Introduction
Acute promyelocytic leukemia (APL) is a subtype of
acute myelogenous leukemia (AML). Despite being a relatively rare
condition, APL is extremely malignant due to the rapid spontaneous
evolution of the disease and the occurrence of sudden hemorrhages
(1,2).
At the genetic level, APL is characterized by a specific
chromosomal rearrangement between the retinoic acid receptor α
(RARα) on chromosome 17, and a number of partners, including the
promyelocytic leukemia (PML), PLZF, NPM, STAT5b and NuMA genes. The
majority of patients (98%) present with the 15;17 translocation,
which results in a fusion between the RARα gene and the PML gene on
chromosome 15 (3). Retinoic acid
(RA)-based chemotherapy is the standard treatment regimen
administered for APL (4,5). However, this regimen only prolongs
survival, and numerous patients develop resistance to the
administered agents and eventually succumb to APL or RA-associated
toxicity and side-effects, particularly the fatal retinoic acid
syndrome (6). Although arsenic
trioxide (ATO) was identified as an effective drug in the treatment
of APL, this agent works only through a peripheral intravenous
route.
Compound realgar natural indigo tablets (CRNITs) are
another type of arsenic compound that are often used to treat
dermatosis. CRNITs have also been used against certain other
disease, including chronic bronchitis, bronchial asthma, herpes
zoster and pterygium in Traditional Chinese Medicine, with the
principal of using a toxic substance to inhibit a toxic disease
(7). In the 1990s, CRNIT treatment
was introduced to the therapeutic regimens for APL and demonstrated
a marked effectiveness in the Northeastern region of China
(8). The clinical complete remission
(CR) rate achieved with orally administered CRNIT treatment
(3.75–7.5 g/day) 30 days was reported as between 98.3 and 100%
(9). In addition, 88.52% of the
patients demonstrated a survival time of >3 years, and the
majority of patients experienced neither bone marrow (BM)
depression nor other severe clinical side-effects during the
treatment (10).
The present study reports the clinical outcome of
CRNIT treatment in 31 patients with refractory APL, and also
reports the cell molecular biology results obtained from the
administration of CRNIT treatment to the RA-resistant APL NB4-R1
cell line. The aim of the present study was to assess the clinical
efficacy and safety of CRNIT treatment applied alternately with
chemotherapy in patients with refractory APL, and indicated that
CRNITs may be a promising agent for the treatment of APL.
Materials and methods
Patients
Patients were eligible for inclusion in the present
study if they were: Admitted to The First Affiliated Hospital,
Xi'an Jiaotong University (Xi'an, Shaanxi, China) between June 2008
and June 2013; diagnosed on the basis of clinical data, such as
history, symptoms and physical findings, and peripheral blood and
BM examinations, according to the French-American-British
classification (11); and not in
remission following two courses of standard treatment, or
experienced recrudescence within 6 months subsequent to remission.
A final number of 31 patients with refractory APL were included in
the present study. The main clinical data of the patients at the
time of diagnosis are summarized in Table
I.
 | Table I.Clinical data of the patients at the
time of diagnosis. |
Table I.
Clinical data of the patients at the
time of diagnosis.
| Patient | Gender | Age, years | Hb level, g/l | WBC count, n
x109 | Platelet count, n
x1012 | APL cells in BM,
% | t (15;17)
PML-RARα | Post-remission
therapy |
|---|
| 1 | M | 33 | 68 | 0.7 |
8.3 | 40.5 | + | CRNIT+DA+HA |
| 2 | M | 40 | 112 | 1.1 |
7.9 | 52.7 | + | CRNIT+DA+HA+MA |
| 3 | F | 35 | 101 | 42.5 |
4.0 | 39.9 | + | CRNIT+DA |
| 4 | M | 42 | 83 | 51.9 | 56.4 | 60.1 | + | CRNIT+TA+MA+MEA |
| 5 | F | 26 | 119 | 4.5 | 79.1 | 70.5 | + | CRNIT+HA |
