Introduction
The presence of malignant effusion is associated
with significant morbidity and a poor quality of life. The most
significant causes of malignant pleural effusion are lung or breast
cancer, and malignant lymphoma. Once malignant pleural effusion has
developed, the average survival time for the patient is often <6
months (1). Malignant ascites is most
common in gastrointestinal and gynecological cancers, with a median
survival time of <20 weeks. Patients with malignant ascites that
result from gastrointestinal cancers have a particularly poor
prognosis and the survival rate is only 12–20 weeks (2). Pericardial effusion is generally
observed in dying patients, which worsens prognosis. The treatment
of malignant effusions is often a challenge for physicians.
Currently, the conventional treatment of malignant effusions is
primarily composed of diuresis, salt restriction, serous cavity
paracentesis, intracavitary chemotherapy, biological response
modifiers, traditional Chinese medicine or thermotherapy, however,
the therapies are not effective. Following treatment with these
methods, there is no significant reduction in effusions and
relapses often occur quickly. Furthermore, almost all of these
treatment methods result in toxic side-effects of various degrees.
Thus, it is important to understand the underlying molecular
mechanisms associated with malignant effusion. Previous studies
have demonstrated that elevated levels of vascular endothelial
growth factor (VEGF), tumor angiogenesis and increased vascular
permeability following tumor invasion or metastasis to the
pleuroperitoneum are important mechanisms of serous cavity
effusions. VEGF requires further study due to its presence in the
pleural fluid and its potential use as a therapeutic target
(3–5).
The VEGF family of proteins is known to promote
angiogenesis during embryonic development or wound healing,
however, the altered expression of VEGF contributes to disease
development, including tumorigenesis and tumor progression
(6). The VEGF family contains a group
of secreted proteins, including VEGF-A, -B, -C, -D and -E, and
placental growth factor (7).
Specifically, VEGF-A is a heparin-binding dimeric cytokine, which
is important in vascular permeability and angiogenesis. VEGF-A is
50,000-fold more potent than histamine in the induction of vascular
permeability (7). Increased VEGF-A
levels produced by tumor, mesothelial and infiltrating immune cells
may lead to increased vascular permeability, which is crucial for
pleural or peritoneal fluid formation. By contrast, VEGF-C and -D
are closely associated with lymph vessel neogenesis (8). Previous studies have demonstrated that
VEGF-A protein is present in significant amounts in peritoneal and
pleural effusions of varying etiologies (9,10). VEGF-A
levels in malignant effusions were found to be significantly
increased compared with those in non-malignant effusions,
indicating that this difference may aid in the differentiation
between malignant and non-malignant effusions (11,12). The
altered expression of VEGF-A has been reported to be associated
with the poor prognosis of various types of human cancer (13,14).
Multiple clinical studies have also demonstrated the potential
benefit of VEGF-A inhibition in patients with malignant effusions
(15). Anti-angiogenic therapy (such
as bevacizumab, a monoclonal antibody targeting VEGF-A) adjuvant to
chemotherapy serves a potential function in the management of
pleural effusions in advanced non-squamous non-small cell lung
cancer (5,16).
VEGF-A is the most important regulatory factor in
tumor angiogenesis, and VEGF-C and -D are the most important in
tumor lymphangiogenesis (16).
Previous studies of VEGF in serous cavity effusions have mainly
focused on VEGF-A. However, suppressing lymphangiogenesis in
malignant effusions formation may provide another therapeutic
strategy for cancer patients with malignant effusions. The most
effective and definitive technique to diagnose malignant effusion
is cytological examination of the pleural or peritoneal fluid. The
specificity of cytological examination is usually high, but the
sensitivity has been reported to range between 30 and 90% (17). Thus, it is important to have molecular
biomarkers available to aid in the diagnosis of malignant effusion.
In the present study, the content and expression levels of VEGF-A,
-C and -D were examined in the sera and supernatants of malignant
effusion and exfoliated cells from patients with malignant serous
cavity effusions that resulted from lung and gastric cancer. The
association between the expression levels of the different VEGF
subtypes and the clinicopathological characteristics and prognosis
of the patients was also examined.
Materials and methods
Study population
In the present study, consecutive patients with
pleural, peritoneal or pericardial fluid were recruited from The
Fourth Hospital of Hebei Medical University (Shijiazhuang, China)
between July 2012 and January 2013. All the cases were diagnosed
pathologically using hematoxylin and eosin staining (H&E) and
immunocytochemistry (ICC). All patients were followed up until
January 2014. Supernatant fluids were collected from 96 patients
(38 primary lung cancer, 30 primary gastric cancer and 28 benign
effusions) and analyzed for levels of VEGF-A, -C and -D proteins.
In addition, serum samples from 79 patients (30 primary lung
cancer, 21 primary gastric cancer and 28 benign effusion) and
cytological smears of the effusions from 71 patients (34 primary
lung cancer, 17 primary gastric cancer and 20 benign effusion) were
prepared for the assaying of VEGF-A, -C and -D levels. The
characteristics of the patients are summarized in Table I. The study was approved by the Ethics
Committee of The Fourth Hospital of Hebei Medical University, and
all participants provided written informed consent to participate
in this study.
 | Table I.Characteristics of patients according
to effusion (n=96), serum (n=79) and cytological samples
(n=51). |
Table I.
Characteristics of patients according
to effusion (n=96), serum (n=79) and cytological samples
(n=51).
