Introduction
Dimethyl sulfoxide (DMSO) is a solvent with
amphiphathic properties that is capable of dissolving a wide range
of substances, and is used as a solvent in biological studies and
as a vehicle for drug therapy (1).
cis-Diamminedichloroplatinum (II) (cisplatin; CDDP) is
widely used in the treatment of various types of cancer.
CDDP-containing chemotherapy regimens have been shown to be
effective in the clinical management of testicular embryonal
carcinomas (ECs) (2). CDDP is not
soluble in water; accordingly, DMSO is often used as a solvent for
it or for other combined agents in biological experiments and in
the clinical setting.
Testicular germ cell tumors (TGCTs) are the most
frequently occurring malignancy in males aged 15–35 years. While
TGCTs are classified into two major histological subgroups, namely
seminomas and non-seminomatous germ cell tumors (NSGCTs), they are
suggested to all arise from the same precursor (3). The clinical outcome of patients with
NSGCTs tends to be worse for those with seminomas. NSGCTs exhibit
embryonal and extraembryonal differentiation patterns, including
primitive zygotic (EC), embryonal-like somatic differentiated
(teratoma) and extraembryonally differentiated (choriocarcinoma and
yolk sac tumor) phenotypes (4). The
majority of NSGCTs contain ECs, with these displaying similarities
to embryonic stem (ES) cells and being able to differentiate into
essentially any tissue (5), including
yolk sac tumors, choriocarcinoma and teratoma. Due to advances in
combination chemotherapy, the 10-year disease-specific survival
rate in patients with metastatic NSGCTs is 88% (6). However, 30% of patients with stage I
NSGCTs relapse during active surveillance (7–9).
Chemotherapy containing CDDP has been introduced as an adjuvant
treatment option for micrometastatic disease, thereby reducing the
risk of relapse to 2% (10). The
percentage of EC component in the primary tumor is an important
predictive factor for occult metastasis in stage I NSGCTs (7,11–13), and adjuvant chemotherapy should be
performed in such a way that resistance is minimized (14). Therefore, EC component control without
induction of chemotherapeutic resistance is crucial for the
appropriate clinical management of NSGCTs when considering the fact
that ECs are a putative cancer stem component of NSGCT (15).
DMSO has been shown to induce differentiation in EC
and ES cells, and to affect other cellular functions, including
progression through the cell cycle and apoptosis (1). If DMSO is utilized to solubilize
chemotherapeutic agents, such as CDDP, in the clinical management
of NSGCTs, it can affect the character of EC components and reduce
drug efficacy. Thus, the present study was designed to clarify the
potential effects of DMSO on EC cells and the associated responses
to CDDP.
Materials and methods
Cell lines and culture conditions
Two EC cell lines, namely NEC8 and NEC14 (Riken Cell
Bank, Tsukuba, Japan), were used in the present study. The cell
lines were maintained in RPMI 1640 (Life technologies, Tokyo,
Japan) containing 10% fetal bovine serum (FBS; GE healthcare Life
Sciences, Chalfont, UK) supplemented with penicillin (100 U/ml),
streptomycin (100 U/ml) and glutamine (300 mg/l) in a humidified
atmosphere of 5% CO2 on dishes coated with type I
collagen. The cells were passaged when they reached 80% confluence.
Experimental procedures using the cell lines were performed within
6 months of receipt from the cell bank.
DMSO treatment
The NEC8 and NEC14 cells were exposed to DMSO
(Sigma-Aldrich, St. Louis, MO, USA) for 72 h after plating and the
effects of DMSO were examined and compared with untreated control
cells. DMSO was diluted in the culture medium, with resulting final
concentrations of 0.2, 0.4, 0.6, 0.8 and 1.0% (v/v),
respectively.
Cell counting kit-8 (CCK-8) assay
The NEC8 and NEC14 cells were seeded at
3×103 cells/well in 96-well plates coated with type I
collagen containing RPMI 1640 with 10% FBS, and incubated at 37°C.
The next day, DMSO was applied at the various aforementioned
concentrations. After 72 h of DMSO treatment, CDDP was applied at a
final concentration of 0, 2.5, 5, 7.5, 10, 15, and 20 µM,
respectively. The cells were incubated in presence or absence of
CDDP for 2 days. Cell viability was subsequently measured by WST-8
assay using CCK-8 (Dojindo Molecular Technologies Inc., Kumamoto,
Japan). WST-8 reagent solution was added to each well, and the cell
plates were incubated for 3 h. Next, the absorbance of each well
was measured using an Infinite M200 microplate reader (Tecan,
Männedorf, Switzerland) at a wavelength of 450 nm.