| 6 | F | 51 | 104 | 17.8 | 16.6 | 81.1 | + | CRNIT+DA+HA |
| 7 | F | 48 | 99 | 64.7 | 22.3 | 31.6 | ND | CRNIT+DA+HA |
| 8 | F | 39 | 126 | 2.7 | 76.3 | 39.8 | + | CRNIT+DA+HA+TEA |
| 9 | M | 39 | 142 | 0.9 | 24.0 | 64.2 | + | CRNIT+DA+TA |
| 10 | M | 40 | 125 | 3.9 | 188.0 | 40.7 | ND |
CRNIT+DA+HA+TA+IDA |
| 11 | M | 35 | 133 | 7.7 | 101.0 | 33.5 | – | CRNIT+HA+IDA |
| 12 | M | 32 | 77 | 48.1 | 12.4 | 92.7 | + |
CRNIT+MA+TA+MA+TA+MA+TEA |
| 13 | M | 46 | 98 | 3.3 | 49.5 | 51.2 | ND | CRNIT+MEA+IDA |
| 14 | F | 23 | 100 | 10.2 | 122.7 | 39.8 | + | CRNIT+HA+DA+MA+
MEA+TA |
| 15 | M | 24 | 122 | 6.2 | 80.5 | 65.2 | + |
CRNIT+MEA+TA+TA+MA |
| 16 | M | 49 | 131 | 3.1 | 102.4 | 33.8 | ND | CRNIT+DA+DA+MA+
TA+IDA |
| 17 | F | 50 | 69 | 50.5 |
24.6 | 86.5 | + | CRNIT+DA+HA |
| 18 | F | 17 | 111 |
4.0 |
42.1 | 42.9 | – | CRNIT+MA+MA+TA |
| 19 | F | 31 | 110 |
4.6 |
89.5 | 49.7 | + |
CRNIT+HA+TA+MEA |
| 20 | F | 29 | 65 |
0.6 |
13.5 | 54.4 | + | CRNIT+TA+IDA |
| 21 | M | 44 | 127 |
1.7 |
18.8 | 67.2 | + | CRNIT+HA+MA |
| 22 | M | 35 | 104 |
4.3 | 100.0 | 78.0 | + | CRNIT+IDA+HA |
| 23 | F | 55 | 65 | 32.4 |
37.8 | 95.5 | + |
CRNIT+TA+MEA+DA |
| 24 | M | 31 | 106 |
2.9 |
76.0 | 83.8 | + |
CRNIT+DA+HA+MA+TA |
| 25 | F | 20 | 115 |
3.4 |
46.4 | 43.6 | ND | CRNIT+TA+HA+DA |
| 26 | F | 33 | 114 | 10.5 |
57.9 | 58.8 | + |
CRNIT+HA+HA+TA+IDA |
| 27 | M | 42 | 98 |
7.7 |
52.0 | 67.1 | + | CRNIT+DA+MA+HA |
| 28 | M | 36 | 102 | 15.2 |
74.0 | 70.6 | + |
CRNIT+HA+TA+HA+DA |
| 29 | M | 52 | 90 | 20.0 |
8.8 | 35.0 | + |
CRNIT+MEA+TA+MA |
| 30 | F | 40 | 76 | 17.6 |
15.4 | 32.8 | + |
CRNIT+MEA+TEA+TA |
| 31 | M | 48 | 123 |
6.7 |
81.0 | 46.6 | + | CRNIT+MA+TA+HA |
Written informed consent was obtained from the
patients or their families upon admission hospital and prior to the
initial treatment. This study was approved by the Medical Ethics
Committee of Xi'an Jiaotong University (Xi'an, China).
Treatment schema
Compound realgar natural indigo tablets
CRNITs were composed of realgar, indigo naturalis,
savia miltiorrhiza and radix pseudostellariae. The tablets were
administered at a daily dose of 3.75–7.5 g, which was taken over
three doses subsequent to meals.
Combination chemotherapy regimens
The combination chemotherapy regimens administered
in the present study were as follows: DA, 40 mg/day daunomycin
(days 1–3) and 150 mg/day Ara-C (days 1–7) administered by
intravenous drip over the 7-day course of treatment; MA, 6 mg/day
mitoxantrone (days 1–3) and 100 mg/day Ara-C (days 1–7)
administered by intravenous drip over the 7-day course of
treatment; HA, 4 mg/day homoharringtonine (days 1–7) and 200 mg/day
Ara-C (days 1–7) administered by intravenous drip over the 7-day
course of treatment; TA, 25 mg/day pirarubicin (days 1–3) and 100
mg/day Ara-C (days 1–7) administered by intravenous drip over the
7-day course of treatment; IDA, 10 mg/day idarubicin (days 1–3) and
100 mg/day Ara-C (days 1–7) administered by intravenous drip over
the 7-day course of treatment; TEA, 20 mg/day pirarubicin (days
1–3), 100 mg/day etoposide (days 4–5) and 100 mg/day Ara-C (days
1–5) administered by intravenous drip over the 5-day course of
treatment; and MEA, 4 mg/day mitoxantrone (days 1–3), 100 mg/day
etoposide (days 1–3) and 100 mg/day Ara-C (days 1–7) administered
by intravenous drip over the 5-day course of treatment.
Combination chemotherapy was applied alternately
with CRNIT treatment. The initial interval between chemotherapy
regimens was 30 days, but extended to 3–4 months following 10
chemotherapeutic cycles. The maintenance therapy lasted ≥2
years.
Complication assessment and
management
Central nervous system leukemia
A routine cerebrospinal fluid (CSF) examination was
performed subsequent to admission. The patients were also
administered with an intrathecal injection of 10 mg methotrexate
and 5 mg dexamethasone once a week, for a total of 4 doses.