| Characteristics | Effusion, n (%) | Serum, n (%) | Cytological samples n
(%) |
|---|
| Disease |
|
|
|
| Gastric
cancer | 30 (31.3) | 21 (26.6) | 17 (33.3) |
| Lung
cancer | 38 (39.6) | 30 (38.0) | 34 (66.7) |
| Benign
tuberculosis | 16 (16.7) | 16 (16.7) | 0 (0.0) |
| Disease
non-tuberculosis | 12 (12.4) | 12 (12.4) | 0 (0.0) |
| Gender |
|
|
|
|
Female | 34 (35.4) | 27 (34.2) | 17 (33.3) |
| Male | 62 (64.6) | 52 (65.8) | 34 (66.7) |
| Age, years |
|
|
|
| ≤45 | 12 (12.5) | 11 (13.9) | 6 (11.7) |
|
46–65 | 52 (54.2) | 43 (54.4) | 24 (47.1) |
|
>65 | 32 (33.3) | 25 (31.7) | 21 (41.2) |
| Site |
|
|
|
| Pleural
effusion | 57 (59.4) | 47 (59.5) | 32 (62.8) |
|
Peritoneal effusion | 38 (39.6) | 32 (40.5) | 16 (31.4) |
|
Pericardial effusion | 1 (1.0) | 0 (0.0) | 3 (5.9) |
| Metastasis |
|
|
|
| Pleural
or peritoneal metastasis | 29 (42.7) | 22 (43.1) | 25 (49.0) |
|
Multiple metastasis | 39 (57.4) | 29 (56.9) | 26 (51.0) |
| Volume,
cm3 |
|
|
|
| ≤5 | 45 (46.9) | 34 (43.0) | 21 (41.2) |
|
5–10 | 37 (38.5) | 34 (43.0) | 21 (41.2) |
|
>10 | 14 (14.6) | 11 (13.9) | 9
(17.6) |
| Feature |
|
|
|
| Clear,
yellow | 61 (63.5) | 51 (64.6) | 27 (52.9) |
|
Bloody | 35 (36.5) | 28 (35.4) | 24 (47.1) |
Enzyme-linked immunosorbent assay
(ELISA) detection of VEGF-A, -C and -D levels in serum and
supernatant fluid
To assess the expression levels of VEGF-A, -C and -D
proteins, 10 ml of fresh pleural, peritoneal or pericardial fluid
was collected from each patient prior to treatment and then
centrifuged at 200 × g for 10 min at 4°C to pellet the cellular
elements. The supernatant was stored at −80°C until use. Serum was
prepared from 5 ml of blood that was extracted from the cubital
vein in the morning after fasting. The serum and supernatant fluid
levels of the VEGF-A, -C and -D proteins were assayed using a
double antibody sandwich ELISA with ELISA kits (VEGF-A kit,
NeoBioscience, Shenzhen, China; VEGF-C kit, RayBiotec, Inc.,
Norcross, GA USA; and VEGF-D kit, ImmunoWay Biotechnology Company,
Newark, DE, USA) according to the manufacturers' instructions. The
values were read by a microplate reader (Anthos Labtec Instruments
GmbH, Salzburg, Austria), and the data are presented as the median
and interquartile range.
ICC detection of VEGF-A, -C and -D
expression in exfoliated cells from effusion
To determine the expression of VEGF-A, -C, and -D
proteins, 50–500 ml of fresh pleural, peritoneal or pericardial
fluid samples were collected from patients using a disposable cell
enrichment collector (pore size, 5–8 µm; Beiing Xincheng
International Exhibit Trading Co., Ltd., Beijing, China). The
exfoliated cells were prepared for cytological smear on glass
slides and then fixed in 95% ethanol for 10 min, stained with
H&E and viewed under a light microscope (BX51; Olympus
Corporation, Tokyo, Japan). The well-distributed and qualified
exfoliated cell smear slides were selected for immunocytochemical
analysis. Briefly, the exfoliated cell smear slides were treated
with H2O2 for 10 min, followed by microwave
treatment for antigen retrieval. The slides were then incubated
with a normal non-immunized serum at room temperature for 30 min
and then with a primary rabbit anti-human VEGF-A, -C or -D antibody
(all from BioWorld Technology, Inc., Dublin, OH, USA) at a dilution
of 1:75, overnight at 4°C. The following day, the slides were
washed with phosphate-buffered saline (PBS) three times and then
further incubated with an anti-rabbit immunoglobulin G antibody at
a dilution of 1:100 (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China). The color reaction was performed using DAB solution
and counterstained with hematoxylin solution briefly.
Evaluation of immunocytochemical
stained slides
A primary monoclonal mouse anti-human
carcinoembryonic antigen antibody (Maixin-Bio, Fuzhou, China) at a
dilution of 1:200 was used as the positive control and the primary
antibody was replaced with PBS for the negative control. The
stained slides were reviewed and scored independently by two
investigators who did not have knowledge of the slide
identification and clinical data. If there was a discrepancy, this
was resolved by consensus. A semi-quantitative method was used to
score the staining of each antibody. The percentage of
immunopositive cells was assigned by a four-point system as
follows: 0 points, no positive cells; 1 point, <25% positive
cells; 2 points, 26–50% positive cells; 3 points, 51–75% positive
cells; and 4 points, >75% positive cells. The staining intensity
was scored similarly: 0 points, negative staining (colorless); 1
point, weak staining (light yellow): 2 points, moderate staining
(brown): and 3 points, strong staining (dark brown).
Immunoreactivity scores for each lesion were calculated by the
summation of the two scores: Negative (–), 0 score; weakly positive
(1), 1–2 scores; positive (2+), 3–4
scores; or strongly positive (3+), 5–7 scores.
Statistical analysis
Statistical analysis was performed using the SPSS
software package, version 17.0 (SPSS, Inc., Chicago, IL, USA).
Comparison of data between the groups was performed with the
non-parametric Kruskal-Wallis test followed by the Mann-Whitney
test. The association of VEGF-A, -C and -D levels with
clinicopathological parameters was determined using multiple linear
regression and multivariate logistic regression analysis. Survival
curves were calculated by the Kaplan-Meier method and the
significance of differences was estimated by the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
VEGF-A
Expression of VEGF-A proteins in sera,
supernatant fluid and cytological samples
The expression of VEGF-A, -C and -D proteins in the
serum (sVEGF-A, -C and -D) and in the pleural, peritoneal or
pericardial supernatant fluid (pVEGF-A, -C and -D) is presented in
Table II.
 | Table II.Levels of VEGF-A, -C and -D proteins
in benign and malignant effusions and sera in pg/ml (median;
interquartile range). |
Table II.