Western blot analysis
The cells were lysed in Laemmli-sodium dodecyl
sulfate (SDS) buffer, and the lysate was sonicated and then boiled
for 5 min. Protein lysate from U-2 OS/DOXO35 (16) cells was used as a positive control for
MDR-1 expression. Samples containing equal amounts of protein were
separated via SDS-polyacrylamide gel electrophoresis on 6–10% gels
prior to transfer to polyvinylidine fluoride membranes (Millipore,
Billerica, MA, USA). The membranes were immunoblotted with the
following primary antibodies: mouse monoclonal anti-MDR-1 (1:100,
JSB-1; Abcam, Cambridge, UK) and mouse monoclonal anti-MRP-1
(1:100; MRPm6; Enzo Life Sciences, Farmingdale, NY, USA), which are
drug efflux pumps; rabbit monoclonal anti-Vimentin (1:1,000; D21H3;
Cell Signaling Technology Inc., Danvers, MA, USA), mouse monoclonal
anti-Fibronectin (1:500; NCL-FIB, #568; Leica Microsystems Inc.,
Buffalo Grove, IL, USA) and mouse monoclonal anti-TRA-1-60 (1:500;
TRA-1-60; eBioscience Inc., San Diego, CA, USA), as markers of
differentiation; rabbit polyclonal anti-SOX2 (1:500, #2748; Cell
Signaling Technology Inc.); and goat polyclonal anti-OCT3/4 (1:250;
C-20; Santa Cruz Biotechnology Inc., Dallas, TX, USA), as a marker
of stemness. Mouse monoclonal anti-DNMT1 (1:500; 60B1220-1; Abcam),
rabbit polyclonal anti-DNMT3A (1:1,000: 64B1446; Novus Biologicals,
LLC, Littleton, CO, USA) and mouse monoclonal anti-DNMT3L (1:1,000;
Novus Biologicals, LLC) (17) were
used for DNMT family screening. Anti-α tubulin (1:2,000; DM1A;
Sigma-Aldrich) was utilized to provide a loading control.
Fluorescence immunocytochemistry
The NEC8 and NEC14 cells were cultured on type I
collagen-coated LabTek™ chamber slides (BD Biosciences, Franklin
Lakes, NJ, USA) to 50% confluence, fixed with 2%
buffered-formaldehyde and 70% ethanol, and then permeabilized with
0.1% Triton X-100. The primary mouse anti-TRA-1-60 (1:200; TRA1-60;
eBioscience), mouse anti-SSEA-1 (1:200; MC-480, Abcam), rat
anti-SSEA-3 (1:50; MC-631, R&D systems, Minneapolis, MN, USA)
and rabbit anti-DNMT3L antibodies (17) were incubated overnight at 4°C.
Appropriate secondary antibodies labeled with Alexa 488 (Life
Technologies) were then applied, followed by washing with
phosphate-buffered saline (PBS) to remove excess fluorescent dye,
and mounting with glycerol. The specimens were observed and images
were captured under identical conditions using a fluorescence
microscope fitted with a charge-coupled device camera (DMI4000 B;
Leica Microsystems Inc.).
DNA extraction
The NEC8 and NEC14 cells were washed with PBS and
suspended in lysis buffer [10 mM Tris-HCl and 50 mM EDTA (pH 8.0),
10 mM NaCl, 2% N-lauryl sarcosine and 200 µg/ml proteinase
K]. The mixture was incubated for 20 h at 55°C, followed by phenol
chloroform extraction and ethanol precipitation. DNA from cell
lines was extracted using QIAamp DNA Blood Mini kits (Qiagen Inc.,
Valencia, CA, USA) and human genomic DNA from peripheral blood
lymphocytes was obtained from Takara Bio Inc. (Otsu, Shiga,
Japan).
Treatment of DNA with sodium
bisulfite
The method described by Clark et al (18) was used to perform bisulfite treatment,
with alterations used as detailed by Frevel et al (19). The bisulfite reaction, under mineral
oil, was performed at 55°C for 16 h in a 525-µl total volume
containing 2.4 M sodium bisulfite (Sigma-Aldrich) and 123 mM
hydroquinone (Sigma-Aldrich). Reactions were desalted using a QIAEX
II Gel Extraction kit (Qiagen Inc.). DNA was eluted in 50 µl
H2O, incubated with 5 µl of 3 M NaOH for 15 min at 37°C,
neutralized with ammonium acetate (final concentration of 3 M) and
ethanol precipitated. The bisulfite-treated DNA was then
resuspended in 25 µl H2O and stored at −20°C.
