malignant tenosynovial giant cell tumor (M-GCTS) (7,8).
D-GCTS demonstrates invasive growth in the local
region, which is considerably different from L-GCTS. D-GCTS usually
develops in the synovium, and often invades the surrounding knee
and soft tissue (2,9). Since it arises mainly from the soft
tissue outside the joints, Weiss et al (10) regarded D-GCTS as a type of PVNS that
appears in the soft tissue outside the joints, and also presents
with intra-articular lesions (2,5). L-GCTS
mainly occurs in the small joints, including the hand, foot and
ankle. Image findings in previous studies reveal these soft tissue
masses occur beside small joints (9,11). If the
results of image analysis demonstrate that the lesions have invaded
the joints, and if there is a concurrent weak signal of T1W and T2W
on a magnetic resonance imaging (MRI) scan, D-GCTS should be
considered as a probable diagnosis (12–15).
GCTS usually occurs in individuals between the ages
of 30 and 50 years (5). As a painless
and slow-growing disease, it has been termed an innocent tumor
(4,5,13).
However, it may recur if incompletely removed (5). D-GCTS has a low incidence of two per
1,000,000 per year in middle-young aged person (5). With a pain-free and long clinical
history, surgery is the standard treatment. With the risk of
recurrence, rare cases may become M-GCTS (5,7,13).
D-GCTS mainly occurs in weight-bearing joints, such
as the knee, ankle and foot, and a small number of lesions occur in
non-weight-bearing joints (16).
D-GCTS of the tendon sheath in the temporal region is extremely
rare (8,17,18).
The present study reports two cases, consisting of
the case of a 33-year-old female patient that presented with D-GCTS
of the temporal fossa, which had invaded the skull base, and the
case of a 30-year-old male patient that presented with D-GCTS that
had invaded the external auditory canal.
Case report
A 33-year-old woman who first felt pain in September
2009 in the left temporomandibular joint pain and limitation of
mouth opening subsequent to biting a hard substance three years
prior to presentation underwent a lateral skull X-ray examination
at the Department of Oral of Bao'an District Hospital of
traditional Chinese medicine (Shenzhen, Guangdong, China), and was
diagnosed with temporomandibular joint dysfunction syndrome (TMDS).
The patient possessed no systemic disease and no associated family
medical history.
For almost one year prior to October 10, 2012, the
patient had experienced pain in the temporomandibular region when
tired or subsequent to chewing for an extended period, but had not
received treatment. The patient experienced an increase in the pain
in the left temporal region, without limited movement of the joint
in July 2012. Subsequent to the imaging examination performed in
the Department of Oral of Bao'an District Hospital of traditional
Chinese medicine, the destruction of the left temporal bone was
revealed, in addition to the presence of soft tissue
space-occupying lesions in the deep left buccal division. The
patient was then referred to the Department of Oral and
Maxillofacial Surgery of Shenzhen Hospital, Peking University
(Shenzhen, Guangdong, China).
The clinical examination revealed that the left
temporal bone was slightly more ridged compared with the right
temporal bone. The oral mucosa, tongue activity, opening of mouth
and lymph nodes in associated regions were all determined to be
normal.
Subsequently, a 30-year-old man was referred to the
Department of Oral and Maxillofacial Surgery of Shenzhen Hospital,
Peking University, with a painless left temporal mass and external
auditory canal bleeding. The patient found the mass incidentally in
April 2013. Since the lesion was painless and had no influence on
daily life, the patient overlooked the mass prior to the
commencement of bleeding of the external auditory canal. The
patient underwent a biopsy due to a rupture in the external
auditory canal. The biopsy results suggested that the lesion may be
a left plane epidermoid cyst associated with infection.
Similarly to the female patient, the male patient
possessed no systemic disease and no relevant family history. The
clinical examination expressed the same findings as the examination
performed on the female patient, with the exception of the bleeding
in the external auditory canal.
The two patients were admitted to hospital and were
diagnosed with a left temporal tumor.