Infection and bleeding
Anti-infective therapy was administered to patients
with APL that experienced infection or fever. In addition, the
isolation of these patients and disinfection of the hospital were
considered important. Nursing was increased, particularly for the
oral cavity and the perineum. Patients with an evident tendency for
bleeding were administered with hemostatic therapy (tranexamic acid
injection of 0.75 g/day, intravenous drip), and once the patients
were diagnosed with disseminated intravascular coagulation (DIC),
small doses of heparin (20,000 units/day, intravenous drip) were
added to the therapy regimen. Platelets or fresh frozen plasma
infusion were also administered when necessary.
Assessment of response
BM aspiration was performed prior to each course of
treatment. Samples of BM fluid (0.2 ml) were taken and, subsequent
to smear and Wright's stain, the cell morphology was observed under
microscopy with oil immersion (cedar oil, C3277; Sigma-Aldrich, St.
Louis, MO, USA). The identification of one of the following three
criteria was considered to indicate recurrence: i) Combined
proportion of myeloblasts and progranulocytes in the BM was >5
and ≤20%, and a CR myelogram was not achieved subsequent to one
course of effective anti-leukemia treatment; ii) proportion of
myeloblasts and progranulocytes in the BM was >20%; and iii)
extramedullary leukemia cells.
Effect of CRNIT treatment on the APL
NB4-R1 cell line
Apoptosis assay
The NB4-R1 cell line is a RA-resistant cell line
that was previously isolated from the RA-sensitive human APL cell
line NB4. The NB4-R1 cell line expresses wild-type RARα and
PML/RARα at the mRNA and protein levels (12). The NB4-R1 cells were cultured in
RPMI-1640 medium (Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% heat-inactivated fetal bovine serum at 37°C
in a humidified incubator containing a 5% CO2
atmosphere. The cells were divided into two groups, the control
group, which consisted of untreated NB4-R1 cells, and the
experimental group, which was administered with 34 mg/l CRNIT
treatment.
A double-labeling system was used to distinguish
between apoptosis and necrosis. In total, 1×106 cells
were harvested and washed twice with pre-chilled phosphate-buffered
saline (PBS; 0.1 mol/l; pH 7.2). In addition, 100 µl incubation
buffer, 2 µl Annexin V-5(6)-carboxyfluorescein-N-hydroxysuccinimide
ester (FLUOS) and 2 µl propidium iodide (PI; Roche, Mannheim,
Germany) were added directly to the culture medium or to the cell
suspension. The mixture was incubated for 15 min at room
temperature in the dark. Subsequently, 500 µl PBS was added to each
sample tube and the samples were analyzed using
fluorescence-activated cell sorting (Becton Dickinson, Franklin
Lakes, NJ, USA) using Cell Quest Research Software (Becton
Dickinson) equipped with a 427 nm argon laser light source, 489 nm
band pass filter (FL1-H) and 582 nm band pass filter (FL2-H).
Electronic compensation of the instrument was performed to exclude
overlapping of the emission spectra. A total of 10,000 events were
acquired. The cells were gated and a dual-parameter dot plot of
FL1-H (x-axis; FLUOS fluorescence) vs. FL2-H (y-axis; PI
fluorescence) revealed a logarithmic fluorescence intensity.
PML-RARα fusion protein assay
Western blot analysis was performed as previously
described (13). Briefly, the cells
were lysed in RIPA buffer in the presence of a proteinase inhibitor
cocktail (Sigma-Aldrich), which included 10 µg/ml phenylmethyl
sulfonyl fluoride, 2 µg/ml aprotinin, 2 µg/ml leupeptin, 100 µg/ml
Pefabloc SC and 100 µg/ml chymostatin. A total of 20 µg protein was
separated by 12% SDS-PAGE and transferred to a nitrocellulose
membrane. The rabbit polyclonal anti-RARα IgG antibody (dilution,
1:700; catalog no. sc-551; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and mouse monoclonal anti-GAPDH IgG1 antibody (dilution,
1:1,000; catalog no. sc-365062; Santa Cruz Biotechnology, Inc.)
were used to confirm the expression level of the PML-RARα fusion
protein.