Combined bisulfite restriction
analysis (COBRA) for LINE1
COBRA was conducted as described previously
(20). Methylation of the LINE1
promoter was investigated as follows: Polymerase chain reaction
(PCR) amplification was performed in 25-µl volumes using Ex Taq
buffer (Takara Bio Inc.) under the following conditions: 2 mM
MgCl2, 200 mM of each deoxynucleotide triphosphate, 0.8
mM final concentration of each primer and 0.6 units of Ex Taq
buffer (Takara Bio Inc.). The primer sequences associated with the
LINE1 promoter region were: 5′-TTGAGTTGTGGT GGGTTTTATTTAG-3′
(496–520, X58075) and 5′-TCATCT CACTAAAAAATACCAAACA-3′ (108–132,
X58075). The PCR cycling conditions were 95°C for 30 sec, 50°C for
30 sec and 72°C for 30 sec for 35 cycles. The final PCR product was
digested with the HinfI restriction enzyme (Takara Bio
Inc.). The digested PCR products were separated by electrophoresis
on 6% polyacrylamide gels. In a COBRA analysis, the lower digested
bands represent methylated repetitive elements and the upper
undigested band represents unmethylated repetitive elements or
repetitive elements in which the restriction site has been mutated.
Following gel electrophoresis and ethidium bromide staining, the
PCR bands were quantitated through densitometric analysis and the
degree of methylation was thereby determined for LINE1 elements in
the NEC8 and NEC14 cells.
Statistical analysis
A two-way factorial analysis of variance and
multiple comparison tests accompanied by Scheffe's significance
test were performed using StatView software for Windows, version