Levels of VEGF-A, -C and -D proteins
in benign and malignant effusions and sera in pg/ml (median;
interquartile range).
| Protein | Gastric cancer | Lung cancer | Benign disease |
|---|
| pVEGF-A | 500.13;
1725.39a | 457.54;
1988.96a | 124.48;
588.56b |
| pVEGF-C | 40.97; 39.63 | 62.92; 61.33 | 50.50;
51.81c |
| pVEGF-D | 4796.32;
2238.91 | 3540.08;
2923.62 | 3938.30;
3243.40c |
| sVEGF-A | 174.04; 201.61 | 147.67; 255.51 | 129.58;
196.88c |
| sVEGF-C | 156.71; 84.23 | 187.11; 88.79 | 178.24;
95.05c |
| sVEGF-D | 4037.43;
776.90 | 4282.18;
1237.71 | 3757.34;
1515.77c |
No statistically significant differences were
observed in the level of sVEGF-A proteins in the patients with
cancer compared with those with benign effusions (P>0.05). The
cancer patients exhibited increased pVEGF-A expression levels
compared with those with benign effusions (P<0.05). The
upregulated level of pVEGF-A was not associated with tumor
histological types (P>0.05). The pVEGF-A level was similar to
the corresponding sVEGF-A level in the patients with benign
effusions. Nevertheless, the pVEGF-A level in the malignant
effusions were significantly increased compared with its
corresponding sVEGF-A level (P<0.05).
Cellular levels of VEGF-A, -C and -D were also
assessed using ICC (cVEGF-A, -C and -D). The expression levels of
VEGF-A, -C and -D were detected in 20 cytological smears from
benign effusions. No positive immunoreactivity was observed for
VEGF-A, -C and -D in the 20 benign cases, with the exception of
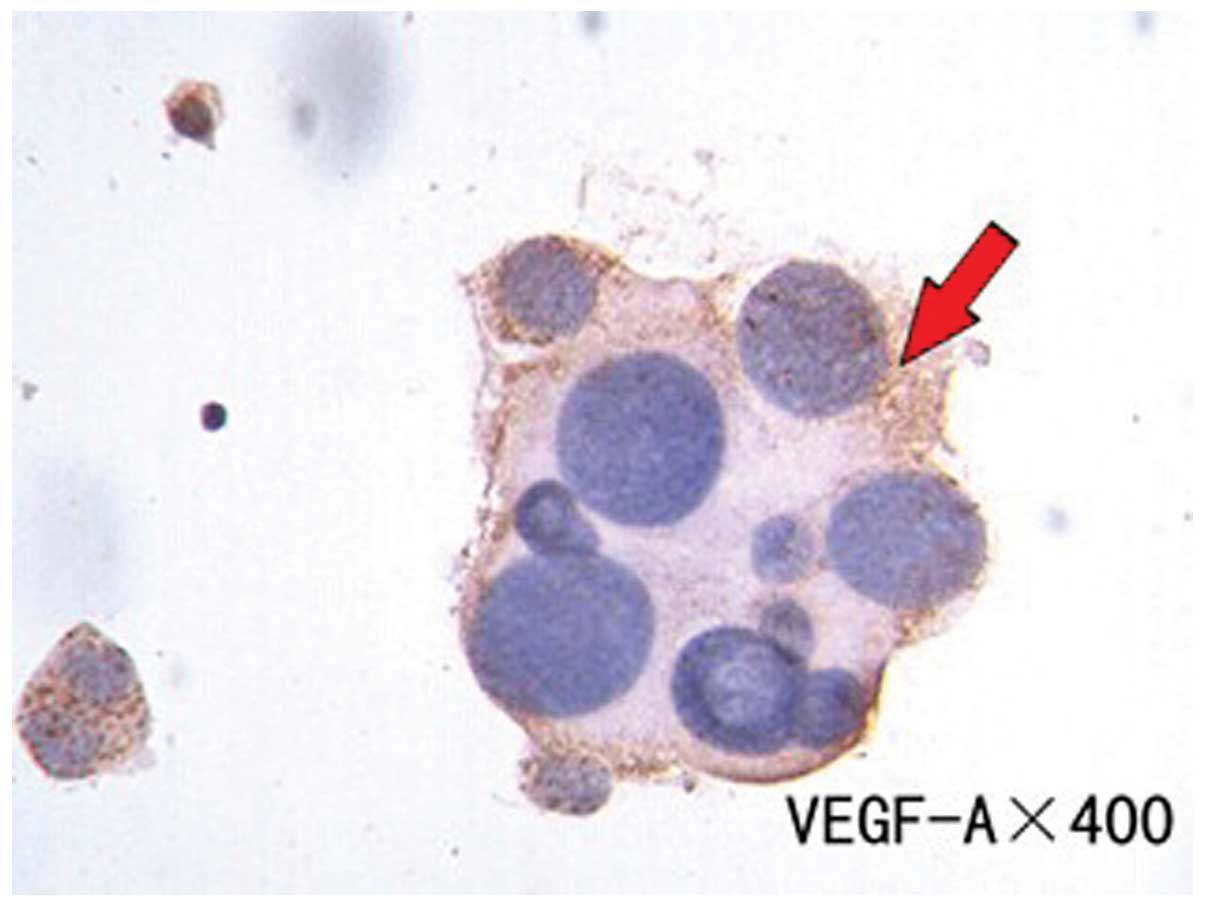
partial staining in the mesothelial cells. The VEGF-A expression
rate was 52.94% and was mainly expressed in the cytoplasm of
positively-expressed tumor cells (Fig.
1).
Association between VEGF-A level and patient
clinicopathological parameters
Using multiple linear regression analysis, the data
demonstrated that pVEGF-A was negatively associated with age, and
positively associated with malignant and bloody effusion, and
cavity only metastasis (P<0.05; Table III). Using multiple logistic
regression analysis, it was demonstrated that cVEGF-A was inversely
associated with patient age.
 | Table III.Multiple linear regression analysis
of the association between pVEGF levels and patient
clinicopathological parameters. |
Table III.