Statistical analysis
All cell molecular biology experiments were
performed at least three times. Statistical analysis was performed
using SPSS software version 15.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Treatment efficacy
The patients were followed up for 12–60 months, with
the median follow-up time being 43 months. In total, 28 out of 31
patients achieved continuous CR (CCR) following CRNIT treatment in
combination with other chemotherapy regimens (Table II), the CCR rate was 90.32%, and the
duration of response was between 10.3 and 60 months (median, 42.4
months). Three patients, consisting of patients 12, 17 and 23,
relapsed at months 8, 13 and 27, respectively, due to treatment
being interrupted in months 6, 10 and 23 of the treatment course,
respectively. Among these three patients, patient 12 succumbed to
AML at month 10, and patients 17 and 23 were subsequently
administered with CRNIT and combination chemotherapy, and achieved
CR again. At the time of writing, patients 17 and 23 remain in
clinical CR, and all remaining 30 patients survived. Immediately
subsequent to CR, the presence of PML-RARα transcripts was examined
using fluorescence in situ hybridization in 26 patients. All
patients demonstrated expression of PML-RARα, with the exception of
patient 29.
 | Table II.Remission induction with CRNIT and
combination chemotherapy. |
Table II.
Remission induction with CRNIT and
combination chemotherapy.
| Patient | Treatment
result | Total doses of
CRNIT, g | Time to CR,
days | Time of sustained
remission, months |
|---|
| 1 | CR | 3.75 | 31 | 10.3 |
| 2 | CR | 6.00 | 31 | 28.0 |
| 3 | CR | 6.00 | 32 | 36.5 |
| 4 | CR | 7.50 | 37 | 37.0 |
| 5 | CR | 7.50 | 57 | 55.0 |
| 6 | CR | 7.50 | 59 | 41.0 |
| 7 | CR | 6.75 | 28 | 56.6 |
| 8 | CR | 6.75 | 30 | 34.0 |
| 9 | CR | 5.25 | 34 | 50.9 |
| 10 | CR | 5.25 | 38 | 38.7 |
| 11 | CR | 7.50 | 40 | 40.8 |
| 12 | NR | 7.50 | 37 | NA |
| 13 | CR | 6.00 | 42 | 60.0 |
| 14 | CR | 6.75 | 49 | 37.0 |
| 15 | CR | 6.75 | 50 | 42.4 |
| 16 | CR | 6.00 | 36 | 41.0 |
| 17 | NR | 6.00 | 30 | NA |
| 18 | CR | 6.00 | 27 | 49.7 |
| 19 | CR | 6.00 | 33 | 31.0 |
| 20 | CR | 7.50 | 52 | 57.1 |
| 21 | CR | 7.50 | 56 | 42.0 |
| 22 | CR | 7.50 | 54 | 47.8 |
| 23 | NR | 7.50 | 60 | NA |
| 24 | CR | 7.50 | 34 | 39.6 |
| 25 | CR | 6.75 | 37 | 53.0 |
| 26 | CR | 4.50 | 44 | 58.5 |
| 27 | CR | 4.50 | 28 | 47.2 |
| 28 | CR | 5.25 | 39 | 52.6 |
| 29 | CR | 5.25 | 53 | 54.2 |
| 30 | CR | 7.50 | 54 | 59.1 |
| 31 | CR | 6.00 | 30 | 44.4 |
Complications
In total, 4 out of 31 patients demonstrated
significant CSF abnormalities, and one patient experienced cerebral
hemorrhage. Subsequent to treatment with intrathecal injection, a