5.0 (SAS Institute, Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
DMSO enhances resistance to CDDP in EC
cells without induction of drug efflux pumps
DMSO significantly induced the resistance to CDDP in
the NEC8 and NEC14 cells. The CDDP half maximal inhibitory
concentration (IC50) values in the NEC8 cells varied
from 4.1 to 11.6 µM at 0 to 0.8% (v/v) of DMSO exposure, conferring
up to 3-fold more resistance to CDDP than the no DMSO control
(P=0.0012; Fig. 1A). Similarly, the
NEC14 cells were ~3-fold more resistant to CDDP when exposed to
0.8% (v/v) DMSO (P<0.001; Fig.
1B). However, DMSO exposure did not result in the induction of
the drug efflux pumps, MDR-1 and MRP-1, in either EC cell line
(Fig. 1C).
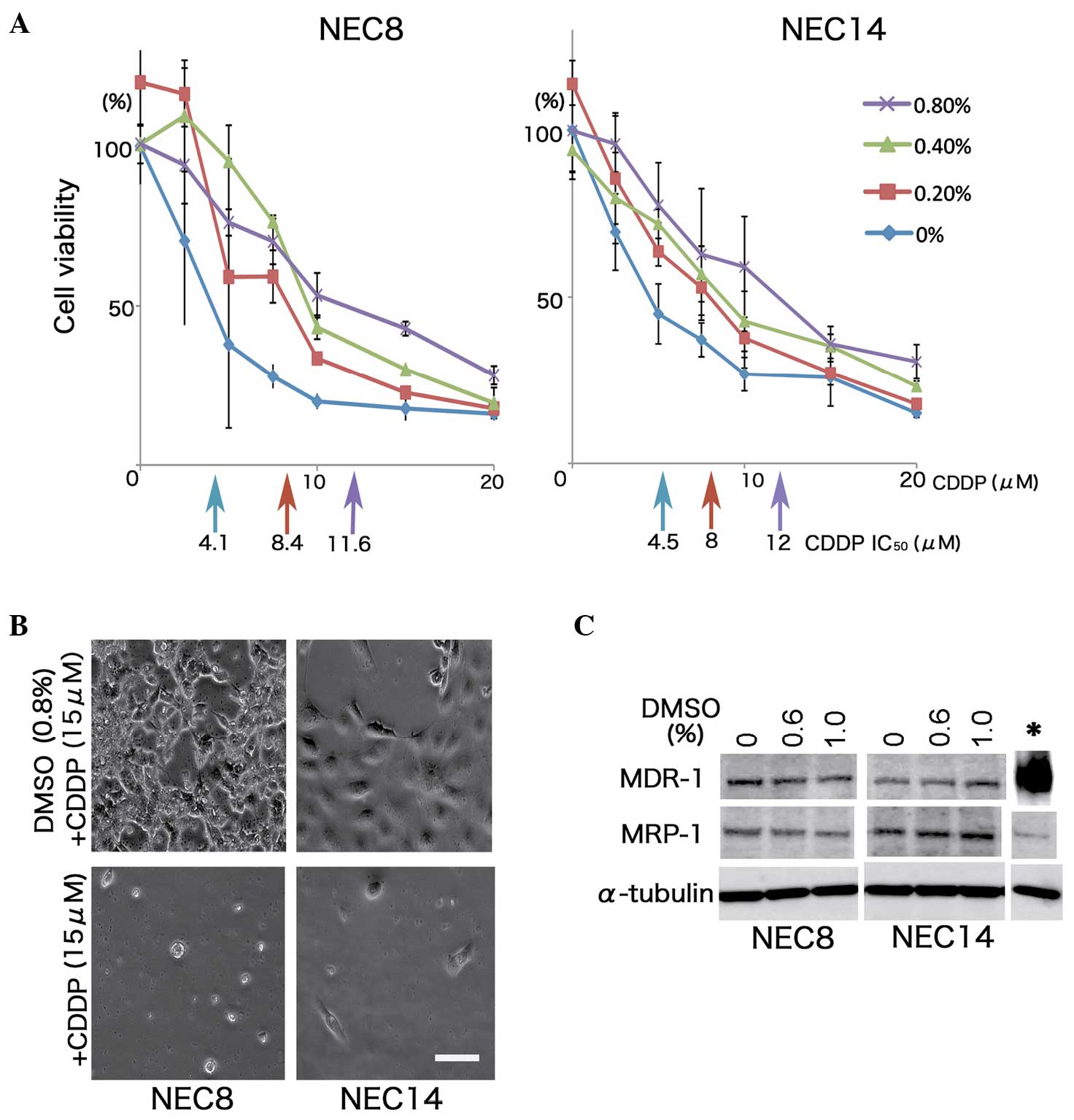 | Figure 1.DMSO enhances resistance to CDDP
without induction of MDR-1 or MRP-1 in human EC cells. (A)
Viability of human EC cells after 48 h of CDDP exposure together
with DMSO treatment. Two EC cell lines, NEC8 and NEC14, were
treated with DMSO at the indicated concentrations for 72 h, and
various doses of CDDP were additionally applied for 48 h. The
effects on cell growth were determined by use of the WST-8 assay
(n=3; vertical bars, standard deviation). IC50 values
were indicated by each colored-arrow and value. DMSO decreased the
cytotoxicity of CDDP in a dose-dependent manner; 0.8% (v/v) DMSO
induced ~3-fold more resistance to CDDP than 0% (v/v) DMSO in each
cell line. (B) Micrographs of NEC8 and NEC14 cells cultured in
medium containing 15 mM CDDP with/without 0.8% (v/v) DMSO. The NEC8
and NEC14 cells retained their morphology at high doses of CDDP