Multiple linear regression analysis
of the association between pVEGF levels and patient
clinicopathological parameters.
| Parameter | B | β | P-value |
|---|
| pVEGF-A |
|
|
|
|
Age | −455.768 | −0.226 | <0.05 |
|
Malignant/benign | −2085.78 | −0.730 | <0.01 |
| Only
cavity metastasis/multiple metastasis | −961.545 | −0.613 | <0.01 |
| Clear,
yellow/bloody effusion | 722.668 | 0.268 | <0.01 |
| pVEGF-C |
|
|
|
|
Age | −22.843 | −0.302 | <0.01 |
|
Malignant/benign | −26.833 | −0.250 | >0.05 |
| Only
cavity metastasis/multiple metastasis | −21.597 | −0.367 | >0.05 |
| Clear,
yellow/bloody effusion |
2.957 | 0.029 | >0.05 |
| pVEGF-D |
|
|
|
|
Age | 1406.21 | 0.394 | <0.01 |
|
Malignant/benign | 2776.59 | 0.548 | <0.01 |
| Only
cavity metastasis/multiple metastasis | 1510.85 | 0.543 | <0.01 |
| Clear,
yellow/bloody effusion | −369.836 | −0.077 | >0.05 |
Association between VEGF-A level and overall
survival
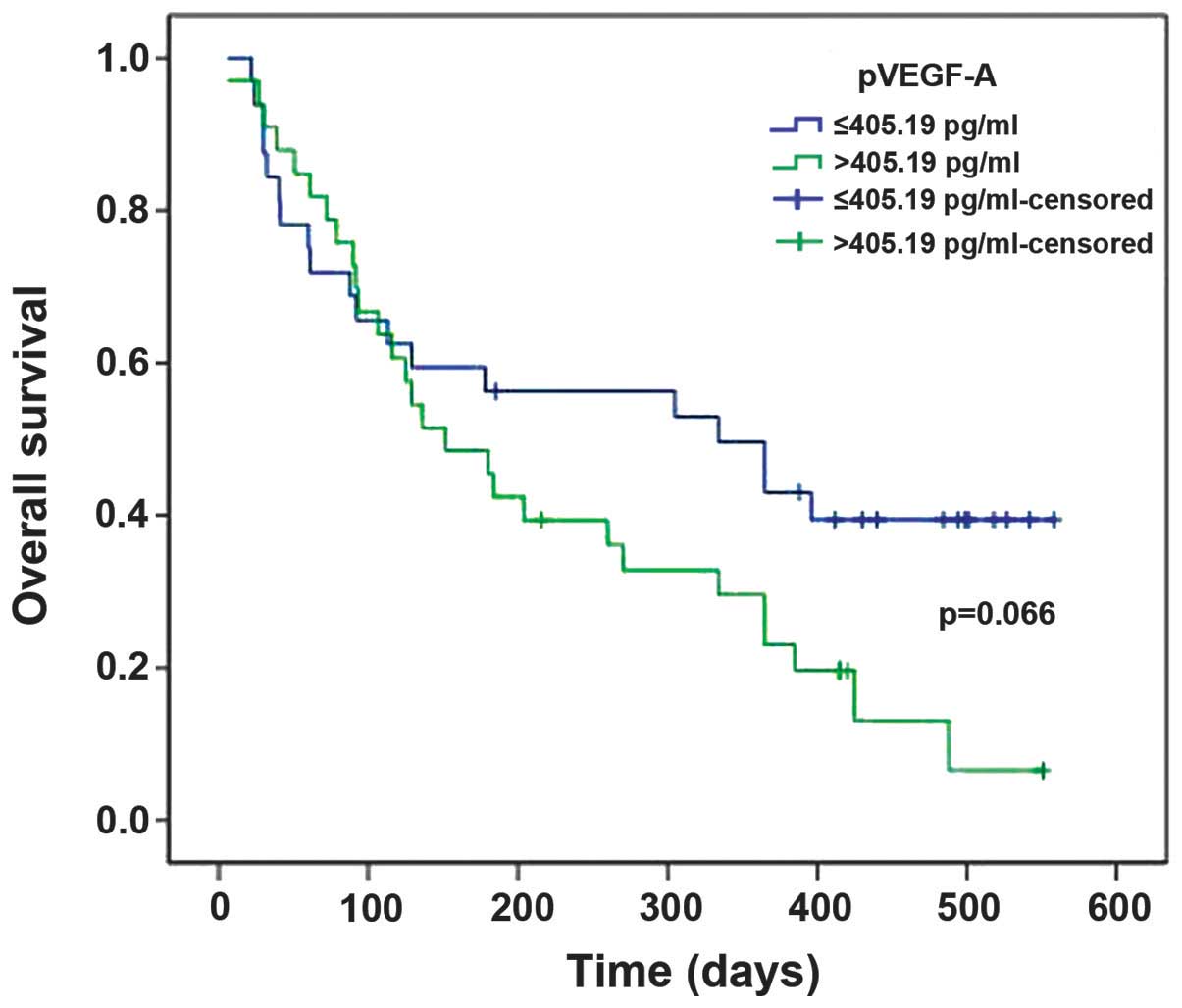
With the median VEGF-A level as the cut-off value,
patients were divided into high and low supernatant fluid parameter
groups. Survival time analysis demonstrated a relatively shorter
survival time for patients with pVEGF-A levels of >406.19 pg/ml
compared with those presenting with pVEGF-A levels of ≤406.19
pg/ml, although this effect was not statistically significant
(P=0.066; Fig. 2). sVEGF-A expression
similarly did not exhibit statistical significance in predicting
survival time for patients with malignant effusions
(P>0.05).
