Introduction
Alkylating agents comprise a major class of
chemotherapeutic agents, widely used in various types of cancer,
including leukemia (1,2). There are two types of alkylating agents:
monofunctional and bifunctional agents. Bifunctional alkylating
agents include cyclophosphamide, ifosfamide, melphalan (MEL) and
carmustine (BCNU; also known as
1,3-bis(2-chloroethyl)-1-nitrosourea). Monofunctional agents
include temozolomide [TMZ; also known as
3,4-dihydro-3-methyl-4-oxoimidazo
(5,1-d)-as-tetrazine-8-carboxamide] and dacarbazine (1–3).
Alkylating agents form a variety of DNA adducts in
cancer cells, including mono-adducts on N1-alkylguanine,
N3-alkyladenine, N7-alkylguanine or
O6-alkylguanine, and di-adducts between or within DNA
strands (1–4). Bifunctional alkylating agents result in
cytotoxicity due to the production of interstrand crosslinks, which
are formed through the intermediate production of
O6-alkylguanine (5,6). These
crosslinks are repaired through nucleotide excision repair (NER)
and recombination. By contrast, monofunctional agents generate
persistent O6-methylguanine adducts that initiate futile
cycling of the DNA mismatch repair (MMR) pathway, which causes DNA
double-strand breaks (7–10). Intact MMR is required for the exertion
of the cytotoxicity of monofunctional agents. The DNA repair
enzyme, O6-methylguanine-DNA methyltransferase (MGMT),
repairs O6-alkylguanine adducts (11,12) and
reverses the cytotoxicity induced by the two types of alkylating
agents.
The cytotoxic effects of alkylating agents are
limited by a number of factors, including DNA repair (2,13,14). In the present study, the cytotoxic
effects of the bifunctional BCNU and MEL agents, as well as the
monofunctional TMZ agent, were evaluated in relation to DNA repair.
The effects were compared in two cultured leukemia cell lines,
HL-60 and MOLT-4. In addition, the sensitivity of the cells was
manipulated by the addition of an MGMT inhibitor,
O6-benzylguanine (BG). The extent of the drug
cytotoxicity was analyzed to determine its correlation with DNA
repair, including any associations with MGMT, NER and MMR (15,16).
Our previous study demonstrated the important role
of MMR in the exertion of the cytotoxicity of monofunctional agent
temozolomide (17). Restored MMR
sensitized the cancer cells to temozolomide. Therefore, the aim of
the present study was to evaluate the cytotoxicity of alkylating
agents from the viewpoint of MGMT.
Materials and methods
Chemicals and reagents
BCNU, MEL and BG (all purchased from Sigma-Aldrich,
St. Louis, MO, USA) were dissolved in 99% ethanol immediately prior
to use. TMZ (Schering-Plough KK, Osaka, Japan) was dissolved in
100% dimethyl sulfoxide immediately prior to use.
Cell culture
Human acute myeloid leukemia cell line, HL-60, and
human acute T lymphoblastic leukemia cell line, MOLT-4, were used
in this study (JCRB Cell Bank, Osaka, Japan). The cells were
cultured in RPMI 1640 medium (Life Technologies Japan, Ltd., Tokyo,
Japan) in a humidified atmosphere with 5% CO2 at
37°C.
Drug treatment and proliferation
assay
To evaluate the growth-inhibitory effect of each
agent on the two cell lines, the trypan blue exclusion assay was
performed (17,18). Briefly, the cells were incubated with
various concentrations of TMZ, BCNU or MEL (10 nM, 100 nM, 1 µM or
10 µM), alone or in combination with BG (10 µM), for 72 h.