with DMSO, with exposure to CDDP alone leading to a significant
reduction in cell numbers. Scale bar, 100 µm. (C) Western blot
analysis of the major drug efflux pumps, MDR-1 and MRP-1. No
induction of either pump was observed in response to DMSO treatment
in the NEC8 and NEC14 cells. Cell lysate (*) of U-2OS/DOXO35 was
used as the positive control for MDR-1. DMSO, dimethyl sulfoxide;
CDDP, cisplatin; EC, embryonal carcinoma; IC50, half
maximal inhibitory concentration. |
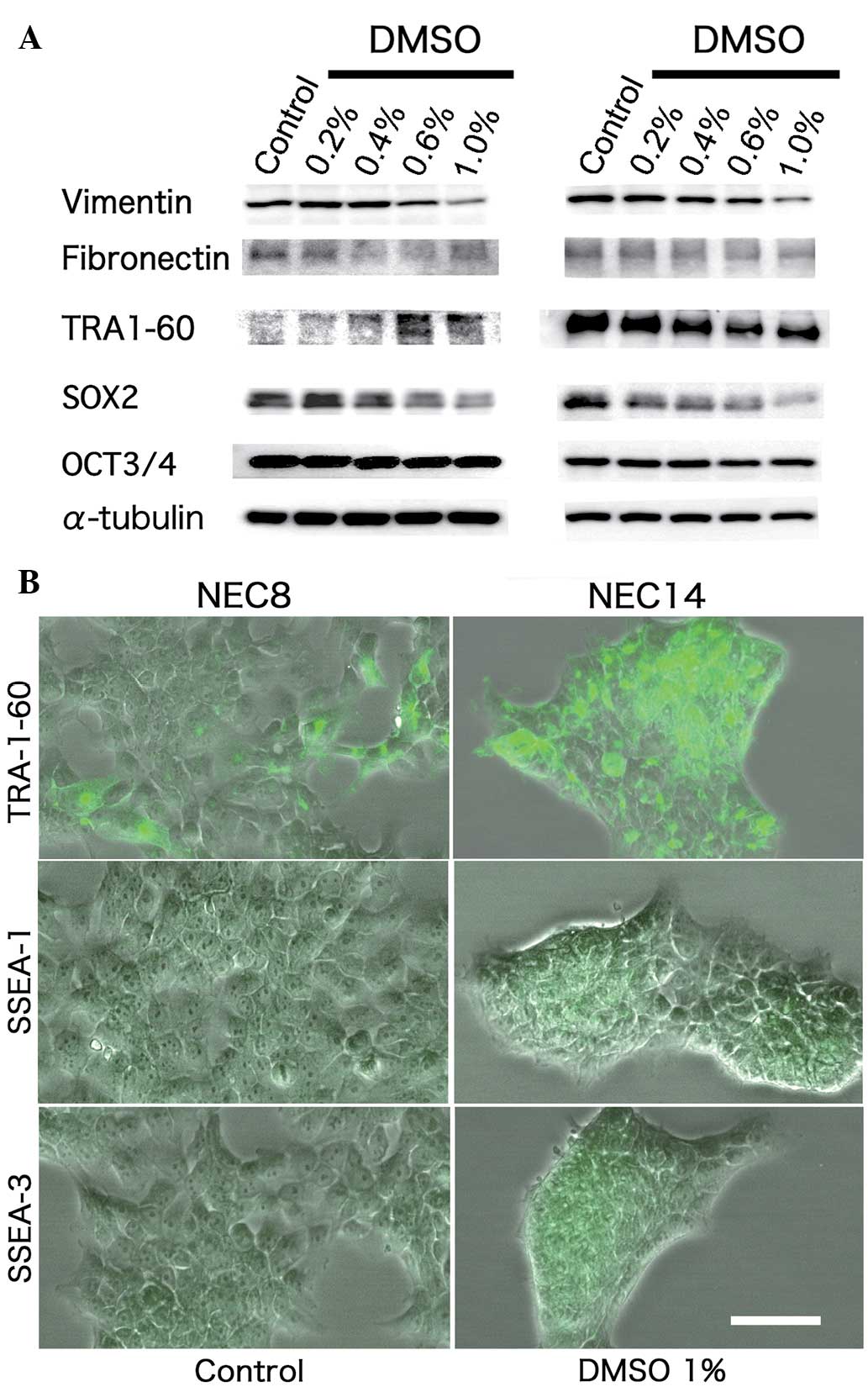
DMSO perturbs differentiation and
reduces stemness-related markers in EC cells
In order to detail the differentiation status of EC
cells exposed to DMSO, the expression levels of Vimentin,
Fibronectin, TRA-1-60 and SSEA-1 and -3 proteins were evaluated.
DMSO dose-dependently induced TRA-1-60 expression in the NEC8
cells, but had no effect on this protein in the NEC14 cells.
Vimentin protein expression was reduced dose-dependently by DMSO in
each cell line (Fig. 2A–B). DMSO did
not alter the expression of Fibronectin protein (Fig. 2A). SSEA-1 and -3 were subtly expressed
in each EC cell line, with no differences in expression detected
following DMSO exposure (Fig. 2A and
B). Collectively, these data indicated that DMSO promoted
aberrant differentiation in the human EC cells. To evaluate whether
DMSO affects the stemness of EC cells, the present study analyzed
several stemness-related markers in the NEC8 and NEC14 cells. DMSO
reduced SOX2 protein expression dose-dependently in the two cell
types, although OCT3/4 protein expression was unchanged (Fig. 2A).
