Introduction
Epithelial-myoepithelial carcinoma (EMCa) is a
malignant biphasic salivary-type tumor, which is typically composed
of large, clear myoepithelial cells surrounding variable
proportions of epithelial-lined ducts that resemble intercalated
ducts (1,2). EMCa predominantly arises in the parotid
gland, accounting for ~1% of all salivary gland tumors (2–4).
Immunohistochemical studies are useful in the differentiation of
EMCa from other similar tumors, highlighting their characteristic
biphasic epithelial-myoepithelial phenotype (1).
However, several cases of EMCa have been reported in
the mucoserous glands of the upper and lower aerodigestive tract
(1,3),
including the paranasal sinuses (2),
nasopharynx (3), nasal cavity
(2), hypopharynx (4), larynx (4),
trachea (2,4), bronchus (2) and lungs (5). Additionally, the development of EMCa at
unusual sites, for example the breast (2) and lacrimal glands (2,4), has been
reported.
The present study reports a rare case of
nasopharyngeal EMCa in an 80-year-old woman treated with concurrent
chemoradiation therapy, followed by systemic chemotherapy.
Case report
An 80-year-old woman with complaints of right-sided
nose blockage and occasional epistaxis for several months was
referred to Sanggye Paik Hospital (Seoul, Korea). The patient had a
history of diabetes mellitus and hypertension for several years,
but no notable history of rhinologic problems other than a
Caldwell-Luc operation to treat chronic maxillary sinusitis 20
years previously. On nasal speculum examination, a hemorrhagic mass
protruding towards the right posterior nasal cavity was observed.
There was no cervical lymphadenopathy detected on physical
examination. The results of laboratory examination including
complete blood count, electrolytes, and urine analysis were normal.
Chest radiographs also revealed no significant findings. Computed
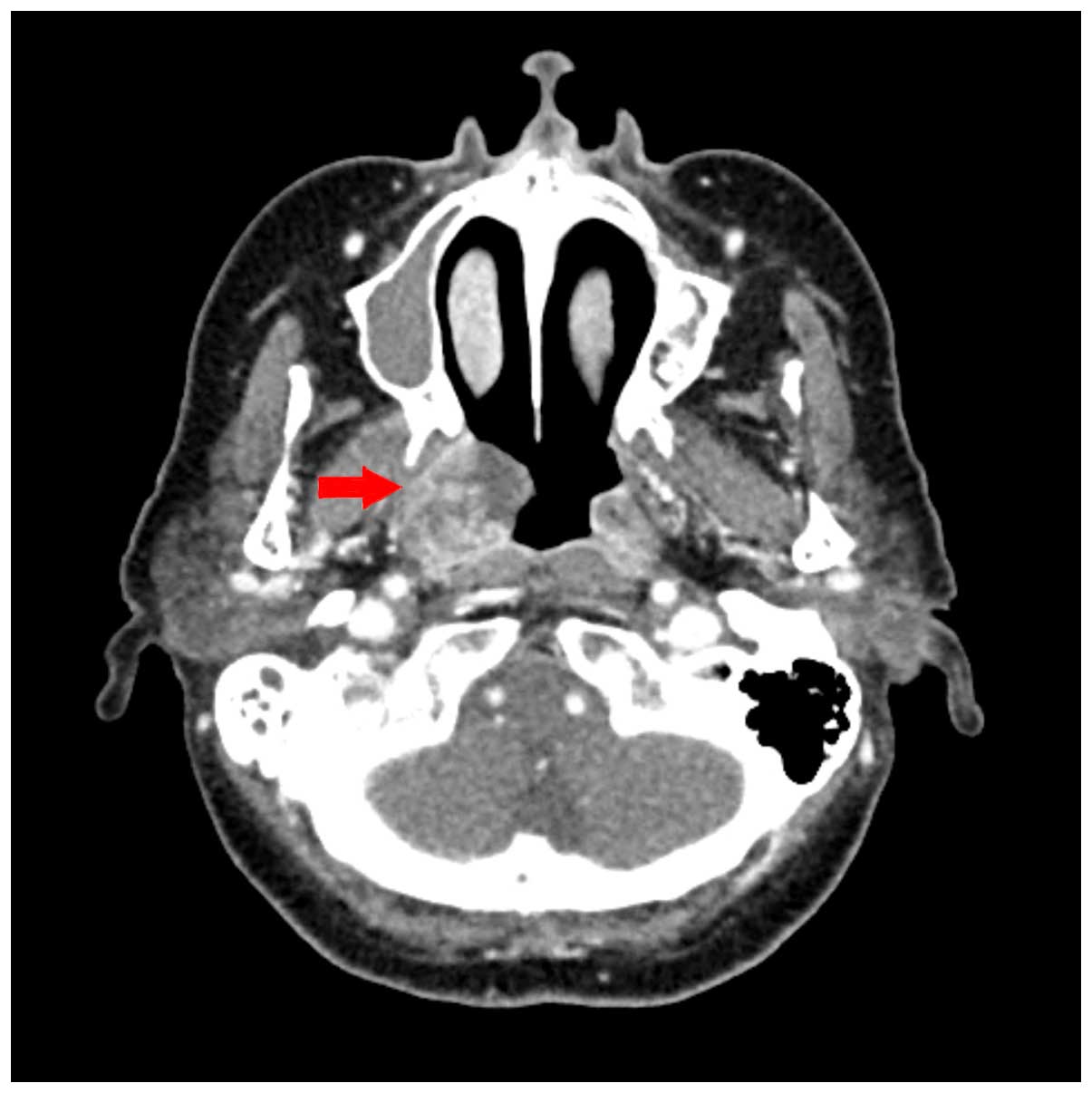
tomography (CT) of the neck revealed a heterogeneously enhancing
soft tissue mass in the right nasopharynx (Fig. 1), and two enhanced ovoid lymph nodes
of <1 cm in the retropharyngeal space, with unknown clinical
significance. There was no hot-uptake lesion except for the right
nasopharyngeal mass in positron emission tomography-CT (PET-CT).