Subsequently, the samples were stained with trypan blue (Wako Pure
Chemical Industries, Ltd., Osaka, Japan), and the viable cells,
which exhibited negative staining, were counted. The 50%
growth-inhibitory concentration (IC50) was the
concentration at which 50% of the growth of the untreated cells was
inhibited. This value was extrapolated from the growth curve drawn
for each drug treatment, with 100% considered to be the condition
of untreated cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
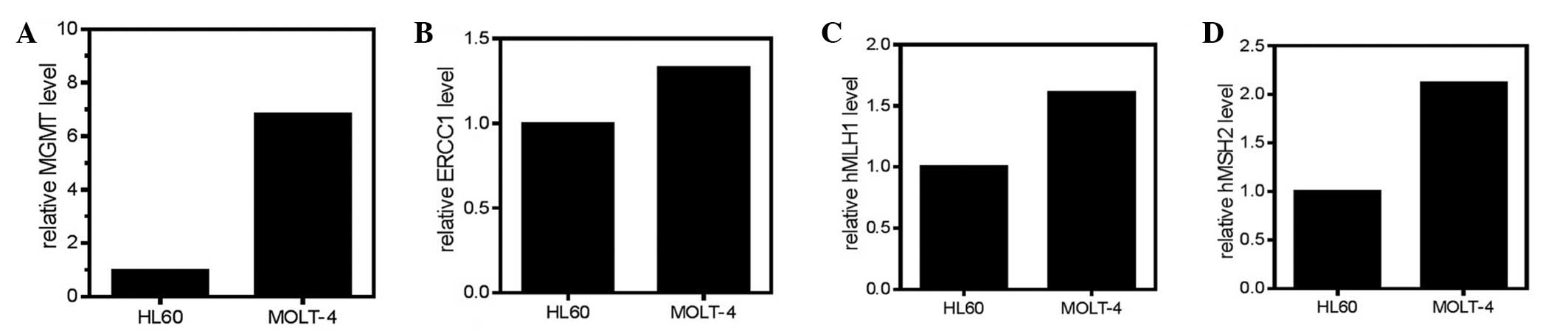
The transcript levels of MGMT, ERCC1, hMLH1 and
hMSH2 were determined by RT-qPCR using the ABI Prism 7700 sequence
detection system (Applied Biosystems Life Technologies, Foster
City, CA, USA). RT-qPCR was performed according to the method of
our previous study (17). ERCC1 is
responsible for incision of the damaged DNA strand in the NER
pathway, while hMLH1 and hMSH2 are two key factors in the MMR
response. For MGMT, the sense primer sequence was
5′-TCCCGTTTTCCAGCAAGAGTC-3′, and the antisense sequence was
5′-GGGCTGCTAATTGCTGGTAAGA-3′. The TaqMan probe DNA sequence was
FAM-CCAGACAGGTGTTATGGAAGCTGCTGAAG-TAMRA (Mitsubishi Kagaku
Bio-Clinical Laboratories, Tokyo, Japan). In addition, the primers
for ERCC1, hMLH1 and hMSH2 were purchased from Mitsubishi Kagaku
Bio-Clinical Laboratories. The absolute standard curve quantitation
method was used for MGMT and ERCC1, and the relative standard curve
quantitation method was used for hMLH1 and hMSH2. The values of
HL-60 cells were set to 1 and relative values were determined for
the MOLT-4 cells.
Statistical analyses
Graphs were generated using the GraphPad Prism
software (version 5.0; GraphPad Software, Inc., San Diego, CA,
USA). Spearman's rank correlation was used for determination of any
correlation between two parameters. All statistical analyses were
performed using Microsoft Excel 2007 (Microsoft Corporation,
Redmond, WA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Growth-inhibitory effects of the
alkylating agents
The growth-inhibitory effects of TMZ, BCNU and MEL
were evaluated in the HL-60 and MOLT-4 cells. Overall, the
sensitivity of the two cell lines to these agents varied; however,
treatment with MEL appeared to be the most effective in inhibiting
the cell growth (Table I).
 | Table I.Drug sensitivities of the two leukemia
cell lines. |
Table I.
Drug sensitivities of the two leukemia
cell lines.
|
| IC50
(µM) |
|---|
|
|
|
|---|
| Drugs | HL-60 | MOLT-4 |
|---|
| TMZ | 49.0 | 191.5 |
| BCNU | 10.0 | 22.0 |
| MEL | 4.5 |
1.5 |
| TMZ+BG |
4.5 | 169.0 |
| BCNU+BG |
3.0 |
1.4 |
| MEL+BG |
0.3 |
1.4 |
Inhibition of MGMT by BG
The cytotoxic effect of alkylating agents is
generally reduced by DNA repair in cancer cells (3–6). Upon
treatment of the HL-60 and MOLT-4 cells with TMZ, BCNU or MEL in
the presence of BG, an MGMT inhibitor, the two cell lines were
apparently sensitized to all these agents (Table I). However, the extent of
sensitization varied among the drugs in the two cell lines
(Table II; Fig. 1). BG was not found to be cytotoxic to
cells in the previous study (17).
 | Table II.Sensitization by the addition of
BG. |
Table II.