VEGF-C and -D
Expression of VEGF-C and -D proteins in sera,
supernatant fluid and cytological samples
No statistically significant differences were
observed in the expression levels of sVEGF-C and -D proteins or
pVEGF-C and -D proteins between cancer and benign patients
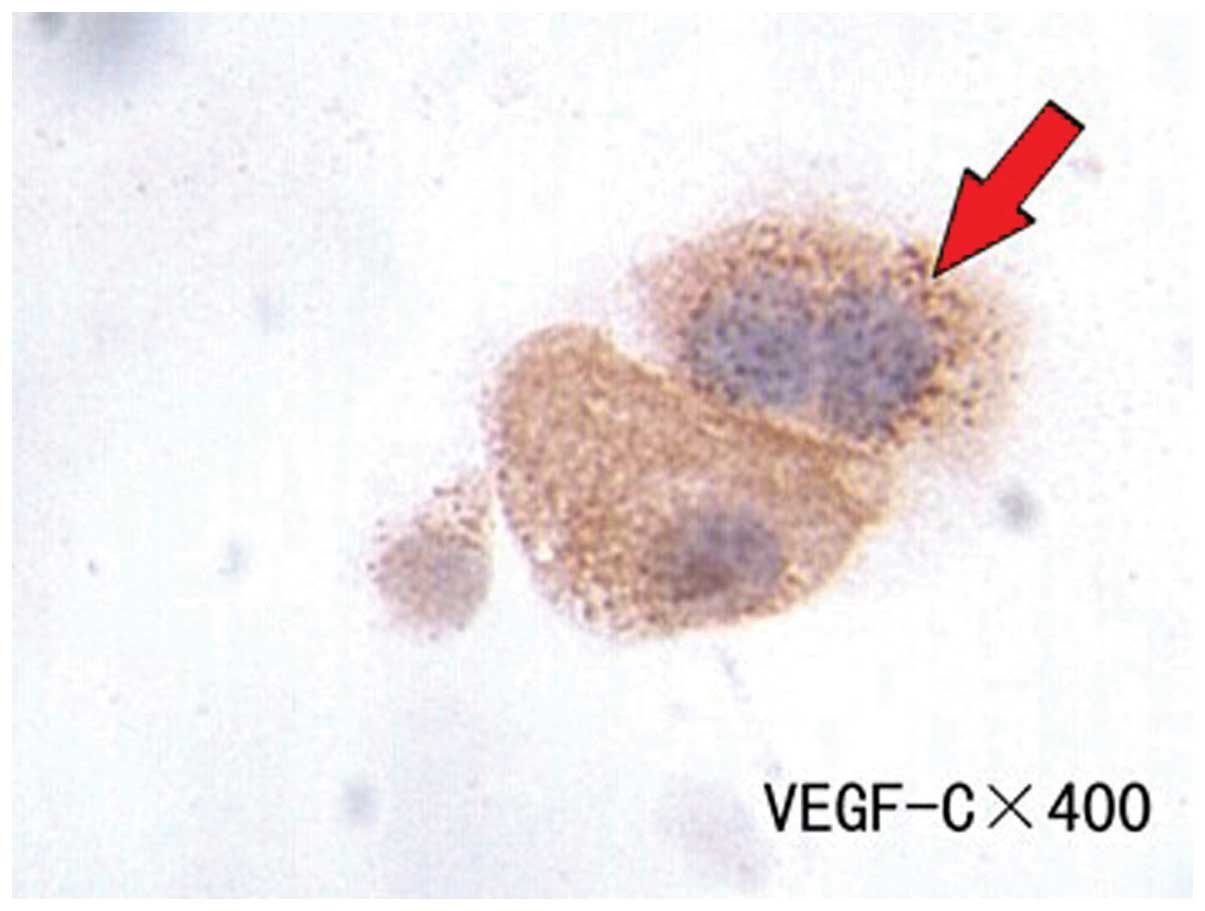
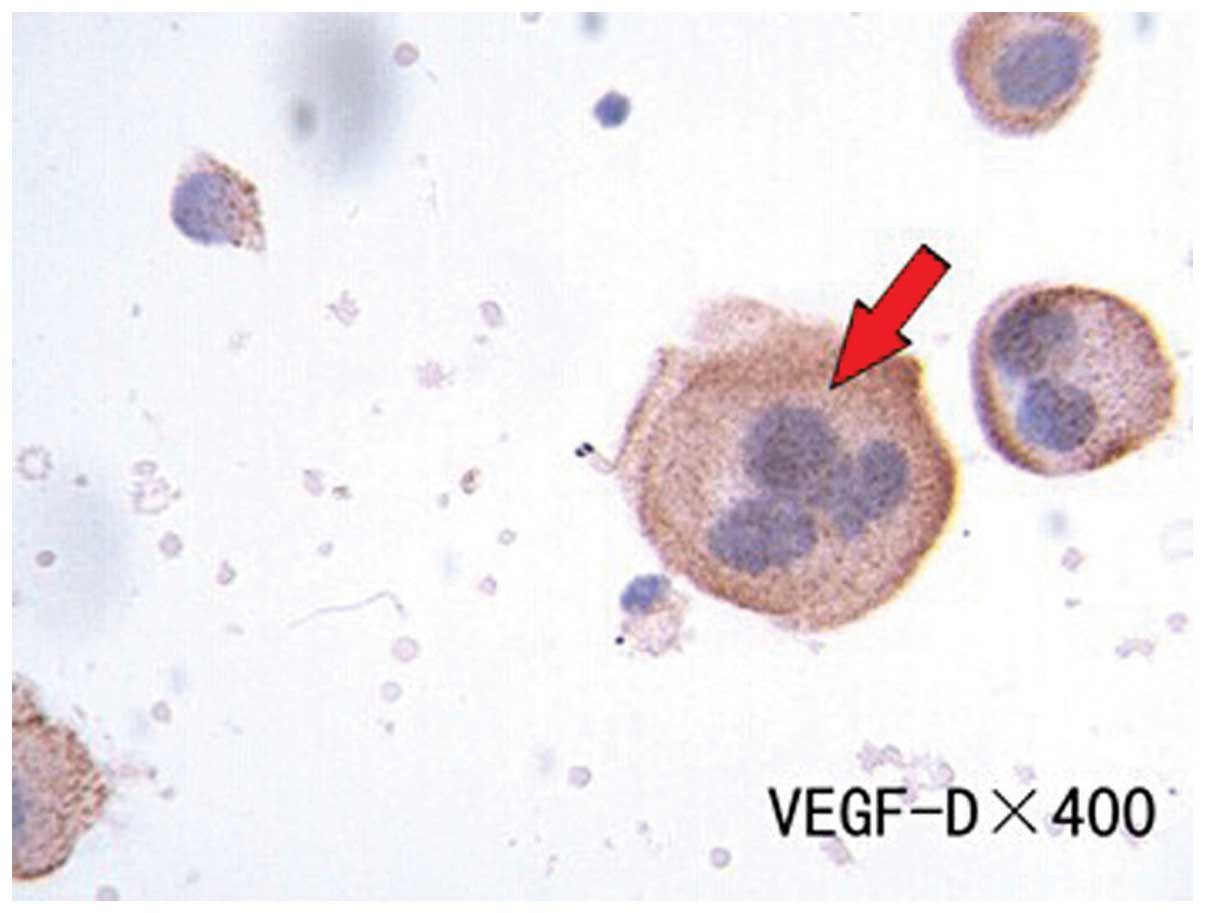
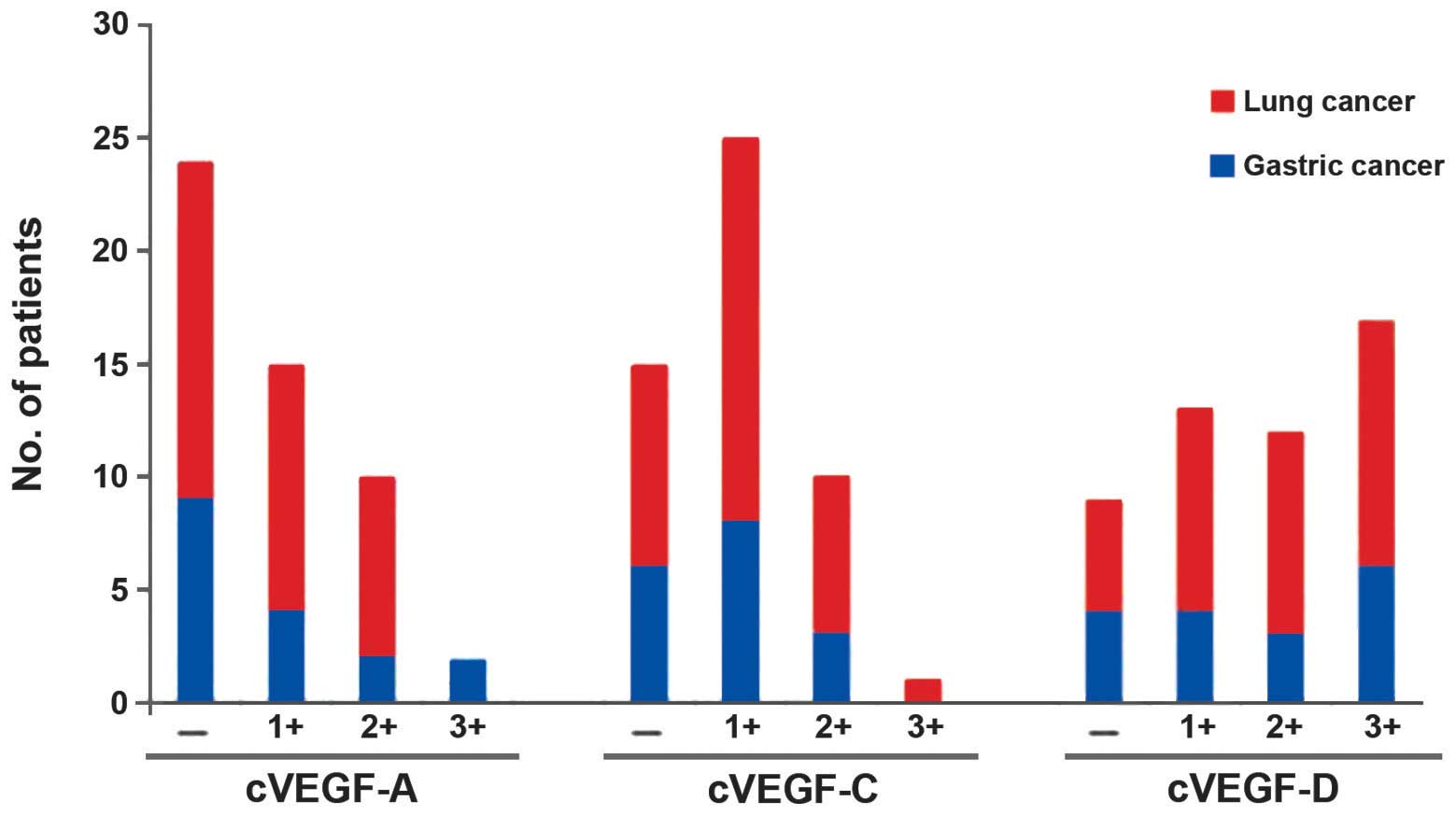
(P>0.05). cVEGF-C and -D expression was 70.58 and 82.35%,
respectively, in the 51 patients with lung or gastric cancer
(Figs. 3 and 4). These proteins were predominantly
expressed in the cytoplasm of positively-expressed tumor cells.
However, the levels of cVEGF-D in the cancer patients were
significantly increased compared with cVEGF-A and -C (P<0.05).
No statistically significant differences were observed in the
cVEGF-A, -C and -D expression levels between lung and gastric
cancer patients (P>0.05; Fig.
5).
Association between VEGF-C and -D levels and
clinicopathological patient parameters
pVEGF-C expression was inversely associated with
patient age. pVEGF-D expression was associated with age, and
inversely associated with malignant effusion and only cavity
metastasis (P<0.05; Table III).
However, no association was observed between the sVEGF-C and -D
proteins and the examined clinicopathological factors
(P>0.05).
Association between VEGF-C and -D levels and the
overall survival of patients
No statistically significant associations were
observed between the expression levels of sVEGF-C and -D, and
pVEGF-C and -D, and the survival rates for patients with malignant
effusions (P>0.05).
Discussion