routine CSF examination of this patient revealed a normal result.
The remaining patients did not experience severe infection,
bleeding or DIC.
Side-effects
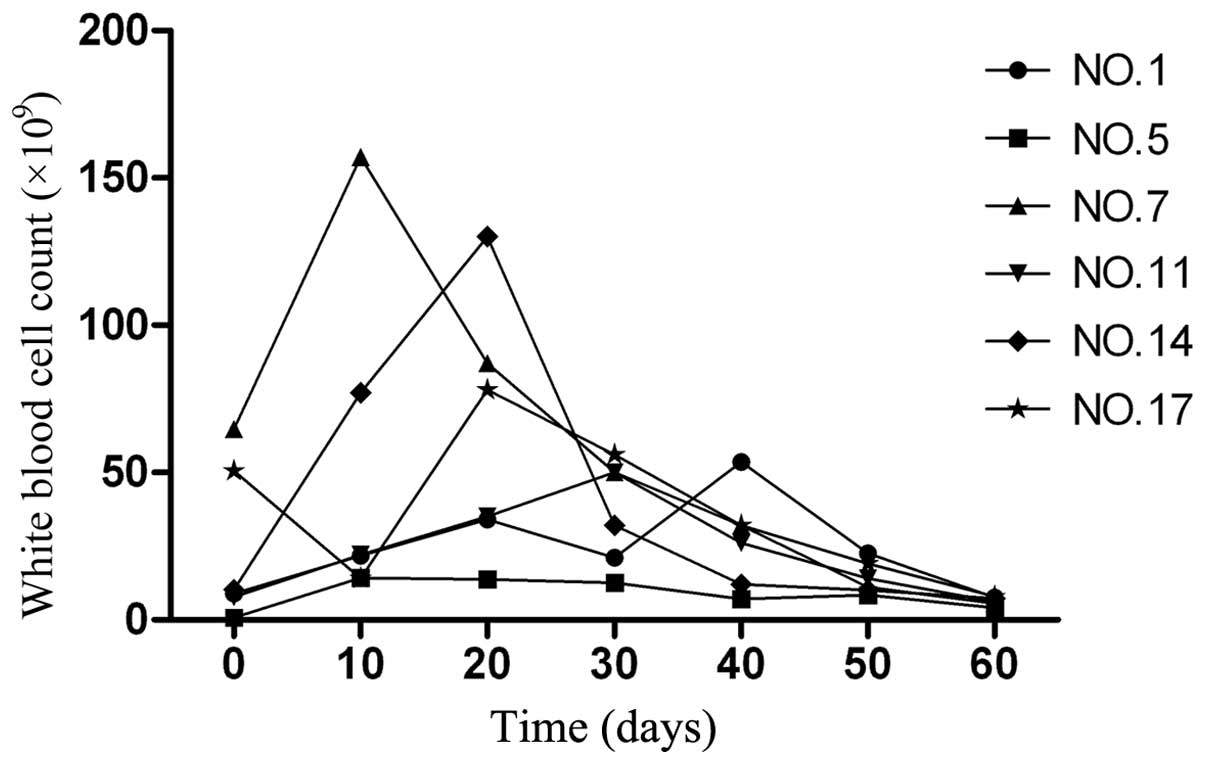
Out of the 31 patients with APL, 6 demonstrated an
increased peripheral WBC count, with peak values of
14.2–187.0×109 cells/l (normal range,
4–10×109 cells/l). The time to reach the peak WBC
numbers subsequent to the initiation of CRNIT treatment was 10–42
days (median, 22 days; Fig. 1). This
situation appears to be similar to the clinical picture observed
following RA treatment in patients with APL. However, the
proportion of patients demonstrating hyperleukocytosis is much
higher during RA remission induction, with 80–90% of patients
developing the condition (14). No
significant changes in hemoglobin (Hb) and platelet levels were
observed in 24 patients, but 7 patients, consisting of patients 1,
4, 12, 17, 20, 23 and 30, demonstrated a slightly decreased Hb and
platelet level following low-dose chemotherapy. Therefore, the
administration of CRNIT treatment was not associated with
significant BM suppression.
The principal side-effects of CRNIT treatment are
summarized in Table III. The
results revealed that the most common side-effects were
gastrointestinal symptoms, including nausea, vomiting and loss of
appetite, and dermatological symptoms, including itching or
erythematous skin changes.
 | Table III.Main side-effects due to the
administration of rifampin and combination chemotherapy (n=31). |
Table III.
Main side-effects due to the
administration of rifampin and combination chemotherapy (n=31).
| Side-effects | Frequency, n
(%) |
|---|
| Skin dryness,
itching or erythematous changes | 7
(22.6) |
| Headache | 2 (6.5) |
| EKG change | 4
(12.9) |
| Nausea, vomiting or
loss of appetite | 8
(25.8) |
| Liver function |
|
| (1) GPT
increased | 2 (6.5) |
| (2) GOT
increased | 3 (9.7) |
| (3) AKP
increased | 1 (3.2) |
| (4)
Total bilirubin increased | 1 (3.2) |
| Toothache | 1 (3.2) |
| Oral ulcer | 1 (3.2) |
| Hemorrhage of
teeth, nose or skin | 2 (6.5) |
In addition, liver function tests revealed moderate
alterations, such as increased serum hepatic enzyme levels in 7
patients, consisting of patients 4, 12, 16, 20, 23, 27 and 29.
Electrocardiogram changes, including T-wave changes, were observed
in 4 patients, consisting of patients 6, 16, 23 and 31. Other
side-effects, usually of a mild nature, were encountered in
isolated cases. All these manifestations, however, were tolerated
by the patients or disappeared rapidly with symptomatic treatment
so that no discontinuation of the drug was required during the
induction of remission.