Sensitization by the addition of
BG.
|
| Ratio of
IC50 |
|---|
|
|
|
|---|
| Drugs | HL-60 | MOLT-4 |
|---|
| TMZ / TMZ+BG | 10.9 |
1.1 |
| BCNU / BCNU+BG |
3.3 | 15.7 |
| MEL / MEL+BG | 15.0 |
1.1 |
Transcript levels of DNA
repair-associated genes
The transcript levels of MGMT, ERCC1, hMLH1 and
hMSH2 were determined in the two cell lines (Fig. 2), and were found to be different
between the two cell lines. Associations between these expression
levels and the drug sensitivity were also investigated (Figs. 3 and 4).
Alkylguanine is repaired primarily by MGMT (12,14,19).
However, no apparent correlation was observed between the
expression levels of MGMT and the drug sensitivity in the presence
or absence of BG (Fig. 3A and B).
Furthermore, the ratio of the IC50 value of each agent
over the IC50 value of each agent + BG was calculated
(Table II). These values were
plotted against the MGMT transcript levels (Fig. 3C). It was expected that the ratio may
be higher in the cell line with a higher MGMT transcript level.
However, the ratio was not correlated with the MGMT expression
levels (Fig. 3C). This indicated that
sensitization through the inhibition of MGMT could not be predicted
based on the levels of MGMT transcript in these cell lines.
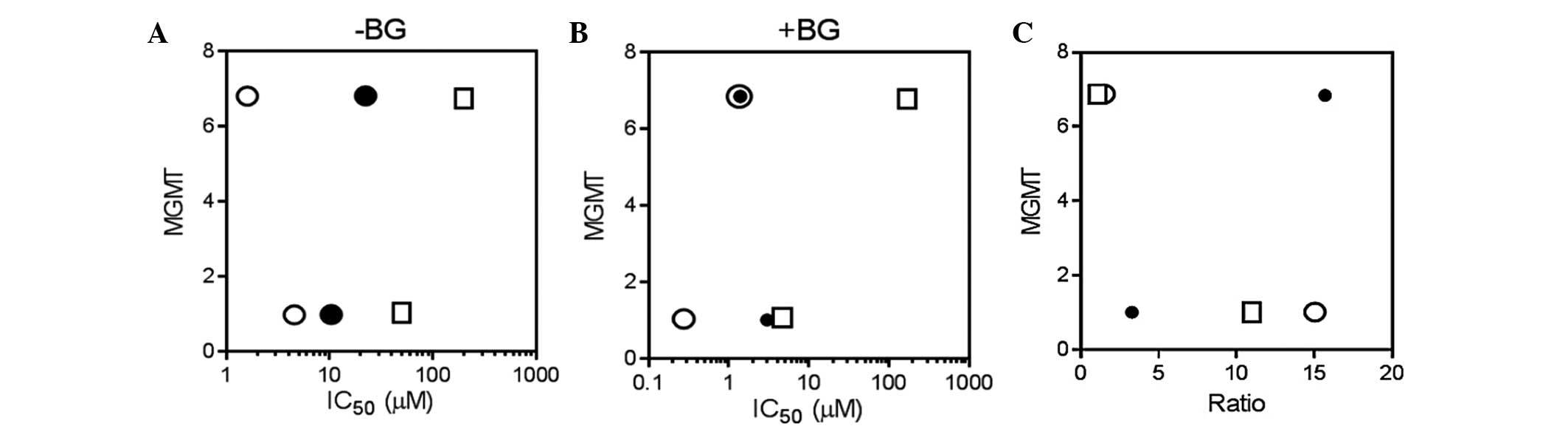 | Figure 3.Correlation between MGMT transcript
levels and drug sensitivity (in terms of IC50 values) in
HL-60 and MOLT-4 cells. Cells were incubated for 72 h with various
concentrations of TMZ, BCNU or MEL, (A) with or (B) without a
minimally toxic concentration of BG (10 µM). The growth inhibition
effects were determined using the trypan blue exclusion assay.