Angiogenesis has a critical effect on cancer growth
and metastasis, and VEGF is a potent angiogenic and lymphangiogenic
mediator. Previous studies demonstrated that an increased level of
pleural VEGF-A was associated with malignancy and that VEGF-A was
considered to be a marker for the diagnosis of malignant effusion
(9,11). VEGF-C and -D are the ligands of VEGF
receptor-3 (VEGFR-3) and the latter is localized on
lymph-endothelial cells; when VEGFR-3 is activated through the
binding of VEGF-C and/or -D, it may to be sufficient to promote the
metastasis of cancer cells (18). In
solid tumors, experimental and clinical evidence has indicated that
the expression of VEGF-C or -D proteins can contribute to increased
lymphatic vessel density and tumor lymphatic metastasis, and that
the expression of VEGF-C or -D is an independent prognostic factor
for patients with oral squamous cell carcinoma (19). Cancer cell lymphatic spread induced by
VEGF-D may be blocked with an antibody against VEGF-D (20). Limited animal studies have been
conducted on effusions, but have demonstrated that VEGF-C and -D
may be important in producing pleural dissemination (21,22). In
the present study, the protein expression levels of VEGF-A, -C and
-D in the supernatant fluid, exfoliated cells and sera from
patients with benign and malignant diseases were determined. The
analysis demonstrated that pVEGF-A levels in the supernatant fluid
from pleural, peritoneal or pericardial effusion were significantly
upregulated compared with that in the benign effusions, indicating
that the detection of pVEGF-A levels may have potential diagnostic
value for malignant effusions.