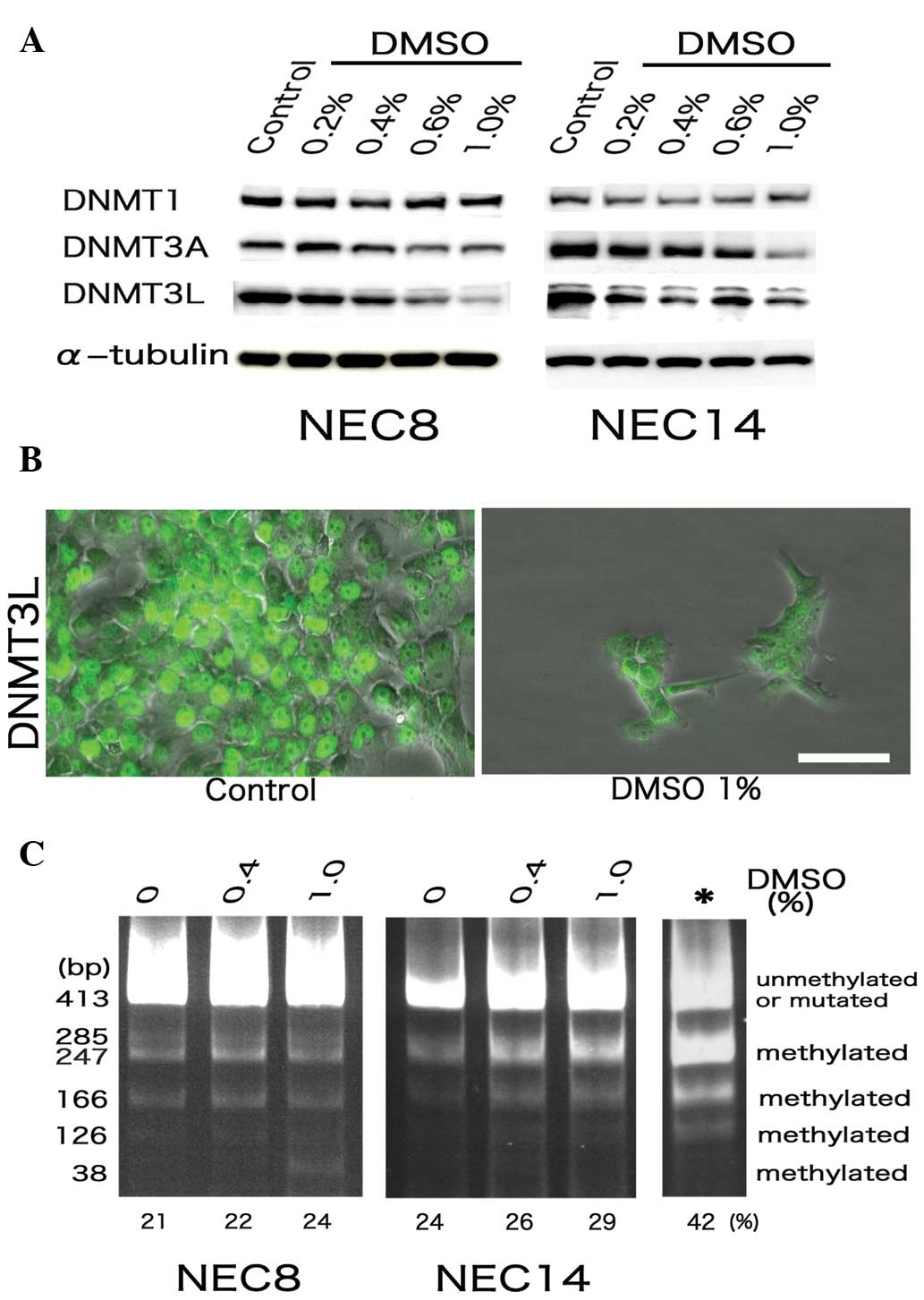
DMSO treatment reduces DNMT-3L and -3A
expression, and up-regulates DNA methylation in EC cells
Testicular EC cells possess a strong tendency to
maintain the entire genome in a demethylated state, distinguishing
them from somatic cancer cells, which commonly display genomic
hypermethylation (21,22). In addition, DNMT-3L is a significant
marker of human embryonic carcinoma (17). Therefore, the expression of several
DNMT family members, as well as the methylated DNA status, was
evaluated in the NEC8 and NEC14 cells following DMSO exposure. DMSO
had no effect on DMNT1 expression, but significantly reduced the
expression of DNMT-3L and -3A (Fig.
3A). Moreover, DNMT3L was aberrantly delocalized from the cell
nuclei by DMSO in the EC cells (Fig.
3B). While conducting COBRA of LINE1 repetitive elements, the
two EC cell lines showed dose-dependent increases in the degree of
LINE1 methylation after 72 h of treatment with DMSO (Fig. 3C).
Discussion
The majority of TGCTs are curable, even in the
metastatic stages, as they are extremely sensitive to CDDP-based
chemotherapy. However, certain NSGCT cases are refractory to any
type of chemotherapy. EC is regarded as a stem cell component for
NSGCT (15). EC cells may acquire
resistance to chemotherapy in parallel with aberrant
differentiation (23,24). Thus, EC component control, without
triggering chemotherapeutic resistance or perturbation of
differentiation, is crucial for the effective clinical management
of NSGCTs (15). The present study
results indicated that DMSO can induce resistance to CDDP and can
perturb the differentiation status of human EC cells. DMSO is
frequently used as a solvent in biological studies and as a vehicle
for drug therapies (25,26). Even if DMSO is used as a vehicle for
other drugs, when these agents are used in combination with CDDP,
DMSO could reduce the chemotherapeutic efficacy of human EC cells.