Values are expressed as the mean of at least three independent
experiments. (C) The ratio between the IC50 values of
TMZ/BCNU/MEL to the values of TMZ/BCNU/MEL with BG was plotted
against the MGMT transcript levels in each cell line. (A):
P>0.99; (B): P>0.99 (Spearman's rank correlation). circle,
MEL; filled circle, BCNU; square, TMZ. MGMT,
O6-methylguanine-DNA methyltransferase; TMZ,
temozolomide; BCNU, carmustine; MEL, melphalan; BG,
O6-benzylguanine; IC50, 50% growth-inhibitory
concentration. |
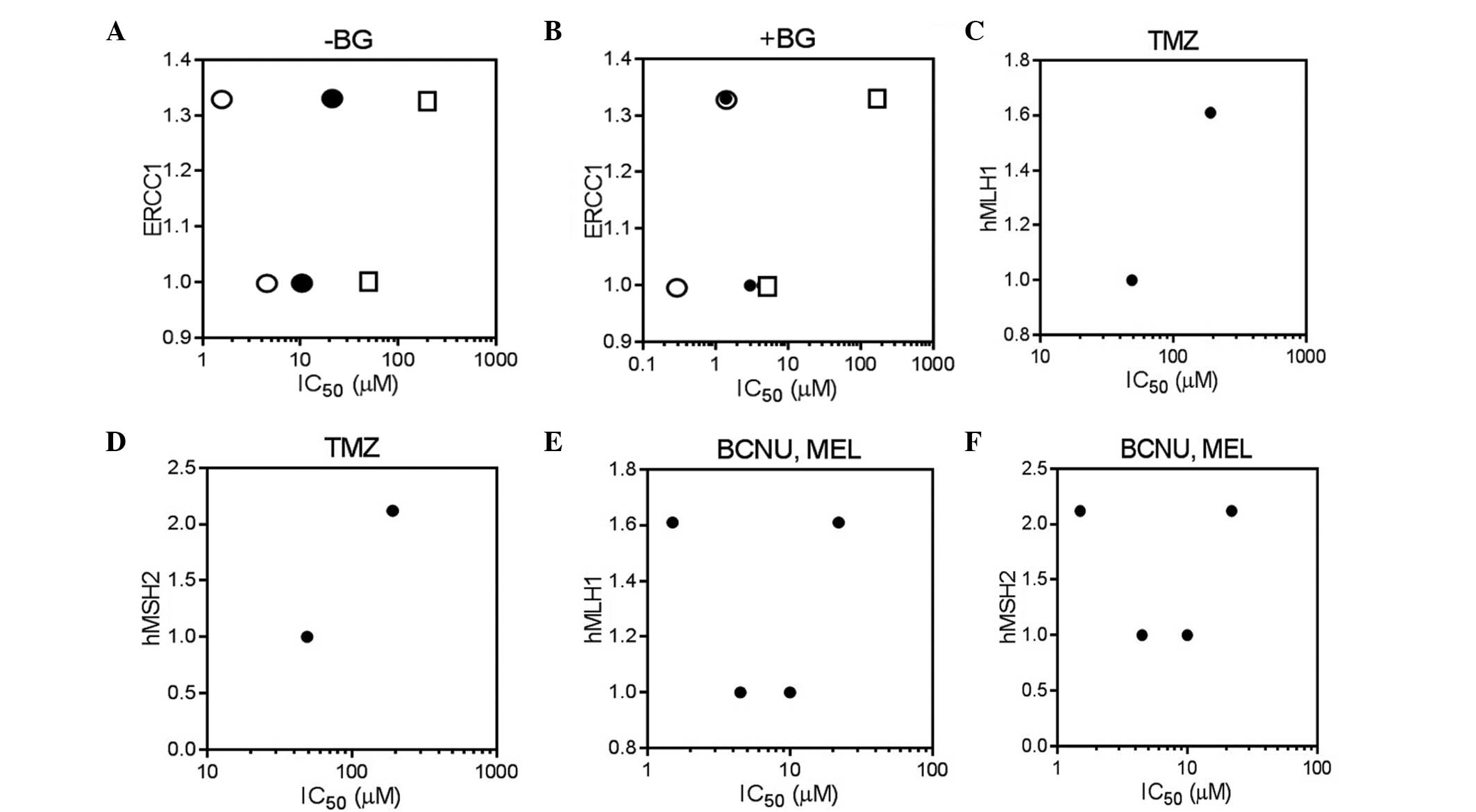 | Figure 4.Correlation between ERCC1/hMLH1/hMSH2
transcript levels and IC50 values of BCNU/TMZ/MEL in
HL-60 and MOLT-4 cells. The IC50 values of TMZ, BCNU and
MEL, (A) with and (B) without a minimally toxic concentration of BG
(10 µM) were plotted against ERCC1 transcript levels in the HL-60
and MOLT-4 cells (circle, MEL; filled circle, BCNU; square, TMZ).