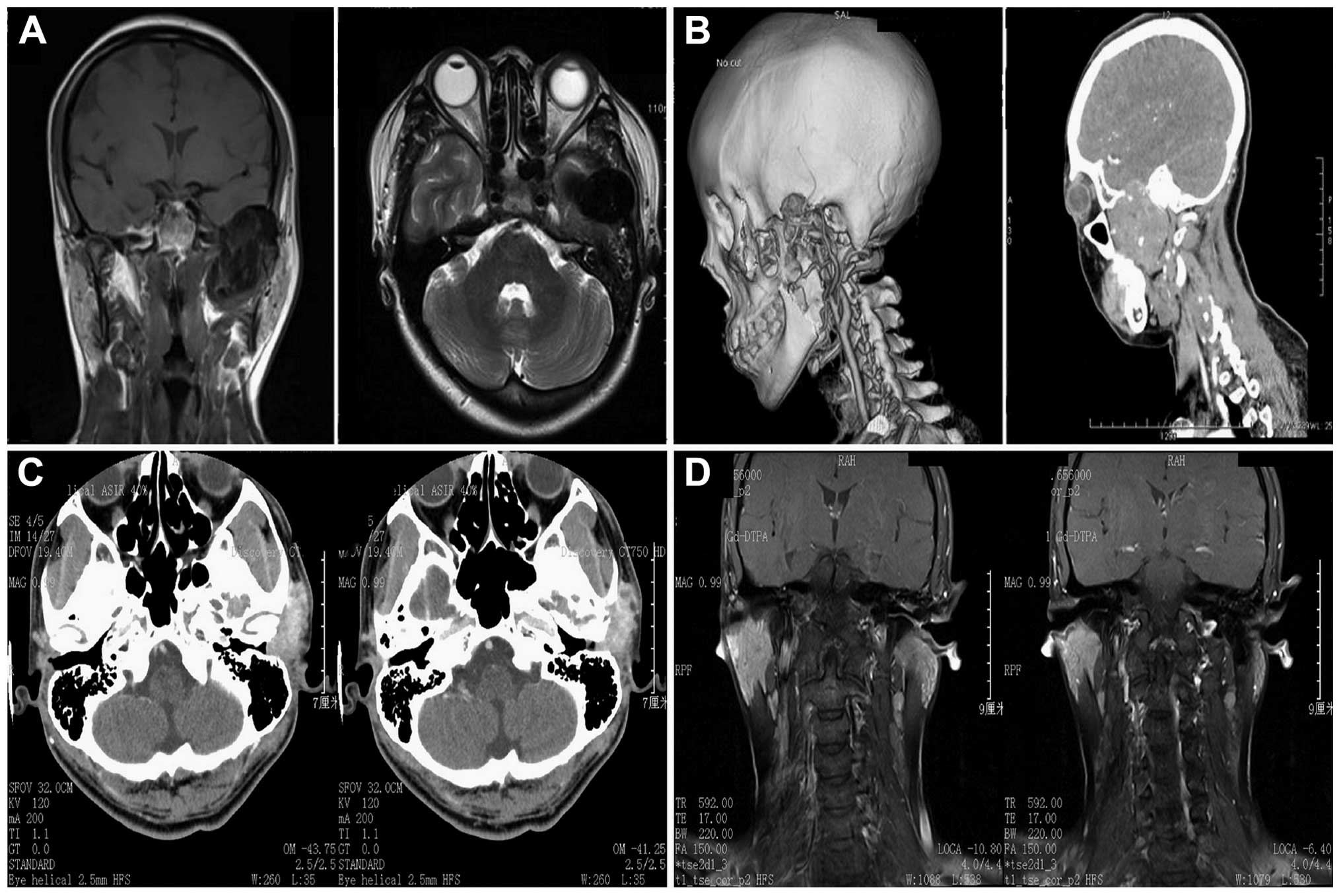
The results of computed tomography (CT) and spiral
CT performed on the female patient revealed the mass lesion in the
left temporal fossa and the destruction of the left temporal,
sphenoid wing and skull base bones. Magnetic resonance imaging
(MRI) revealed that the mass lesion under the left temporal fossa
and skull base in this patient broke through the left temporal bone
to push the left temporal lobe. The lesion also pushed the medical
and lateral pterygoid muscle. The range of destruction and
absorption of the left temporal and sphenoid wing bone was ~6.2×3.8
cm. The lesion exhibited characteristic hypointense and
heterogeneous hypointense signals on T1W and T2W images (Fig. 1).