To analyze whether these markers were associated
with tumor histological types, patients were recruited with
malignant effusions from two types of malignancies, primary lung
and gastric cancer, which most often lead to pleural and peritoneal
effusion. No significant differences were observed in pVEGF-A
levels between lung and gastric cancer patients, which is
consistent with other studies that observed no significant
difference in supernatant fluid VEGF-A levels in patients with
different histological types or clinical stages of lung cancers
(12,23). Multiple linear regression analysis was
used to demonstrate that the levels of VEGF-A in the supernatant
fluid of the patients with bloody effusion were increased compared
with the patients with non-bloody effusion, which is consistent
with other previous studies that demonstrated that the pleural
VEGF-A level is associated with the number of red blood cells
(14,24,25). In
the present study, the levels of pVEGF-A protein were inversely
associated with age, indicating that age is a protective factor and
reflecting the reduced angiogenesis capacity in aging individuals.
In addition, the supernatant fluid levels of VEGF-A in the patients
with only cavity metastasis were increased compared with the
patients with metastases other than only cavity metastasis.
In the current study, statistically significant
differences in the levels of pVEGF-C and -D were not observed
between cancerous and benign disease, which is in accordance with
the results reported by Croghan et al (26). Using multiple linear regression
analysis, it was demonstrated that supernatant fluid levels of
pVEGF-C proteins were inversely associated with age, and that
levels of pVEGF-A and -D were positively associated with a number
of clinicopathological parameters, including malignant effusion and
only cavity metastasis. However, the underlying mechanisms remain
to be determined.
The prognostic significance of pVEGF-A has been
estimated in several previous studies (13,14).
Hirayama et al followed 28 malignant pleural mesothelioma
patients closely for up to 600 days (13) and demonstrated that a VEGF level of
>2,000 pg/ml was a significant predictor of patient survival
(13). Hsu et al (14) retrospectively studied 97 NSCLC
patients with only malignant pleural effusion and observed that a
VEGF level of >1,350 ng/ml was a significant negative predictor
of patient survival. In the present prospective study that followed
66 patients with malignant serous cavity effusions from lung and
gastric cancer closely for up to 550 days, pVEGF-A levels with a
median level of 406.19 pg/ml as a cut-off value did not reach
statistical significance as a potential predictor of poor clinical
outcome (P=0.066). The Kaplan-Meier method demonstrated that
pVEGF-C and -D did not exhibit statistical significance in
predicting survival for patients with malignant effusions.
Prospective studies with long-term follow-up of malignant effusion
patients are required.
In addition, the present study also assessed the
expression levels of VEGF-A, -C and -D proteins in exfoliated cells
from the effusion, and observed that these proteins were most
highly expressed in the cytoplasm of tumor and mesothelial cells,
which is consistent with the literature on VEGF-A in effusion wax
blocks (10,14). Multiple logistic regression analysis
demonstrated that the expression of cVEGF-A proteins was inversely
associated with age, indicating that age is a protective factor and
reflects the reduced angiogenesis capacity in aging individuals.
Since the number of cytological samples was limited in the present
study, the association between cVEGF-A, -C and -D expression and
overall survival was not examined. The present study also
demonstrated that cVEGF-D expression was increased compared with
cVEGF-A and -C expression in exfoliated cells from malignant
effusion, particularly in strongly positive cells. This result
indicates that VEGF-D-mediated lymphangiogenesis may be important
in the formation of malignant effusion and may provide a novel
targeted therapy for cancer patients. No statistically significant
differences were observed in pVEGF-C and -D expression between
benign and malignant effusions, however, the proteins were highly
expressed at the cellular level. Additional clinical samples are
required to further study the diagnostic value of pVEGF-C and -D
following disease stratification.
In the present study, serum VEGF-A, -C and -D levels
exhibited no marked clinical significance in the diagnosis and
prognosis of serous cavity effusions, and were also not
significantly associated with the examined clinicopathological
parameters. Certain previous studies have demonstrated that
supernatant fluid VEGF-A levels in malignant effusions are
consistently increased compared with serum levels, while other
studies have observed no correlation between the levels of
supernatant fluid VEGF-A in malignant effusions and plasma
(27–30). In the present study, the pVEGF-A
levels were similar to the corresponding sVEGF-A levels in the
patients with benign effusions. However, the pVEGF-A levels in the
patients with malignant effusions were significantly increased
compared with their corresponding sVEGF-A levels. Analysis of this
phenomenon demonstrated that serous cavity markers do not easily
enter the blood circulation inactivated by the liver, however,
serological markers are easily affected by numerous factors, such
as body metabolism, which results in concentration of the
serological markers being reduced compared with the serous cavity
markers. It has previously been indicated that serological markers
may not be as effective for the diagnosis and prognostic values of
serous cavity effusions compared with local effusion markers.
The present study applied ELISA to detect local
effusion markers from the supernatant and ICC to the cells. Using
ELISA for the detection was convenient due to the small amount of
effusion required and the quantitative analysis provided, but the
method is easily affected by the whole body disease and reagent
instruments. The ICC analysis may be performed on wax blocks and
fresh exfoliated cells. The former has been used in previous
studies, with the advantage of long sample storage and the
disadvantage of a long, complex production process that is easily
affected by impurities. The latter was used in the present study
and has the advantage of using fresh cells, not being easily
affected by impurities and possibly providing a rapid clinical
diagnosis. However, the method has the disadvantage of requiring at
least 50 ml of sample and has no long-term sample preservation.
Notably, the wax block method is suitable for retrospective studies
and fresh exfoliated cells are suitable for prospective studies,
with timely clinical diagnosis and treatment. The methods of using
ELISA and ICC to identify local effusion markers require
improvement by multicenter, large sample, randomized, prospective
clinical trials and adequate follow-up, and this may be used to
determine which detected form is active and suitable for VEGF-A,
-C, and -D in clinical to timely diagnosis and target therapy.
In conclusion, the present study demonstrated that
the pVEGF-A expression level may be useful in the differential
diagnosis of malignant effusion from lung or gastric cancer
samples. cVEGF-C and -D may be important in the formation of
malignant effusion. The levels of VEGF-A and -C protein in the
supernatant fluid and VEGF-A protein in the cells were negatively
associated with age, while supernatant fluid VEGF-A was positively
associated with malignant and bloody effusion, and only cavity
metastasis. Serum VEGF-A, -C and -D levels exhibited no marked
clinical significance in the diagnosis or prognosis of serous
cavity effusions. Therefore, future studies with a larger sample
size and long-term follow-up are required to establish the role of
VEGF-A, -C and -D for the diagnosis, prognosis and targeted therapy
of malignant effusions.