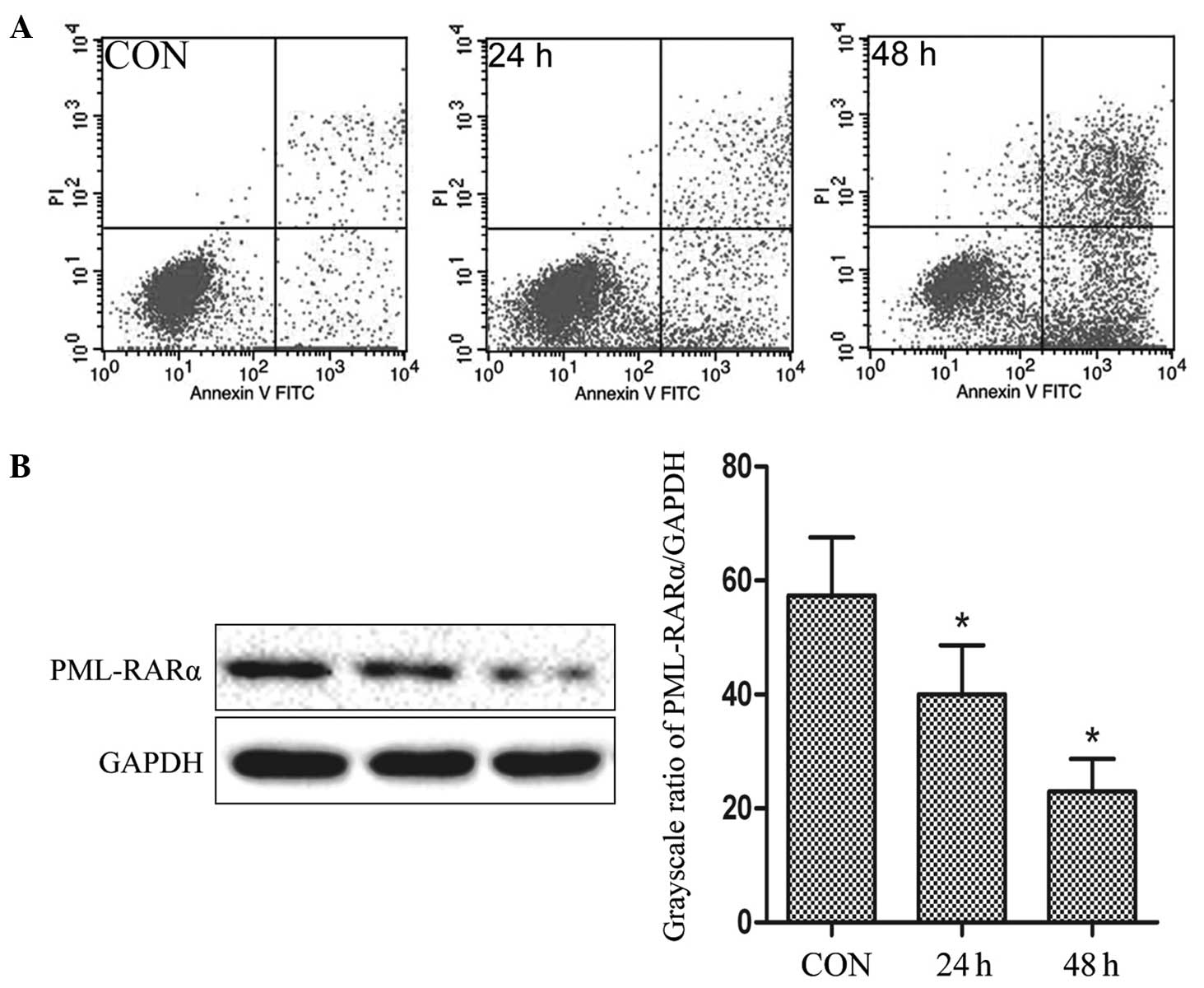
CRNIT treatment in NB4-R1 cells
resulted in the induction of apoptosis and degradation of the
PML-RARα fusion protein in vitro
Subsequent to the administration of 34 mg/l CRNIT
treatment, flow cytometry data revealed that 4.6±0.14% of the
NB4-R1 cells administered with CRNIT treatment for 24 h were in the
upper right (UR) quadrant and 18.45±0.82% of the cells were in
lower right (LR) quadrant. Also, 13.02±0.62% of the NB4-R1 cells
administered with CRNIT treatment for 48 h were in the LR quadrant
and 53.24±0.98% of the cells were in the UR quadrant. By contrast,
only 1.59±0.22% and 4.26±0.41% of the untreated control cells were
in the LR and UR quadrants, respectively (Fig. 2A).
Representative western blot analysis and comparative
grayscale ratios are shown in Fig.
2B. Compared with the expression in the control group
(57.35±10.21%), the expression of the PML-RARα fusion protein was
downregulated by 17.34 and 34.41% in the cells administered with
CRNIT treatment for 24 h and 48 h, respectively (40.01±8.64% and
22.94±5.73%; P<0.05). The data indicated that the administration
of CRNIT treatment may result in the degradation of the PML-RARα
fusion protein in NB4-R1 cells.
Discussion
Realgar, indigo naturalis, savia miltiorrhiza and
radix pseudostellariae are clinically active antitumor compounds
(15–18). In 2006, the Phase II Clinical Trials
Cooperative Group of China reported a similar curative effect
between the administration of RA and CRNIT treatment in patients
with incipient APL (19). However,
few clinical studies have been performed investigating the efficacy
and safety of CRNIT treatment in patients with refractory APL.