The IC50 values of TMZ were plotted against the (C)
hMLH1 and (D) hMSH2 transcript levels of the HL-60 and MOLT-4
cells. The IC50 values of BCNU and MEL were plotted
against the (E) hMLH1 and (F) hMSH2 transcript levels of the HL-60
and MOLT-4 cells. TMZ, temozolomide; BCNU, carmustine; MEL,
melphalan; BG, O6-benzylguanine; IC50, 50%
growth-inhibitory concentration. |
BCNU-mediated interstrand crosslinks are repaired by
NER and recombination, while O6-alkylguanine is repaired
by NER (20). The ERCC1-XPF
heterodimer is an endonuclease that is involved in the NER pathway
(15). In the present study, the
expression levels of ERCC1 were not found to be correlated to the
drug sensitivity (Fig. 4A). No
correlation was observed using the IC50 values for the
co-treatment with BG, which is an inhibitor of the activity of MGMT
(Fig. 4B). Deficiency in MMR is known
to result in cellular insensitivity to TMZ (10), while MMR is suggested to be involved
in crosslink repair induced by BCNU (21). Based on these previous observations, a
lower MMR function may be associated with lower sensitivity to a
monofunctional TMZ, whereas a higher MMR function may confer the
higher resistance to bifunctional BCNU and MEL. However, in the
present study, no association was detected between the expression
levels of the MMR-associated hMLH1/hMSH2 and the sensitivity to
these two agents (Fig. 4C–F).
Discussion
The MMR response triggered by
O6-alkylguanine-mediated mismatch is indispensable to
the exertion of the cytotoxicity of TMZ. Interstrand crosslinks are
formed through the intermediate production of
O6-alkylguanine in the process of the cytotoxic action
of BCNU and MEL (22). Therefore,
MGMT is closely associated with the mechanisms of resistance to
these agents. In the present study, two different leukemia cell
lines were sensitized to monofunctional (TMZ) and bifunctional
alkylating agents (BCNU and MEL) by an MGMT inhibitor, BG. The
results indicated that O6-alkylguanine was the major
cytotoxic lesion generated by these alkylators. However, the
sensitivity of the cells to these agents and the extent of the
sensitization by BG were not found to be correlated with the MGMT
transcript levels.
A previous study evaluated the role of BG in
restoring TMZ sensitivity in patients with recurrent or progressive
TMZ-resistant malignant glioma in a phase II trial (23). Both TMZ and BG were administered on
day 1 of a 28-day treatment cycle. Patients were administered a 1-h
BG infusion at a dose of 120 mg/m2, immediately followed
by a 48-h infusion at a dose of 30 mg/m2. TMZ was
administered orally within 60 min after the end of the 1-h BG
infusion at a dose of 472 mg/m2. Out of the 66 patients
treated with TMZ and BG, only six patients responded to the
treatment, indicating that the efficacy of this combination was
limited. In addition, the Children Oncology Group evaluated the
combination treatment with BCNU and BG in pediatric patients with
central nervous system tumors in a phase I study (24). The toxicity of this treatment was
evaluated in 25 patients, and the maximum tolerated dose of BCNU
administered with BG (120 mg/m2) was 58
mg/m2. Furthermore, the response to this treatment was
evaluated in 24 patients, and only six patients were found to
present stable disease, while one patient exhibited a minor
response (24). A study by Hegi et
al investigated the association between MGMT silencing and the
survival of patients with glioblastoma, treated with radiotherapy
alone or radiotherapy combined with TMZ (25). The MGMT promoter was methylated in 45%
of the 206 assessed cases and the methylation was an independent
favorable prognostic factor (25).
The authors concluded that patients with glioblastoma containing a
methylated MGMT promoter benefited from TMZ, whereas patients
without a methylated MGMT promoter did not benefit from the
treatment (25). Therefore, the
critical role of MGMT was demonstrated in the therapeutic outcome
of alkylator-based cancer treatment (25,26).
