Introduction
The wingless-type mouse mammary tumor virus
intergration site family (Wnt) signaling pathway plays a major role
in modulating cellular processes involved in development,
differentiation and tissue homeostasis (1,2).
Interestingly, an aberrant Wnt-signaling pathway is generally
implicated in cancer and other disease states, including the
development of colorectal cancer (CRC) (1,2). In fact,
Wnt-signaling effectors regulate different processes that are
essential for cancer progression, including tumor initiation and
growth as well as differentiation and metastasis (1–3).
In the center of the Wnt signaling pathway is the
flexible β-catenin which is tightly controlled mainly by Wnt-1,
that stabilizes free pools of β-catenin and activates
β-catenin-dependent transcription (4). Nuclear accumulation of β-catenin, is
believed to be a crucial step in the carcinogenesis of CRC
(1–3).
Besides Wnt-1, the adenomatous polyposis coli (APC) gene is another
important effector of the Wnt signaling pathway, and evidence
suggest that APC is a negative regulator of β-catenin stability
(5,6).
Of note, APC and β-catenin are frequently mutated in patients with
CRC and overexpression of β-catenin or loss of APC function can
lead to the development of CRC (5,6).
The Wnt signaling pathway is also involved in the
metastatic progression of CRC (1–3).
Activation of the pathway in the tumor itself frequently maintains
a transcriptional course that is reminiscent of an
epithelial-mesenchymal transition (EMT) which can provide cell
migration and invasiveness (2).
Additionally to the induction of EMT, β-catenin also modulates the
expression of other factors that are important for metastatic
progression, particularly matrix metalloproteinases (MMPs) and
other factors that are essential for the regulation of the
extracellular matrix (2). Finally,
effectors of the Wnt signaling pathway directly control changes in
cell morphology or signaling that are important for migration and
invasion (2).
However, little is known regarding the exact
interaction and context-sensitive expression of Wnt pathway
effectors in the primary tumor and corresponding metastasis.
Therefore, this study assessed the expression of the three most
important effectors of the Wnt pathway, β-catenin, APC and Wnt-1,
in the primary tumor and corresponding metastasis of patients with
CRC.
Patients and methods
Twenty-four patients with metastatic CRC were
included in this study. All study patients underwent surgical
resection between 1996 and 2005 at the Surgical Department of the
University of Düsseldorf (Düsseldorf, Germany). The formalin-fixed,
paraffin-embedded tissue samples of the primary tumors and their
corresponding liver metastases as well as 20 synchronous lymph node
metastases were analyzed immunohistochemically. The pathological
tumor stage and disease grades were classified according to the
seventh edition of the tumor, node and metastasis classification of
the International Union against Cancer (Geneva, Switzerland). The
retrospective study regarding the immunohistochemical analyses on
formalin-fixed, paraffin-embedded tissue samples was performed with
approval by the ethics committee of the Medical Faculty of the
University of Düsseldorf.
Tissue samples
Serial sections (4 µm thick) were deparaffinised in
xylene (Z.E.U.S. GmbH, Soltau, Germany) and dehydrated in a
decreasing alcohol gradient, then incubated with different primary
rabbit antibodies for β-catenin, APC and Wnt-1 (Thermo Scientific,
Fremont, CA, USA). For immunohistochemistry, the ABC procedure
(Vectastain® ABC kit, Vector Laboratories, Inc., Burlingame, CA,
USA) was used. Firstly, the slides were heated in a microwave oven
in a Target Retrieval solution (DakoCytomation GmbH, Hamburg,
Germany) at 95°C for 20 min prior to immunostaining. Subsequently,
endogenous peroxidase activity was quenched in 0.3% hydrogen
peroxide for 30 min (Paul W. Beyvers GmbH, Berlin, Germa.