Subsequent to the CT and MRI examination performed
on the male patient, the results revealed that the lesion occurred
in the left temporal fossa and extended into the external auditory
canal. The junction of the zygomatic arch and temporal bone
exhibited signs of destruction, and the report indicated that a
diagnosis of malignant tumor should be considered (Fig. 1).
The female patient underwent expanded lesion sites
resection of the neoplasm in the left temporal fossa and skull
base, osteotomy of the left mandible and resection of the left
zygomatic arch. Internal fixation with a titanium plate was
performed, along with transmyocardial revascularization of the
zygomatic arch. Subsequently, the temporalis myofascial flap and
adjacent gingival flap was used to repair the surgical defect. The
lesion excised during the procedure was ~1.6×1.6 cm, and the edges
of the resection margin did not contain tumor cells.
The male patient underwent expanded lesion sites
resection of the left temporal neoplasm and decompression of the
facial nerve. Subsequently, anastomosis of the facial nerve was
conducted to rebuild the function of facial nerve. The external
auditory canal was then repaired and the surgical defect was
repaired with the opisthotic free flap and the temporalis
myofascial flap. The neoplasm that developed in the external
auditory canal had been removed at the same time. The lesion
removed during the procedure was ~2.5×2.0×1.5 cm in size, and the
edges of the resection margin were negative for tumor cells.
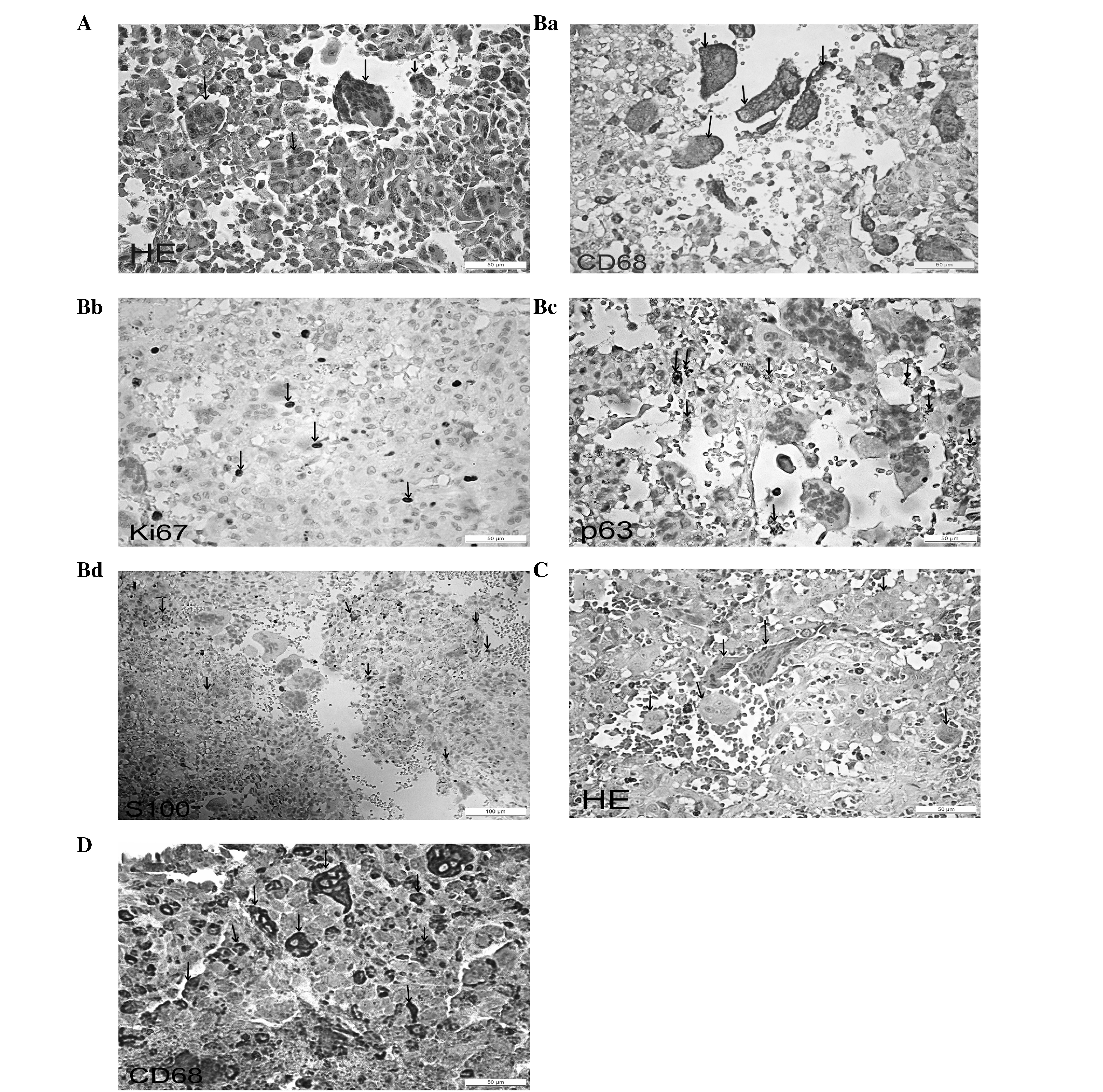
Post-operative pathological examination of the
resected specimen from the female patient revealed that the tissue
section was rich in giant cells. The cells were round or polygonal
in shape, and numerous cells with a clear perimeter were not
mature. Chondroblasts accompanied by individual or several
multi-core osteoclastic-like giant cells were identified under
microscopy (x400 magnification, Leica DM4000B). In addition, the