In the present study, 28 out of 31 patients with
refractory APL achieved CCR during the administration of CRNIT
treatment in combination with other chemotherapy regimens, with a
CCR rate of 90.32%. This result has particular clinical
significance as previous studies have suggested that patients with
refractory APL patients possess a relatively poor prognosis,
achieving CCR rates of ~80% (20,21).
According to a previous multicentric study in China, the remission
rate achieved with the administration of RA and chemotherapy alone
or in combination in patients at first relapse was 40%, and even
the CR duration was generally short (22). CRNIT may demonstrate no significant
cross-resistance with the drugs that are currently used drugs for
the treatment of APL, as all patients in the present study received
RA and chemotherapy during the previous induction of remission or
post-remissional therapy.
As an oral therapy, clinical observations revealed
that the currently used dose of 3.75–7.5 g/day CRNIT treatment was,
in general, well tolerated and associated with only mild to
moderate side-effects. Although neutropenia and thrombocytopenia
were observed in a subset of patients, neither BM suppression nor
bleeding was problematic in the heavily pre-treated group of
patients. Overall, the present data provide strong support for
future investigation into the administration of CRNIT treatment in
combination with the other agents currently used to treat APL.
The high CR rate reported in the present study was
due to the rational combination of CRNIT treatment with
chemotherapy. The present in vitro experiment revealed that
realgar may induce apoptosis and degradation of the PML-RARα fusion
protein in NB4-R1 cells, which was consistent with previous studies
(23). CRNITs contain the core
components of realgar and indigo naturalis, which act
synergistically. Although the compounds do not directly induce
apoptosis, the synergistic effects strengthen the anti-APL function
of realgar. In addition, salvia miltiorrhiza and radix
pseudostellariae may promote the recovery of normal hematopoietic
function, and prevent DIC, bleeding and infection. Additionally,
salvia miltiorrhiza and radix pseudostellariae were revealed to be
able to protect the function of the heart, liver, kidney and other
visceral organs (24). Lan et
al also confirmed that the mechanism of CRNIT treatment
involved multiple components and targets (17). It was concluded that the combination
of multiple drugs may not only enhance the CR rate, but also reduce
incidence of complications and drug toxicity.
Notably, 2 patients in the present study that did
not possess the t (15;17) chromosome translocation or express the
PML-RARα fusion gene transcripts, patients 11 and 18, demonstrated
an excellent response to CRNIT treatment, which was different from
the curative effect of ATO. The latter was reported to yield a
relatively poor result in patients without the PML-RARα fusion
protein (24). It is possible that
PML-RARα fusion gene transcripts was not the only target of CRNITs,
and CRNITs may exert anti-leukemia effects through other methods,
not only through the breakdown of the PML-RARα fusion gene or
protein. Therefore, the patients that lack the t (15,17)
chromosome translocation demonstrated an extremely high CCR rate.
Therefore, it was concluded that CRNIT treatment possesses
considerable potential as a future therapy for APL.
It should be noted that the long-term effect and
specific mechanism of CRNITs requires additional investigation,
despite the studies investigating long-term CRNIT administration as
a second-line agent, and the use of CRNIT treatment should be
reserved for patients with APL refractory to RA and conventional
chemotherapy (20,21,25). If
the relative safety of the long-term use of CRNITs is assured in
the future, CRNIT may be incorporated into a multidrug protocol for
de novo patients.
Acknowledgements
This study was supported by the Fundamental Research
Funds for the Central Universities, Shaanxi Province Science and
Technology Development Fund (grant no., 2012KTCL03-12). The authors
thank Dr. Xinyang Wang for his technological assistance.
References
|
1
|
Dos Santos GA, Kats L and Pandolfi PP:
Synergy against PML-RARa: targeting transcription, proteolysis,
differentiation and self-renewal in acute promyelocytic leukemia. J
Exp Med. 210:2793–2802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanz MA and Lo-Coco F: Modern approaches
to treating acute promyelocytic leukemia. J Clin Oncol. 29:495–503.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lallemand-Breitenbach V, Zhu J, Chen Z and
de Thé H: Curing APL through PML/RARA degradation by As2O3. Trends
Mol Med. 18:36–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adés L, Guerci A, Raffoux E, et al: Very
long-term outcome of acute promyelocytic leukemia after treatment
with all-trans retinoic acid and chemotherapy: The European APL
Group experience. Blood. 115:1690–1696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lo-Coco F, Avvisati G, Vignetti M, et al:
Retinoic acid and arsenic trioxide for acute promyelocytic
leukemia. New Engl J Med. 369:111–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montesinos P, Bergua JM, Vellenga E, et
al: Differentiation syndrome in patients with acute promyelocytic
leukemia treated with all-trans retinoic acid and anthracycline
chemotherapy: Characteristics, outcome and prognostic factors.
Blood. 113:775–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Lu C, Jiang M, et al: Traditional
chinese medicine-based network pharmacology could lead to new
multicompound drug discovery. Evid Based Complement Alternat Med.