Accordingly, the co-occurrence of CDDP and DMSO in treatment
regimens may mediate accidental chemotherapeutic resistance or
aberrant differentiation in EC cell components, with consequent
negative effects on pharmacological efficacies in the clinical
management of NSGCTs.
In the present study, DMSO reduced the expression of
stemness-related markers, such as SOX2, in the human EC cells,
while also reducing DNMT3L, which is a specific marker for human EC
cells. DMSO is known to induce differentiation in ES cell lines
(25,26). Together with the perturbed
differentiation of the human EC cells observed in response to DMSO
within the present study, the findings also support a role for DMSO
in modifying the stemness characteristics of EC cells and in
mediating differentiation from pure EC cells to the other
phenotypic components, including teratoma, choriocarcinoma and yolk
sac tumors. Due to the acquisition of resistance to chemotherapy in
parallel with aberrant differentiation (23,24), the
use of DMSO may be problematic when formulating drugs used to treat
human EC cells (23,24).
To evaluate whether the genomic demethylated status
of EC cells could be affected by DMSO, the present study conducted
COBRA for LINE1 repeats. This showed that DMSO increased the level
of genomic DNA methylation in the human EC cells. These findings
also suggested that the aberrant differentiation triggered by DMSO
in the EC cells may be due, at least in part, to increased genomic
methylation. Indeed, responsiveness to chemotherapy in TGCTs has
been associated with a strong tendency to maintain the entire
genome in a demethylated state (27).
The lack of induction of the major drug efflux pumps in the study
is supportive of a possible association between DNA methylation and
the chemotherapeutic resistance phenomena observed in DMSO-exposed
EC cells. Reduction of DNMT-3A and -3L expression following DMSO
treatment in EC cells was correlated with the increased methylation
of LINE1 elements; this is in agreement with a previous study that
inhibited DNMT3L (28). In germ
cells, DNMT3L expression occurs during a short perinatal period
within non-dividing precursors of spermatogonial stem cells. The
expression of DNMT3L declines rapidly after birth, together with
reduction of DNMT-3A and -3B expression; in addition,
retrotransposons undergo de novo methylation when the
majority of prospermatogonia have differentiated into dividing
spermatogonial stem cells (29).
Taken together, the reduction in DNMT-3A and -3L expression is
consistent with the increased methylation of LINE1 elements
following treatment of human EC cells with DMSO.
In conclusion, the present study showed that DMSO
can induce CDDP resistance in human EC cells, accompanied by
perturbed differentiation and increased genomic methylation. DMSO
could reduce chemotherapeutic efficacy in human EC cells, such that
care is warranted with regard to its use during the clinical
management of NSGCTs.
Acknowledgements
This study was supported by KAKENHI (Grant-in-Aids
for Scientific Research) from the Ministry of Education, Culture,
Sports, Science and Technology, Japan (grant no. 25293130).
References
|
1
|
Santos NC, Figueira-Coelho J,
Martins-Silva J and Saldanha C: Multidisciplinary utilization of
dimethyl sulfoxide: Pharmacological, cellular and molecular
aspects. Biochem Pharmacol. 65:1035–1041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pectasides D, Pectasides E, Constantinidou
A and Aravantinos G: Current management of stage I testicular
non-seminomatous germ cell tumours. Crit Rev Oncol Hematol.
70:114–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Skakkebaek N: Possible carcinoma-in-situ
of the testis. Lancet. 2:516–517. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ulbright TM: Germ cell neoplasms of the
testis. Am J Surg Pathol. 17:1075–1091. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masters JR and Köberle B: Curing
metastatic cancer: Lessons from testicular germ-cell tumours. Nat
Rev Cancer. 3:517–525. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sonneveld DJ, Hoekstra HJ, van der Graaf
WT, et al: Improved long term survival of patients with metastatic
nonseminomatous testicular germ cell carcinoma in relation to
prognostic classification systems during the cisplatin era. Cancer.
91:1304–1315. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freedman LS, Parkinson MC, Jones WG, et
al: Histopathology in the prediction of relapse of patients with
stage I testicular teratoma treated by orchidectomy alone. Lancet.
2:294–298. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Read G, Stenning SP, Cullen MH, et al:
Medical Research Council prospective study of surveillance for
stage I testicular teratoma. Medical Research Council Testicular
Tumors Working Party. J Clin Oncol. 10:1762–1768. 1992.PubMed/NCBI
|
|
9
|
Oliver RT, Ong J, Shamash J, et al:
Long-term follow-up of Anglian Germ Cell Cancer Group surveillance
versus patients with stage 1 nonseminoma treated with adjuvant
chemotherapy. Urology. 63:556–561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cullen MH, Stenning SP, Parkinson MC, et
al: Short-course adjuvant chemotherapy in high-risk stage I
nonseminomatous germ cell tumors of the testis: A Medical Research
Council report. J Clin Oncol. 14:1106–1113. 1996.PubMed/NCBI
|
|
11
|
Guney S, Guney N, Sonmez NC and Ergenekon
E: Risk-adapted management for patients with clinical stage I
non-seminomatous germ cell tumour of the testis. Med Oncol.