Non-specific antibody binding was blocked with 10% normal serum
(Vector Laboratories, Inc.) at room temperature. This was followed
by incubating each section with the primary antibody at the favored
concentrations for 30 min (rabbit monoclonal anti-β-catenin at
1:250 dilution; clone E247, APC and Wnt-1 (at 1:50 dilution; all
rabbit polyclonal). Subsequent to washing with phosphate-buffered
saline (PBS), the slides were incubated with 10% biotinylated
secondary antibody for 30 min at room temperature, and finally
incubated with ABC reagent for 30 min. The immunoperoxidase
reaction was carried out with diaminobenzidine and nuclear
counterstaining was carried out using Mayer's hematoxylin
(Sigma-Aldrich, St. Louis, MI, USA). Membrane expression in the
normal adjacent colonic epithelium was used as a positive control
for each antibody, and sections from each block were incubated
without the primary antibody as a negative control.
Serial sections were used to analyse the expression
of β-catenin, APC and Wnt-1. In the primary tumors, central areas
and the invasion front were analyzed separately. The percentage of
tumor cells showing a positive staining was scored and rounded up
or down to the nearest 10% in 10 steps. The mean intensity of
staining was scored between 0 and +3 (0, no staining; +1, weak; +2,
moderate; and +3 strong). This was performed according to the
procedure to attain the immunoreactive score (IRS). Based on the
percentage of positive tumor cells, four classes were defined
(<10, 11–50, 51–80, and >80%), while the mean staining
intensity was separately evaluated as belonging to one of three
classes (weak, moderate and strong). By multiplying the score
values, an IRS scale ranging between 1 and 12 was obtained.
Statistical analysis
All statistical analyses were performed using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). Correlations between
parameters and the resulting P-values were calculated by applying
the non-parametric Mann-Whitney U test or the Fisher's exact test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patients
Table I shows the
clinicopathological characteristics of the study patients.
 | Table I.Clinicopathological characteristics of
the study patients (n=24). |
Table I.
Clinicopathological characteristics of
the study patients (n=24).
| Patient
characteristics | n (%) |
|---|
| Gender |
|
| Male | 13 (54.16) |
|
Female | 11 (45.83) |