However, the efficacy of combination treatment with BG has not yet
been confirmed clinically.
A number of proteins, including hMLH1 and hMSH2,
participate in the process of MMR, which involves the mismatch
recognition, excision of the DNA-containing error and resynthesis
of the correct DNA (3,10). Intact MMR is required for the exertion
of the cytotoxicity of TMZ, while interstrand crosslinks formed by
bifunctional agents are, in part, repaired by MMR (21,27). Our
previous study evaluated TMZ cytotoxicity in a BCNU-resistant
variant leukemia cell line, in comparison with a BCNU-sensitive
cell line (17). The study identified
that the BCNU-resistant cells were more sensitive to TMZ compared
with the BCNU-sensitive cells (17).
In addition, the BCNU-resistant cells possessed increased hMLH1 and
hMSH2 transcript levels (17).
However, when the cells were transfected with shRNA against hMLH1,
the sensitivity to TMZ was partially reversed. Therefore, the study
suggested inverse roles of MMR on the cytotoxicity between TMZ and
BCNU (17). The present study
investigated only two leukemia cell lines and, therefore, it may be
difficult to clarify the role of MMR in the sensitivity of leukemia
cells to alkylating agents.
In conclusion, the present study evaluated the
cytotoxic effects of monofunctional and bifunctional alkylating
agents in relation to DNA repair in two different leukemic cell
lines. The results revealed that the inhibition of MGMT appeared to
sensitize the two leukemia cell lines to TMZ, BCNU and MEL.
However, no correlation was identified between the drug sensitivity
and MGMT transcript levels.
Glossary
Abbreviations
Abbreviations:
|
IC50
|
50% growth-inhibitory
concentration
|
|
MMR
|
mismatch repair
|
|
MGMT
|
O6-methylguanine-DNA
methyltransferase
|
|
NER
|
nucleotide excision repair
|
|
BG
|
O6-benzylguanine
|
|
TMZ
|
temozolomide or
3,4-dihydro-3-methyl-4-oxoimidazo
(5,1-d)-as-tetrazine-8-carboxamide
|
|
BCNU
|
carmustine or
1,3-bis(2-chloroethyl)-1-nitrosourea
|
|
MEL
|
melphalan
|
References
|
1
|
Tew KD, Colvin M and Chabner BA:
Alkylating agentsCancer Chemotherapy and Biotherapy: Principles and
Practice. Chabner BA and Longo DL: Lippincott-Raven Publishers;
Philadelphia: pp. 297–317. 1996
|
|
2
|
Fu D, Calvo JA and Samson LD: Balancing
repair and tolerance of DNA damage caused by alkylating agents. Nat
Rev Cancer. 12:104–120. 2012.PubMed/NCBI
|
|
3
|
Geleziunas R, McQuillan A, Malapetsa A,
Hutchinson M, Kopriva D, Wainberg MA, Hiscott J, Bramson J and
Panasci L: Increased DNA synthesis and repair-enzyme expression in
lymphocytes from patients with chronic lymphocytic leukemia
resistant to nitrogen mustards. J Natl Cancer Inst. 83:557–564.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drabløs F, Feyzi E, Aas PA, Vaagbø CB,
Kavli B, Bratlie MS, Peña-Diaz J, Otterlei M, Slupphaug G and
Krokan HE: Alkylation damage in DNA and RNA - repair mechanisms and
medical significance. DNA Repair (Amst). 3:1389–1407. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wiencke JK and Wiemels J: Genotoxicity of
1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU). Mutat Res. 339:91–119.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Povirk LF and Shuker DE: DNA damage and
mutagenesis induced by nitrogen mustards. Mutat Res. 318:205–226.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedman HS, Kerby T and Calvert H:
Temozolomide and treatment of malignant glioma. Clin Cancer Res.
6:2585–2597. 2000.PubMed/NCBI
|
|
8
|
Stupp R, Gander M, Leyvraz S and Newlands
E: Current and future developments in the use of temozolomide for
the treatment of brain tumours. Lancet Oncol. 2:552–560. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mason WP and Cairncross JG: Drug Insight:
Temozolomide as a treatment for malignant glioma - impact of a
recent trial. Nat Clin Pract Neurol. 1:88–95. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fink D, Aebi S and Howell SB: The role of
DNA mismatch repair in drug resistance. Clin Cancer Res. 4:1–6.