immunohistochemical staining for the section of the lesion revealed
that the cells did not express cytokeratin, and were weakly
posistive for cluster of differentiation (CD)68, S-100 and
cytoplasmic p63. In total, 5–10% of cells stained for Ki-67
expression. Microscopically, the cell population mostly consisted
of single nuclear cells and multinucleated giant cells, and the
prescence of multifocal irregular calcification regions within
pane-like calcification was common. The shape of cells remained
well-differentiated, and mitotic structures were rarely identified
(Fig. 2).
The histopathological examination on the tumor
excised from the male patient provided reasonable support for the
diagnosis of D-GCTS, which was the same diagnosis made for the
female patient. The results for the male patient of
histopathological examination and immunohistochemical staining are
listed as follows. The section of mass of the left temporal region
revealed that histocytes and multinucleated giant cells constituted
the tumor tissue. Cartilage tissue was also present. The
immunohistochemical staining identified that the three
immunological indicators of the section were CD68(+), S-100(–) and
CD1a(–) (Fig. 2).
Discussion
The clinical manifestations of D-GCTS are local
swelling, limitation of movement and joint closure (19). The incidence of D-GCTS is observed
more often in female patients compared with male patients (4,5). The age
at onset of patients with D-GCTS is, overall, younger compared with
the age at onset of patients with L-GCTS, but the course of the
disease is much longer. Compared with the common pathogenic sites
of L-GCTS, weight-bearing joints may be involved in D-GCTS.
However, it is extremely infrequent that the lesion involves the
temporal fossa (8,18). For this reason, the correct diagnosis
of the disease is a considerable challenge (2,9,13). Additional auxiliary examinations
should be performed, and detailed information from patients should
be recorded, which is vital for the confirmation of a therapeutic
schedule (4,5,13,15).