| Tumor location |
|
| Ascending
colon | 7
(29.16) |
|
Sigma | 6
(25) |
|
Rectum | 6
(25) |
|
Cecum | 4
(16.6) |
|
Descending colon | 1
(4.16) |
| Tumor
differentiation |
|
| G1 | 0 |
| G2 | 17 (70.83) |
| G3 | 6
(25) |
| G4 | 1
(4.16) |
| T-stage |
|
| pT1 | 2
(8.3) |
| pT2 | 1
(4.16) |
| pT3 | 13 (54.16) |
| pT4 | 8
(33.3) |
| N-stage |
|
| pN0 | 4
(16.6) |
| pN1 | 1
(4.16) |
| pN2 | 19 (79.16) |
| M-Stage |
|
| pM0 | 10 (41.6) |
| pM1
(HEP) | 14 (58.3) |
| R-stage |
|
| R0 | 19 (79.16) |
| R1 | 5
(20.83) |
| UICC-classification
(stage) |
|
| I | 3
(12.5) |
| II | 1
(4.16) |
| III | 6
(25) |
| IV | 14 (58.3) |
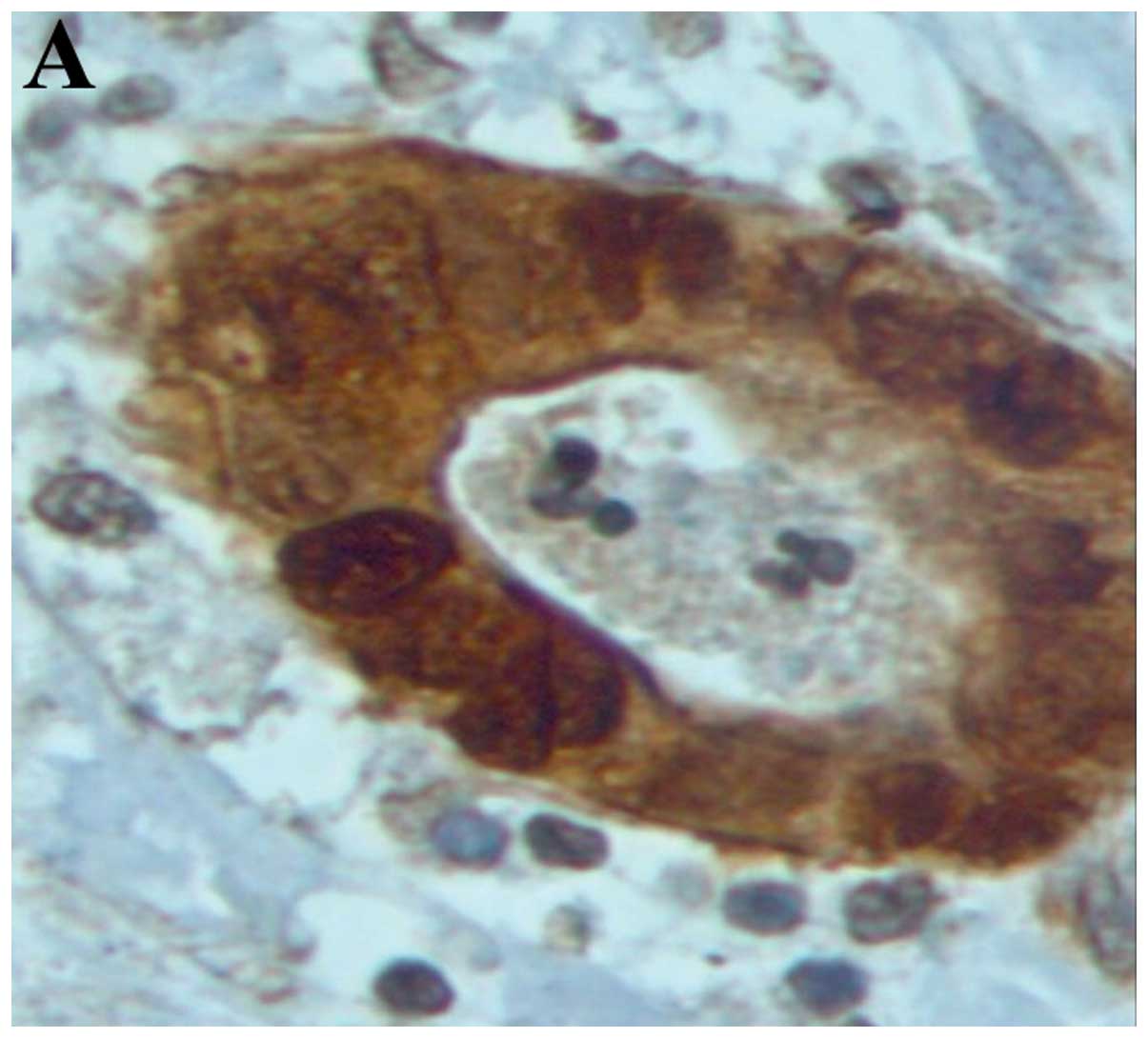
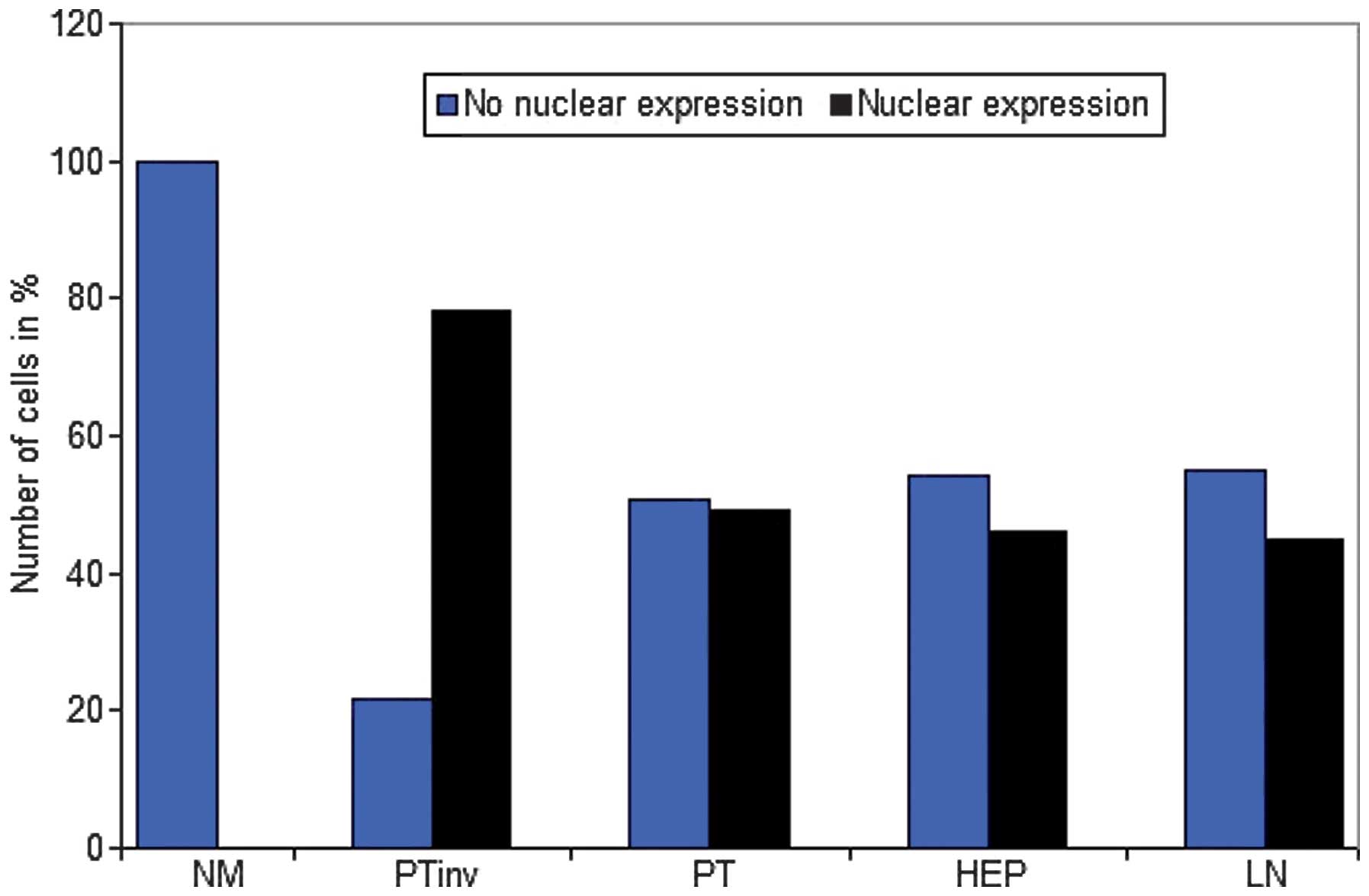
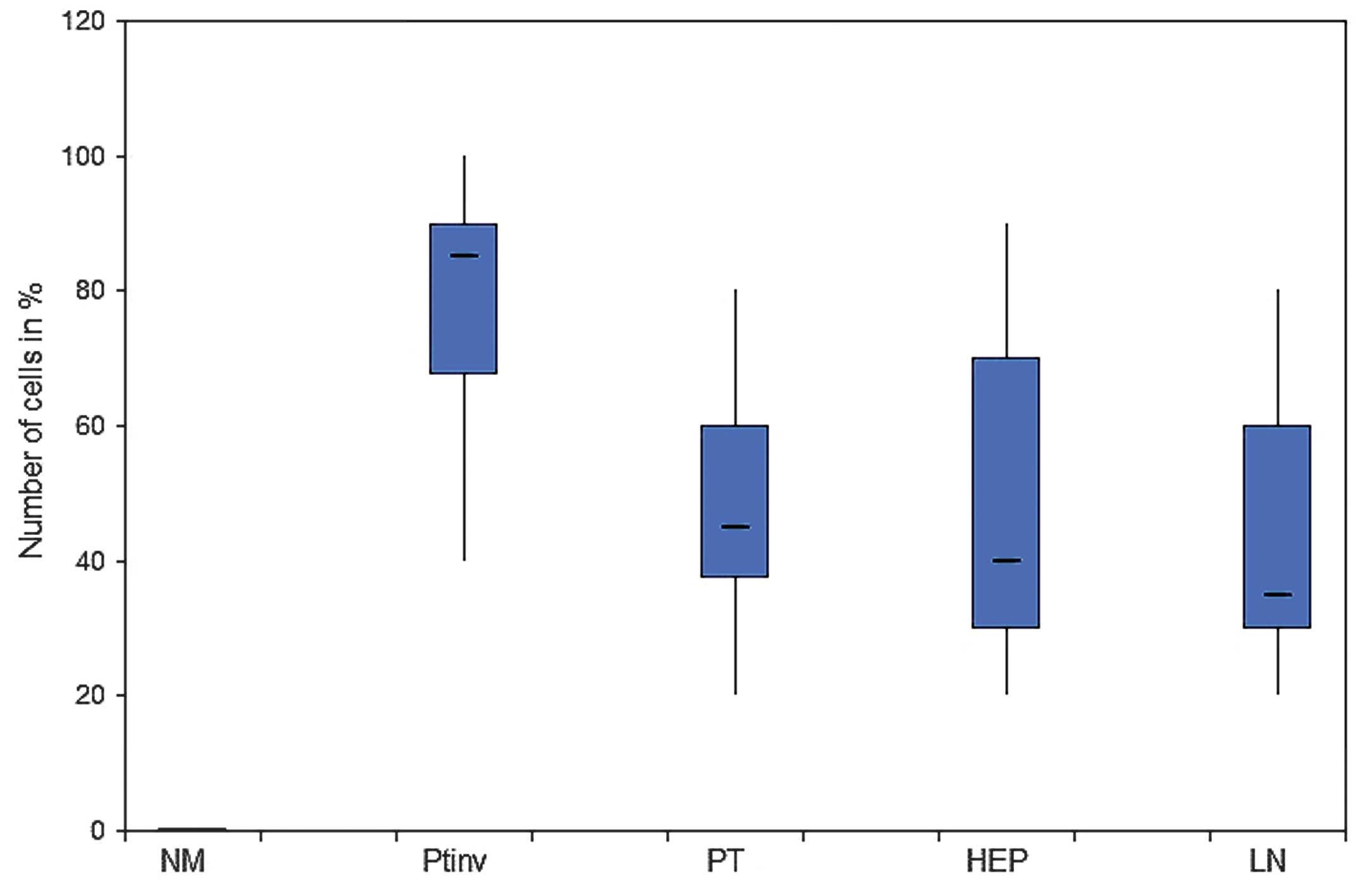
Nuclear β-catenin protein
expression
There was a strong nuclear expression of β-catenin
in 78.34% of the primary tumors, predominantly localized at the
invasion front (Figs. 1A, 2 and 3). By
contrast, cells in the tumor center often showed lower levels of
nuclear staining (49.17%, P<0.0001). The normal colonic
epithelium exhibited no nuclear staining. In liver (45.84%) and
lymph node (45%) metastases, the expression pattern was
homogeneously associated with the tumor center.