2012:1497622012.PubMed/NCBI
|
|
8
|
Huang SL, Guo A, Xiang Y, et al: Clinical
study nf the treatment of acute promyelocytic leukemia mainly with
composite indigo naturalis tablets. Zhonghua Xue Ye Xue Za Zhi.
1:26–28. 1995.(In Chinese).
|
|
9
|
Xiang Y, Huang S, Guo A, et al: The
analysis of therapeutic efficiency on treating acute promyelocytic
leukemia (APL) with Compound Huangdai Tablets. Zhonghua Xue Ye Xue
Za Zhi. 13:11–12. 2000.(In Chinese).
|
|
10
|
Xiang Y, Huang S, Guo A, et al: The
influence on long-term survey of the patients with acute
promyelocytic leukemia treated alternatively with compound huang
dai tablets and chemotherapy. Zhonghua Xue Ye Xue Za Zhi. 16:11–12.
2003.(In Chinese).
|
|
11
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosenauer A, Raelson JV, Nervi C, Eydoux
P, DeBlasio A and Miller WH Jr: Alterations in expression, binding
to ligand and DNA and transcriptional activity of rearranged and
wild-type retinoid receptors in retinoid-resistant acute
promyelocytic leukemia cell lines. Blood. 88:2671–2682.
1996.PubMed/NCBI
|
|
13
|
Liu Y, He P, Zhang M, et al: Silencing of
the human SET gene in vitro with lentivirus-mediated RNA
interference. Mol Med Rep. 7:843–847. 2013.PubMed/NCBI
|
|
14
|
Roberts TF, Sprague K, Schenkein D, Miller
KB and Relias V: Hyperleukocytosis during induction therapy with
arsenic trioxide for relapsed acute promyelocytic leukemia
associated with central nervous system infarction. Blood.
96:4000–4001. 2000.PubMed/NCBI
|
|
15
|
Wu J, Shao Y, Liu J, Chen G and Ho PC: The
medicinal use of realgar (As4 S4) and its recent development as an
anticancer agent. J Ethnopharmacol. 135:595–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gong Y, Li Y, Lu Y, et al: Bioactive
tanshinones in Salvia miltiorrhiza inhibit the growth of prostate
cancer cells in vitro and in mice. Int J Cancer. 129:1042–1052.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Zhou GB, Liu P, et al: Dissection
of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis
as an effective treatment for promyelocytic leukemia. Proc Natl
Acad Sci USA. 105:4826–4831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang HN, Pang JH, Yang SH, et al:
Inhibitory effect of indigo naturalis on tumor necrosis
factor-alpha-induced vascular cell adhesion molecule-1 expression
in human umbilical vein endothelial cells. Molecules. 15:6423–6435.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
The Coorperation Group of Phase II
Clinical Trial of Compound Huangdai Tablet, . Phase II clinical
trial of compound Huangdai tablet in newly diagnosed acute
promyelocytic leukemia. Chin J Hematol. 27:801–804. 2006.
|
|
20
|
Gong JX, Meng JB and Ma Y: Effects of
all-trans retinoic acid and compound huangdai tablet sequential
maintenance treatment on the long-term efficacy of acute
promyelocytic leukemia patients. Zhongguo Zhong Xi Yi Jie He Za
Zhi. 32:1473–1476. 2012.(In Chinese). PubMed/NCBI
|
|
21
|
Xiang Y, Chang XH and Cheng YB: Effect of
post-remission therapy mainly with compound huangdai tablet on
long-term survival of patients with acute promyelocytic leukemia.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 30:1253–1256. 2010.(In
Chinese). PubMed/NCBI
|
|
22
|
Sun GL: Treatment of acute promyelocytic
leukemia (APL) with all-trans retinoic acid (ATRA): A report of
five-year experience. Zhonghua Zhong Liu Za Zhi. 15:125–129.
1993.(In Chinese). PubMed/NCBI
|
|
23
|
Chen S, Fang Y, Ma L, Liu S and Li X:
Realgar-induced apoptosis and differentiation in all-trans retinoic
acid (ATRA)-sensitive NB4 and ATRA-resistant MR2 cells. Int J
Oncol. 40:1089–1096. 2012.PubMed/NCBI
|
|
24
|
Chan E, Tan M, Xin J, Sudarsanam S and
Johnson DE: Interactions between traditional Chinese medicines and
Western therapeutics. Curr Opin Drug Discov Devel. 13:50–65.
2010.PubMed/NCBI
|
|
25
|
Tang D, Zhang Z, Gao Y, Wei Y and Han L:
Protective effects of serum containing Ginkgo biloba extract
on glomerulosclerosis in rat mesangial cells. J Ethnopharmacol.
124:26–33. 2009. View Article : Google Scholar : PubMed/NCBI
|