In imaging examinations, X-rays may reveal joint
capsular swelling or the increased density of circular shadow, and
also reveal the defect, hardening and hyperplasia at the edge of
the joint, where the lesions erode the articular surface (13,20,21). When
the lesions extend intra-articularly, the X-ray may reveal a
widened joint space and oppressive involvement in the adjacent bone
cortex (13). CT demonstrates an
advantage in revealing the extent of disease, particularly in the
bone cortex. The differences between D-GCTS and osteosarcoma or
chondrosarcoma may be determined from the CT results (17,21), as
diffuse thickening of soft tissues around the joints, oppressive
absorption of the affected bones, the presence of a clear boundary,
the lack of periosteal reaction and the presence of a soft tissue
mass also without calcification or ossification may be observed on
CT (13,21,22). MRI
has an extremely high sensitivity and specificity for the diagnosis
of G-GCTS (16,23). MRI can reveal the diffuse and invasive
growth of the irregular mass lesion adjacent to the joints, the
origin and scope of the lesions, and the level of intra-articular
invasion. Generally the MRI signal is weak and this is one of the
characteristics of the imaging diagnosis of D-GCTS (12,19,21).
The cell components of D-GCTS include the large
synovial-like mononuclear, small histiocytoid, foamy histiocyte and
inflammatory cells, fibroblasts and the osteoclast-like
multinucleated giant cells (5,24,25). The number of large synovial-like
mononuclear cells that are positive for clusterin in D-GCTS are
more than that in L-GCTS (P<0.01) and PVNS (P<0.05) (26).
D-GCTS is challenging to diagnose correctly as the
lesions may invade the temporal fossa and extend to the external
auditory canal or skull base (18).
D-GCTS should be distinguished from temporomandibular joint
dysfunction syndrome, synovial sarcoma, xanthomatous inflammatory
malignant fibrous histiocytoma and M-GCTS (2,5,13). Occasionally, the clinical symptoms and
imaging examination does not result in a final precise diagnosis;
therefore, the post-operative histopathological examination is the
gold standard for the confirmation of diagnosis (9).
The pathogenesis of D-GCTS has not been clarified,
and certain studies have concluded that the cause of disease is
associated with bleeding subsequent to trauma and lipid metabolism
disorders (5,13,15).
D-GCTS in the temporal fossa may result in the
limitation of the activity of the temporomudibular joint,
difficulty in opening the mouth, local pain and numerous notable
consequences when the lesion extends into the cranial cavity.
Therefore, early detection and treatment possesses a considerable
significance. In addition, due to the high recurrence rate,
complete surgical treatment and post-operative follow-up review is
required (11,14).
References
|
1
|
Chassaignac M: Cancer de la gaîne des
tendons. Gazette Hôpital Civil Militaire. 47(2): 1852(In
French).
|
|
2
|
Stevenson JD, Jaiswal A, Gregory JJ,
Mangham DC, Cribb G and Cool P: Diffuse pigmented villonodular
synovitis (diffuse-type giant cell tumour) of the foot and ankle.
Bone Joint J. 95-B:384–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heurtaux MA: Myelome des gaines
tendineuses. Arch Gen Med. 167:40–54. 1891.(In French).
|
|
4
|
Di Grazia S, Succi G, Fraggetta F and
Perrotta RE: Giant cell tumor of tendon sheath: Study of 64 cases
and review of literature. G Chir. 34:149–152. 2013.PubMed/NCBI
|
|
5
|
van der Heijden L, Gibbons CL, Dijkstra
PD, et al: The management of diffuse-type giant cell tumour
(pigmented villonodular synovitis) and giant cell tumour of tendon
sheat (nodular tenosynovitis). J Bone Joint Surg Br. 94:882–888.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Caputo V, Fiorella S and Orlando E:
Postsurgical paracicatricial cutaneous satellitosis of giant cell
tumour of the tendon sheath, localized type. Case Rep Dermatol.
3:118–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Theunissen CI, Bras J, Lienden KP and
Obdeijn MC: Malignant giant cell tumor in the carpal tunnel: a case
report and review of literature. J Wrist Surg. 2:271–275. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leonard J, Gökden M, Kyriakos M, Derdeyn
CP and Rich KM: Malignant giant-cell tumor of the parietal bone:
Case report and review of the literature. Neurosurgery. 48:429.