Cytoplasmic β-catenin protein
expression
In 74.5% of the primary tumors, 71.67% of the liver
and 62% of the lymph node metastases, cytoplasmic β-catenin
expression was strong (Table II).
The homogenous expression pattern in primary tumors and metastatic
lesions were statistically significant compared with the normal
colon epithelium.
 | Table II.Cytoplasmic β-catenin expression. |
Table II.
Cytoplasmic β-catenin expression.
|
| Staining intensity
score (% cells) |
|---|
|
|
|
|---|
| Location of
cells | 0 | 1+ | 2+ | 3+ | P-value |
|---|
| Normal
epithelium | 0.00 | 8.75 | 49.58 | 41.67 |
|
| Primary tumor | 0.42 | 3.75 | 21.25 | 74.58 | <0.001 |
| Liver metastases | 0.00 | 2.92 | 25.42 | 71.67 | 0.001 |
| Lymph node
metastases | 0.00 | 4.50 | 33.50 | 62.00 | 0.017 |
Membranous β-catenin protein
expression
As shown in Table
III, membranous β-catenin expression was almost homogenous in
the normal colonic epithelium, primary tumor and metastatic lesions
without any statistical significance (Fig. 1B and Table
III). No significant associations were identified between
β-catenin expression patterns and clinicopathological factors.
 | Table III.Membranous β-catenin expression. |
Table III.