Glossary
Abbreviations
Abbreviations:
|
VEGF
|
vascular endothelial growth factor
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
ICC
|
immunocytochemistry
|
|
H&E
|
hematoxylin and eosin
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Lombardi G, Zustovich F, Nicoletto MO, et
al: Diagnosis and treatment of malignant pleural effusion: A
systematic literature review and new approaches. Am J Clin Oncol.
33:420–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung M and Kozuch P: Treatment of
malignant ascites. Curr Treat Options Oncol. 215–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pichelmayer O, Gruenberger B, Zielinski C,
et al: Bevacizumab is active in malignant effusion. Ann Oncol.
17:18532006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitamura K, Kubota K, Ando M, et al:
Bevacizumab plus chemotherapy for advanced non-squamous
non-small-cell lung cancer with malignant pleural effusion. Cancer
Chemother Pharmacol. 71:457–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du N, Li X, Li F, Zhao H, et al:
Intrapleural combination therapy with bevacizumab and cisplatin for
non-small cell lung cancer-mediated malignant pleural effusion.
Oncol Rep. 29:2332–2340. 2013.PubMed/NCBI
|
|
6
|
Hoeben A, Landuyt B, Highley MS, et al:
Vascular endothelial growth factor and angiogenesis. Pharmacol Rev.
56:549–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dvorak HF: Vascular permeability
factor/vascular endothelial growth factor: A critical cytokine in
tumor angiogenesis and a potential target for diagnosis and
therapy. J Clin Oncol. 20:4368–4380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lohela M, Saaristo A, Veikkola T, et al:
Lymphangiogenic growth factors, receptors and therapies. Thromb
Haemost. 90:167–184. 2003.PubMed/NCBI
|
|
9
|
Shen YC, Liu MQ, Wan C, et al: Diagnostic
accuracy of vascular endothelial growth factor for malignant
pleural effusion: A meta-analysis. Exp Ther Med. 3:1072–1076.
2012.PubMed/NCBI
|
|
10
|
Ishimoto O, Saijo Y, Narumi K, et al: High
level of vascular endothelial growth factor in hemorrhagic pleural
effusion of cancer. Oncology. 63:70–75. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karatolios K, Pankuweit S, Moosdorf RG, et
al: Vascular endothelial growth factor in malignant and benign
pericardial effusion. Clin Cardiol. 35:377–381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim SC, Jung SI, Kim YC, et al: Vascular
endothelial growth factor in malignant and tuberculous pleural
effusions. J Korean Med Sci. 15:279–283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirayama N, Tabata C, Tabata R, et al:
Pleural effusion VEGF levels as a prognostic factor of malignant
pleural mesothelioma. Respir Med. 105:137–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu IL, Su WC, Yan JJ, et al: Angiogenetic
biomarkers in non-small cell lung cancer with malignant pleural
effusion: Correlations with patient survival and pleural effusion
control. Lung Cancer. 65:371–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verheul HM, Hoekman K, Jorna AS, et al:
Targeting vascular endothelial growth factor blockade: Ascites and
pleural effusion formation. Oncologist. 5:45–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahashi S: Vascular endothelial growth
factor (VEGF), VEGF receptors and their inhibitors for
antiangiogenic tumor therapy. Biol Pharm Bull. 34:1785–1788. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
American Thoracic Society, . Management of
malignant pleural effusions. Am J Respir Crit Care Med.
162:1987–2001. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mäkinen T, Veikkola T, Mustjoki S, et al:
Isolated lymphatic endothelial cells transduce growth, survival and
migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J.
20:4762–4773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sugiura T, Inoue Y, Matsuki R, et al:
VEGF-C and VEGF-D expression is correlated with lymphatic vessel
density and lymph node metastasis in oral squamous cell carcinoma:
Implications for use as a prognostic marker. Int J Oncol.
34:673–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stacker SA, Caesar C, Baldwin ME, et al:
VEGF-D promotes the metastatic spread of tumor cells via the
lymphatics. Nat Med. 7:186–191. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishii H, Yazawa T, Sato H, et al:
Enhancement of pleural dissemination and lymph node metastasis of
intrathoracic lung cancer cells by vascular endothelial growth
factors (VEGFs). Lung Cancer. 45:325–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma X, Yao Y, Yuan D, et al: Recombinant
human endostatin endostar suppresses angiogenesis and
lymphangiogenesis of malignant pleural effusion in mice. PLoS One.
7:e534492012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yanagawa H, Takeuchi E, Suzuki Y, et al:
Vascular endothelial growth factor in malignant pleural effusion
associated with lung cancer. Cancer Immunol Immunother. 48:396–400.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yano S, Shinohara H, Herbst RS, et al:
Production of experimental malignant pleural effusions is dependent
on invasion of the pleura and expression of vascular endothelial
growth factor/vascular permeability factor by human lung cancer
cells. Am J Pathol. 157:1893–1903. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gary Lee YC, Melkerneker D, Thompson PJ,
et al: Transforming growth factor beta induces vascular endothelial
growth factor elaboration from pleural mesothelial cells in vivo
and in vitro. Am J Respir Crit Care Med. 165:88–94. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Croghan GA, Nichols F, Cassivi S, et al:
VEGF A, C and D levels in malignant pleural effusions. J Clin
Oncol. 26 (Suppl 20):221262008.
|
|
27
|
Sack U, Hoffmann M, Zhao XJ, et al:
Vascular endothelial growth factor in pleural effusions of
different origin. Eur Respir J. 25:600–604. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kraft A, Weindel K, Ochs A, et al:
Vascular endothelial growth factor in the sera and effusions of
patients with malignant and nonmalignant disease. Cancer.
85:178–187. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Strizzi L, Catalano A, Vianale G, et al:
Vascular endothelial growth factor is an autocrine growth factor in
human malignant mesothelioma. J Pathol. 193:468–475. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yeo KT, Wang HH, Nagy JA, et al: A
prospective, observational study describing the haematological
response in patients undergoing chemotherapy treated by tri-weekly
darbepoetin alpha for anaemia. Cancer Res. 53:2912–2918.
1993.PubMed/NCBI
|