26:136–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vergouwe Y, Steyerberg EW, Eijkemans MJ,
et al: Predictors of occult metastasis in clinical stage I
nonseminoma: A systematic review. J Clin Oncol. 21:4092–4099. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amato RJ, Ro JY, Ayala AG and Swanson DA:
Risk-adapted treatment for patients with clinical stage I
nonseminomatous germ cell tumor of the testis. Urology. 63:144–149.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dunn TA, Schmoll HJ, Grünwald V, Bokemeyer
C and Casper J: Comparative cytotoxicity of oxaliplatin and
cisplatin in non-seminomatous germ cell cancer cell lines. Invest
New Drugs. 15:109–114. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Looijenga LH, Gillis AJ, Stoop HJ, Hersmus
R and Oosterhuis JW: Chromosomes and expression in human testicular
germ-cell tumors: Insight into their cell of origin and
pathogenesis. Ann NY Acad Sci. 1120:187–214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chano T, Ikegawa S, Kontani K, Okabe H,
Baldini N and Saeki Y: Identification of RB1CC1, a novel human gene
that can induce RB1 in various human cells. Oncogene. 21:1295–1298.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Minami K, Chano T, Kawakami T, Ushida H,
Kushima R, Okabe H, Okada Y and Okamoto K: DNMT3L is a novel marker
and is essential for the growth of human embryonal carcinoma. Clin
Cancer Res. 16:2751–2759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clark SJ, Harrison J, Paul CL and Frommer
M: High sensitivity mapping of methylated cytosines. Nucleic Acids
Res. 22:2990–2997. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frevel MA, Sowerby SJ, Petersen GB and
Reeve AE: Methylation sequencing analysis refines the region of H19
epimutation in Wilms tumor. J Biol Chem. 274:29331–29340. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang AS, Estécio MR, Doshi K, Kondo Y,
Tajara EH and Issa JP: A simple method for estimating global DNA
methylation using bisulfite PCR of repetitive DNA elements. Nucleic
Acids Res. 32:e382004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ushida H, Chano T, Minami K, Kita H,
Kawakami T, Okabe H, Okada Y and Okamoto K: Therapeutic potential
of SOX2 inhibition for embryonal carcinoma. J Urol. 187:1876–1881.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wohrle D, Salat U, Hameister H, Vogel W
and Steinbach P: Demethylation, reactivation and destabilization of
human fragile X full-mutation alleles in mouse embryocarcinoma
cells. Am J Hum Genet. 69:504–515. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wermann H, Stoop H, Gillis AJ, Honecker F,
van Gurp RJ, Ammerpohl O, Richter J, Oosterhuis JW, Bokemeyer C and
Looijenga LH: Global DNA methylation in fetal human germ cells and
germ cell tumours: Association with differentiation and cisplatin
resistance. J Pathol. 221:433–442. 2010.PubMed/NCBI
|
|
24
|
Koul S, McKiernan JM, Narayan G,
Houldsworth J, Bacik J, Dobrzynski DL, Assaad AM, Mansukhani M,
Reuter VE, Bosl GJ, Chaganti RS and Murty VV: Role of promoter
hypermethylation in cisplatin treatment response of male germ cell
tumors. Mol Cancer. 3:162004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adler S, Pellizzer C, Paparella M, Hartung
T and Bremer S: The effects of solvents on embryonic stem cell
differentiation. Toxicol In Vitro. 20:265–271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pal R, Mamidi MK, Das AK and Bhonde R:
Diverse effects of dimethyl sulfoxide (DMSO) on the differentiation
potential of human embryonic stem cells. Arch Toxicol. 86:651–661.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okamoto K: Epigenetics: A way to
understand the origin and biology of testicular germ cell tumors.
Int J Urol. 19:504–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ushida H, Kawakami T, Minami K, Chano T,
Okabe H, Okada Y and Okamoto K: Methylation profile of DNA
repetitive elements in human testicular germ cell tumor. Mol
Carcinog. 51:711–722. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bourc'his D and Bestor TH: Meiotic
catastrophe and retrotransposon reactivation in male germ cells
lacking Dnmt3L. Nature. 431:96–99. 2004. View Article : Google Scholar : PubMed/NCBI
|