Membranous β-catenin expression.
|
| Staining intensity
score (% cells) |
|---|
|
|
|
|---|
| Location of
cells | 0 | 1+ | 2+ | 3+ |
|---|
| Normal
epithelium | 0.00 | 1.25 | 22.08 | 76.67 |
| Primary tumor | 0.83 | 2.08 | 24.17 | 72.92 |
| Liver
metastases | 0.00 | 0.00 | 18.75 | 81.25 |
| Lymph node
metastases | 0.00 | 0.00 | 16.50 | 83.50 |
Wnt-1 protein expression
In 25.72% of the normal colonic epithelium and
20.52% of the primary tumors, tumor cells showed a strong staining
(3+; Table IV). In liver metastases,
expression of Wnt-1 (3.32%) was significantly reduced (P=0.003)
compared with the normal colonic epithelium.
 | Table IV.Wingless-type mouse mammary tumor
virus integration site family, member 1 expression. |
Table IV.
Wingless-type mouse mammary tumor
virus integration site family, member 1 expression.
|
| Staining intensity
score (% cells) |
|
|---|
|
|
|
|
|---|
| Location of
cells | 0 | 1+ | 2+ | 3+ | P-value |
|---|
| Normal
epithelium |
5.20 | 28.04 | 37.68 | 25.72 |
|
| Primary tumor |
5.60 | 29.64 | 40.48 | 20.52 |
|
| Liver
metastases | 20.80 | 55.64 | 16.48 |
3.32 | 0.003 |
| Lymph node
metastases |
4.23 | 33.86 | 46.77 | 11.09 |
|
APC protein expression
The APC expression patterns were homogenous in the
primary tumor and metastatic lesions with no statistically
significant differences. The strongest staining (35.42%) was
detected in the normal colonic epithelium which was significantly
higher compared with the primary tumors (P=0.022), liver (P=0.006)
and lymph node (P=0.012) metastases (Table V).
 | Table V.Adenomatous polyposis coli
expression. |
Table V.
Adenomatous polyposis coli
expression.
|
| Staining intensity
score (% cells) |
|
|---|
|
|
|
|
|---|
| Location of
cells | 0 | 1+ | 2+ | 3+ | P-value |
|---|
| Normal
epithelium | 19.17 | 44.58 | 35.42 | 0.83 |
|
| Primary tumor | 24.17 | 65.42 | 10.42 | 0.00 | 0.022 |
| Liver
metastases | 59.17 | 39.17 |
1.67 | 0.00 | 0.006 |
| Lymph node
metastases | 35.00 | 59.50 |
5.50 | 0.00 | 0.012 |
Discussion
By assessing the protein expression of the three
most important effectors of the Wnt pathway, β-catenin, APC and
Wnt-1, the present study was able to demonstrate that these major
Wnt-effectors are heterogeneously expressed in the primary tumor
and corresponding hepatic as well as nodal metastases of patients
with CRC. This context-sensitive diverse expression of Wnt-effector
proteins may be important for future individualized targeted
therapies. In the center of the Wnt signaling pathway is the
flexible β-catenin. Nuclear accumulation of β-catenin is believed
to be a crucial step in the carcinogenesis of CRC (1–3). In fact,
≤80% of colorectal tumors exhibit nuclear accumulation of β-catenin
(7–9).
Furthermore, in ≤90% of all CRCs, a mutation in a key regulator of
the Wnt signaling pathway, such as β-catenin, can be detected
(10). The present results confirm
that primary colorectal tumors present a high nuclear staining of
β-catenin, whereas no relevant staining is found in normal colonic
mucosa. In further detail, the present study revealed β-catenin to
exhibit significantly higher expression at the tumor invasion front
than in the tumor center, while in liver and lymph node metastases,
the expression pattern was homogeneously associated with the tumor
center. Similar results were reported by Brabletz et al
(11) using immunohistochemistry in
the analysis of CRC. This study group showed that the distribution
of overexpressed β-catenin was not homogeneous, but a strong
nuclear expression of β-catenin was predominantly localized at the
invasion front, while in the tumor center often no nuclear staining
was detected. Based on these results it can be hypothesized that
due to the strong activation of the Wnt signaling pathway, an EMT
of the tumor cells is induced which enables them to have a high
metastatic potential (12). In
addition, the relatively reduced staining of β-catenin in the liver
and lymph node metastases suggests that during the metastatic
process, tumor cells run through a mesenchymal-epithelial
transition. Brabletz et al (10) also demonstrated in colorectal
metastases a reduced β-catenin expression compared with the
invasion front of the primary tumor. Therefore, cancer
dissemination appears to be a dynamic process where tumor cells are
interacting at different ‘Wnt activity levels’ with each other
(13).