2001. View Article : Google Scholar
|
|
9
|
Somerhausen NS and Fletcher CD:
Diffuse-type giant cell tumor clinicopathologic and
immunohistochemical analysis of 50 cases with extraarticular
disease. Am J Surg Pathol. 24:479–492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weiss SW, Goldblum JR and Enzinger FM:
Benign tumors and tumor-like lesions of synovial tissueEnzinger and
Weiss's Soft Tissue Tumors. 5th. Elsevier; Philadelphia, PA, USA:
pp. 20–2007
|
|
11
|
Kotwal PP, Gupta V and Malhotra R:
Giant-cell tumour of the tendon sheath: Is radio therapy indicated
to prevent recurrence after surgery? J Bone Joint Surg. 82:571–573.
2000. View Article : Google Scholar
|
|
12
|
Kim KW, Han MH, Park SW, et al: Pigmented
villonodular synovitis of the temporomandibular joint: conventional
findings in four cases. Eur J Radiol. 49:229–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang K, Liu X, Liu Z and Yu M: Diagnosis
and treatment of primary diffuse-type tenosynovial giant cell
tumors of the cervical spine. Chin Med J (Engl). 2:791–792.
2014.
|
|
14
|
Nakahara H, Matsuda S, Harimaya K, et al:
Clinical results of open synovectomy for treatment of diffuse
pigmented villonodular synovitis of the knee: Case series and
review of literature. Knee. 19:684–687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Griffin AM, Ferguson PC, Catton CN, et al:
Long-term outcome of the treatment of high-risk tenosynovial giant
cell tumor/pigmented villonodular synovitis with radiotherapy and
surgery. Cancer. 118:4901–4909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gibbons CL, Khwaja HA, Cole AS, Cooke PH
and Athanasou NA: Giant-cell tumour of the tendon sheath in the
foot and ankle. J Bone Joint Surg Br. 84:1000–1003. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Isaacson B, Berryhill W and Arts HA:
Giant-cell tumors of the temporal bone: management strategies.
Skull Base. 19:291–301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Day JD, Yoo A and Muckle R: Pigmented
villonodular synovitis of the temporomandibular joint: a rare tumor
of the temporal skull base. J Neurosurg. 109:140–143. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Llauger J, Palmer J, Rosón N, Cremades R
and Bagué S: Pigmented villonodular synovitis and giant cell tumors
of the tendon sheath: Radiologic and pathologic features. AJR Am J
Roentgenol. 172:1087–1091. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bemporad AJ, Chaloupka JC, Putman CM, et
al: Pigmented villonodular synovitis of the temporomandibular
joint: Diagnostic imaging and endovascular therapeutic embolization
of a rare head and neck tumor. AJNR Am J Neuroradiol. 20:159–162.
1999.PubMed/NCBI
|
|
21
|
Murphey MD, Nomikos GC, Fleming JD, Gannon
FH, Temple HT and Kransdorf MJ: Imaging of giant cell tumor and
giant cell reparative granuloma of bone: Radiologic-pathologic
correlation. Radiographics. 21:1283–1309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siribumrungwong K, Tangtrakulwanich B and
Nitiruangjaras A: Unusual presentation of giant cell tumor
originating from a facet joint of the thoracic spine in a child: A
case report and review of the literature. J Med Case Reports.
7:1782013. View Article : Google Scholar
|
|
23
|
Zhao H, Maheshwari AV, Kumar D and Malawer
MM: Giant cell tumor of the pes anserine bursa (extra-articular
pigmented villonodular bursitis): A case report and review of the
literature. Case Rep Med. 2011:4914702011.PubMed/NCBI
|
|
24
|
Athanasou NA, Quinn J, Ferguson DJ and
McGee JO: Bone resorption by macrophage polykaryons of giant cell
tumor of tendon sheath. Br J Cancer. 63:527–533. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ho CY and Maleki Z: Giant cell tumor of
tendon sheath: Cytomorphologic and radiologic findings in 41
patients. Diagn Cytopathol. 40 (Suppl 2):E94–E98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Darling JM, Goldring SR, Harada Y, Handel
ML, Glowacki J and Gravallese EM: Multinucleated cells in pigmented
villonodular synovitis and giant cell tumor of tendon sheath
express features of osteoclasts. Am J Pathol. 150:1383–193.
1997.PubMed/NCBI
|