1998.PubMed/NCBI
|
|
11
|
Fan CH, Liu WL, Cao H, Wen C, Chen L and
Jiang G: O6-methylguanine DNA methyltransferase as a
promising target for the treatment of temozolomide-resistant
gliomas. Cell Death Dis. 4:e8762013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fahrer J and Kaina B:
O6-methylguanine-DNA methyltransferase in the defense
against N-nitroso compounds and colorectal cancer. Carcinogenesis.
34:2435–2442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chaney SG and Sancar A: DNA repair:
Enzymatic mechanisms and relevance to drug response. J Natl Cancer
Inst. 88:1346–1360. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Panasci L, Paiement JP, Christodoulopoulos
G, Belenkov A, Malapetsa A and Aloyz R: Chlorambucil drug
resistance in chronic lymphocytic leukemia: The emerging role of
DNA repair. Clin Cancer Res. 7:454–461. 2001.PubMed/NCBI
|
|
15
|
Bowden NA: Nucleotide excision repair: Why
is it not used to predict response to platinum-based chemotherapy?
Cancer Lett. 346:163–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin SA, Lord CJ and Ashworth A:
Therapeutic targeting of the DNA mismatch repair pathway. Clin
Cancer Res. 16:5107–5113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamauchi T, Ogawa M and Ueda T:
Carmustine-resistant cancer cells are sensitized to temozolomide as
a result of enhanced mismatch repair during the development of
carmustine resistance. Mol Pharmacol. 74:82–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto S, Yamauchi T, Kawai Y, Takemura
H, Kishi S, Yoshida A, Urasaki Y, Iwasaki H and Ueda T:
Fludarabine-mediated circumvention of cytarabine resistance is
associated with fludarabine triphosphate accumulation in
cytarabine-resistant leukemic cells. Int J Hematol. 85:108–115.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Esteller M, Garcia-Foncillas J, Andion E,
Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB and Herman JG:
Inactivation of the DNA-repair gene MGMT and the clinical response
of gliomas to alkylating agents. N Engl J Med. 343:1350–1354. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shrivastav N, Li D and Essigmann JM:
Chemical biology of mutagenesis and DNA repair: Cellular responses
to DNA alkylation. Carcinogenesis. 31:59–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fiumicino S, Martinelli S, Colussi C,
Aquilina G, Leonetti C, Crescenzi M and Bignami M: Sensitivity to
DNA cross-linking chemotherapeutic agents in mismatch
repair-defective cells in vitro and in xenografts. Int J Cancer.
85:590–596. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Loeber R, Michaelson E, Fang Q, Campbell
C, Pegg AE and Tretyakova N: Cross-linking of the DNA repair
protein O6-alkylguanine DNA alkyltransferase to DNA in
the presence of antitumor nitrogen mustards. Chem Res Toxicol.
21:787–795. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quinn JA, Jiang SX, Reardon DA, Desjardins
A, Vredenburgh JJ, Rich JN, Gururangan S, Friedman AH, Bigner DD,
Sampson JH, et al: Phase II trial of temozolomide plus
O6-benzylguanine in adults with recurrent,
temozolomide-resistant malignant glioma. J Clin Oncol.
27:1262–1267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adams DM, Zhou T, Berg SL, Bernstein M,
Neville K and Blaney SMChildren's Oncology Group: Phase 1 trial of
O6-benzylguanine and BCNU in children with CNS tumors: A
Children's Oncology Group study. Pediatr Blood Cancer. 50:549–553.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balaña C, Ramirez JL, Taron M, Roussos Y,
Ariza A, Ballester R, Sarries C, Mendez P, Sanchez JJ and Rosell R:
O6-methyl-guanine-DNA methyltransferase methylation in
serum and tumor DNA predicts response to
1,3-bis(2-chloroethyl)-1-nitrosourea but not to temozolamide plus
cisplatin in glioblastoma multiforme. Clin Cancer Res. 9:1461–1468.
2003.PubMed/NCBI
|
|
27
|
Aquilina G, Ceccotti S, Martinelli S,
Hampson R and Bignami M:
N-(2-chloroethyl)-N'-cyclohexyl-N-nitrosourea sensitivity in
mismatch repair-defective human cells. Cancer Res. 58:135–141.
1998.PubMed/NCBI
|