Wnt-1, a glycoprotein that associates with cell
membranes and likely functions as a key regulator of cellular
adhesions, is another important factor of the Wnt signaling pathway
which stabilizes free pools of β-catenin and activates
β-catenin-dependent transcription (14). Of note, the present study was not able
to show an overexpression of Wnt-1 in tumor tissue compared with
normal colonic mucosa. In fact, Wnt-1 expression was actually
significantly reduced in liver metastases. Even the current
literature shows conflicting results regarding the expression
profiles of Wnt-1 in CRC. For example, Stanczak et al
(15) determined the expression and
localization of E-cadherin, β-catenin and Wnt-1 proteins in
advanced CRC immunohistochemically. They demonstrated a strong
Wnt-1 staining in the normal colonic epithelium, while in the
majority of primary tumors the expression was decreased. By
contrast, Khor et al (16)
revealed in 47 colorectal tissue samples that intratumoral protein
expression of Wnt-1 was significantly increased in the primary
tumor compared with the normal epithelium. Finally, Holcombe et
al (17) revealed in CRC that
Wnt-1 is expressed equally and strongly in normal and malignant
colon tissues. Currently, only speculation is possible as to the
reasons for these discrepant findings. Possible reasons are: i)
There are Wnt-1 independent mechanisms that increase the nuclear
accumulation of β-catenin, such as APC mutation; ii) besides the
canonical Wnt/β-catenin pathway, other non-canonical Wnt pathways
have been described which are β-catenin and Wnt-1 independent, and
appear also to play an important role in cell adhesion and cell
migration (18).
The present study results demonstrate that the
highest APC protein expression was detected in the normal colonic
mucosa, whereas the expression in the primary tumors and
particularly in the metastases was significantly lower. These
findings are partially in agreement with a study by Chen et
al (19) that performed a
molecular analysis of APC in 39 primary CRC and 24 liver metastases
samples. While this previous study also showed a decreased APC
expression in primary tumor tissues compared with normal colonic
mucosa, the expression in the liver metastases remained strong. The
reduced APC expression in the primary tumors may be explained by
the following reasons: i) Due to the high rate of APC mutations in
CRC, the APC gene is generally inactivated in this type of cancer
and consequently its protein expression is decreased; ii) APC
inactivation can also emerge due to promoter methylation which is
frequently described in CRC (20).
In conclusion, the present study results demonstrate
heterogeneously expressed major Wnt effectors in the primary tumor
and corresponding hepatic as well as nodal metastases of patients
with CRC. Similar findings were reported by Wu et al
(21) comparing the therapeutic
target expression and promoter methylation between primary breast
tumors and their multifocal metastases. Notably, the study showed
that therapeutic targets identified in the primary breast tumors do
not reflect targets present in the metastatic sites. Therefore, the
data about heterogenous expression in the primary tumor and
corresponding metastasis is an important aspect regarding the
accepted linear progression model which assumed similar molecular
events in the primary tumor and metastasis. In fact, in this model
tumor development is based on a stepwise progression from an early
pattern to invasive and finally metastatic cancer (22). On the contrary, the present and
previous studies support the model of parallel progression
(23). Dissemination of single tumor
cells is an early step in the metastatic process and leads to an
allopatric selection of variant tumor cells, adjusted to their
specific microenvironment (24).
Therefore, the parallel progression model predicts greater
disparity between metastatic lesions and primary tumor cells than
does the linear progression model.
References
|
1
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Caner. 8:387–398.
2008. View
Article : Google Scholar
|
|
2
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Caner. 13:11–26.
2013. View
Article : Google Scholar
|
|
3
|
White BD, Chien AJ and Dawson DW:
Dysregulation of Wnt/β-catenin signalling in gastrointestinal
cancers. Gastroenterology. 142:219–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Papkoff J, Rubinfeld B, Schryver B and
Polakis P: Wnt-1 regulates free pools of catenins and stabilizes
APC-catenin complexes. Mol Cell Biol. 16:2128–2134. 1996.PubMed/NCBI
|
|
5
|
Korinek V, Barker N, Morin PJ, et al:
Constitutive transcriptional activation by a β-catenin-Tcf complex
in APC-/-colon carcinoma. Science. 275:1784–1787. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morin PJ, Sparks AB, Korinek V, et al:
Activation of β-catenin-Tcf signaling in colon cancer by mutations
in β-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martensson A, Oberg A, Jung A, et al:
Beta-catenin expression in relation to genetic instability and
prognosis in colorectal cancer. Oncol Rep. 17:447–452.
2007.PubMed/NCBI
|
|
8
|
Wanitsuwan W, Kanngurn S,
Boonpipattanapong T, et al: Overall expression of beta-catenin
outperforms its nuclear accumulation in predicting outcomes of
colorectal cancers. World J Gastroenterol. 14:6052–6059. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elzagheid A, Buhmeida A, Korkeila E, et
al: Nuclear beta-catenin expression as a prognostic factor in
advanced colorectal carcinoma. World J Gastroenterol. 14:3866–3871.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brabletz T, Jung A, Reu S, et al: Variable
beta-catenin expression in colorectal cancers indicates tumor
progression driven by the tumor environment. Proc Natl Acad Sci
USA. 98:10356–10361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brabletz T, Jung A, Hermann K, Günther K,
Hohenberger W and Kirchner T: Nuclear overexpression of the
oncoprotein beta-catenin in colorectal cancer is localized
predominantly at the invasion front. Pathol Res Pract. 194:701–704.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gabbert H, Wagner R, Moll R and Gerharz
CD: Tumor dedifferentiation: an important step in tumor invasion.
Clin Exp Metastasis. 3:257–279. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hlubek F, Brabletz T, Budczies J, Pfeiffer
S, Jung A and Kirchner T: Heterogeneous expression of
Wnt/beta-catenin target genes within colorectal cancer. Int J
Cancer. 121:1941–1948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Behrens J: The role of Wnt signalling
pathway in colorectal tumorigenesis. Biochem Soc Trans. 33:672–675.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stanczak A, Stec R, Bodnar L, et al:
Prognostic significance of Wnt-1, beta-Catenin and E-Cadherin
expression in advanced colorectal carcinoma. Pathol Oncol Res.
17:955–963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khor TO, Gul YA, Ithnin H and Seow HF: A
comparative study of expression of Wnt-1, WISP-1, survivin and
cyclin-D1 in colorectal carcinoma. Int J Colorectal Dis.
21:291–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holcombe RF, Marsh JL, Waterman ML, Lin F,
et al: Expression of Wnt ligands and Frizzled receptors in colonic
mucosa and in colon carcinoma. Mol Pathol. 55:220–226. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De A: Wnt/Ca2+ signaling pathway: a brief
overview. Acta Biochim Biophys Shanghai 2011. 43:745–756. 2011.
|
|
19
|
Chen J, Röcken C, Lofton-Day C, Schulz HU,
et al: Molecular analysis of APC promoter methylation and protein
expression in colorectal cancer metastasis. Carcinogenesis.
26:37–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baylin SB and Herman JG: DNA
hypermethylation in tumorigenesis: epigenetics joins genetics.
Trends Genet. 16:168–174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu JM, Fackler MJ, Halushka MK, Molavi DW,
et al: Heterogeneity of breast cancer metastases: comparison of
therapeutic target expression and promoter methylation between
primary tumors and their multifocal metastases. Clin Cancer Res.
14:1938–1946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Collins VP, Loeffler RK and Tivey H:
Observations on growth rates of human tumors. Am J Roentgenol
Radium Ther Nucl Med. 76:988–1000. 1956.PubMed/NCBI
|
|
24
|
Klein CA: Parallel progression of primary
tumours and metastases. Nat Rev Cancer. 9:302–312. 2009. View Article : Google Scholar : PubMed/NCBI
|