Excisional biopsy was performed under general anesthesia.
Histopathological findings
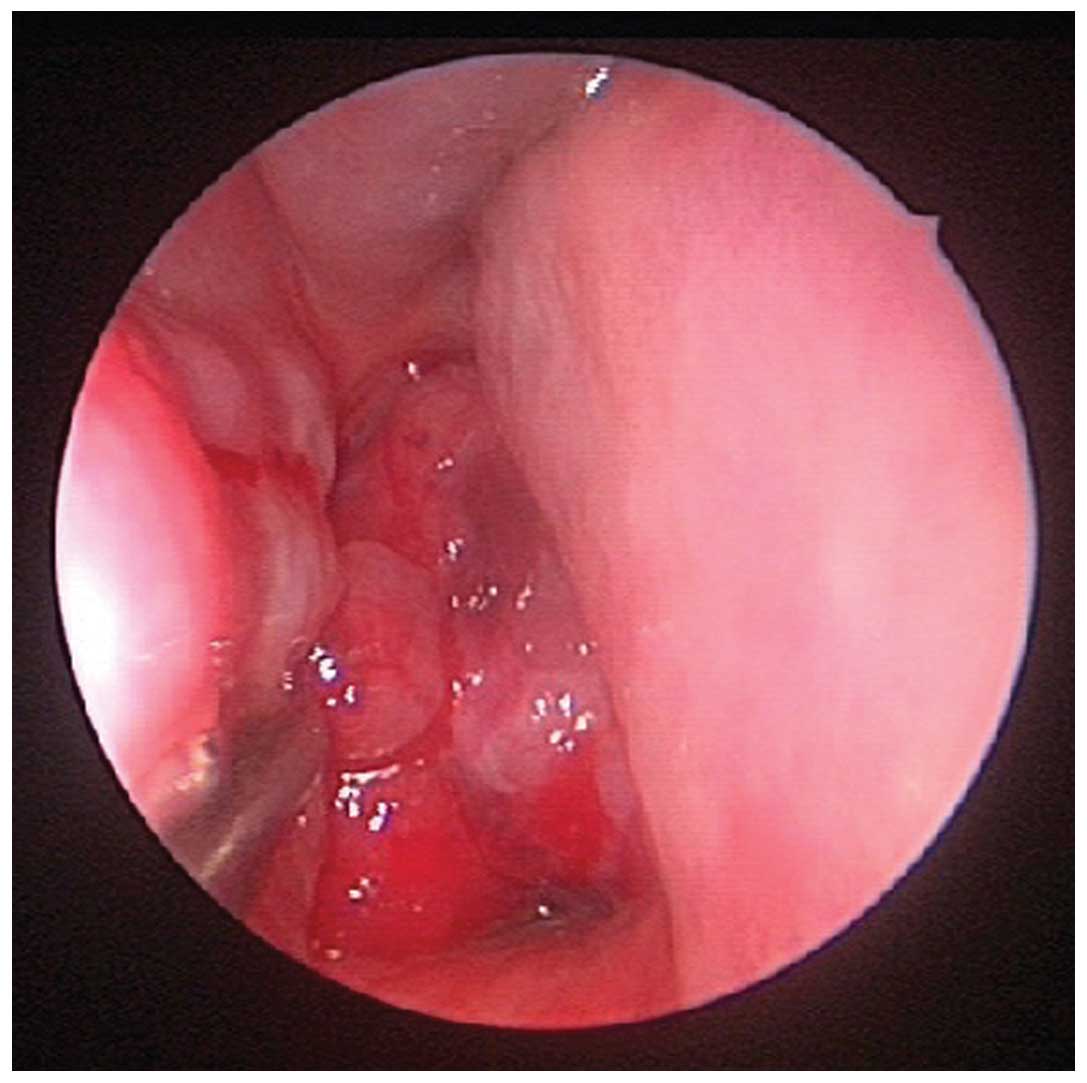
Nasal endoscopic examination revealed an easily
bleeding, friable polypoid mass in the nasopharynx, which
completely obstructed the right choana (Fig. 2). The histopathological diagnosis of
the specimen from excisional biopsy was EMCa. Grossly, multiple
fragments of the cellular, lobulated mass were separated by
sclerotic septae, against a background indicative of recent
hemorrhage, and the tissue was lined by respiratory epithelium. The
majority of the mass was composed of uniform clear cells and
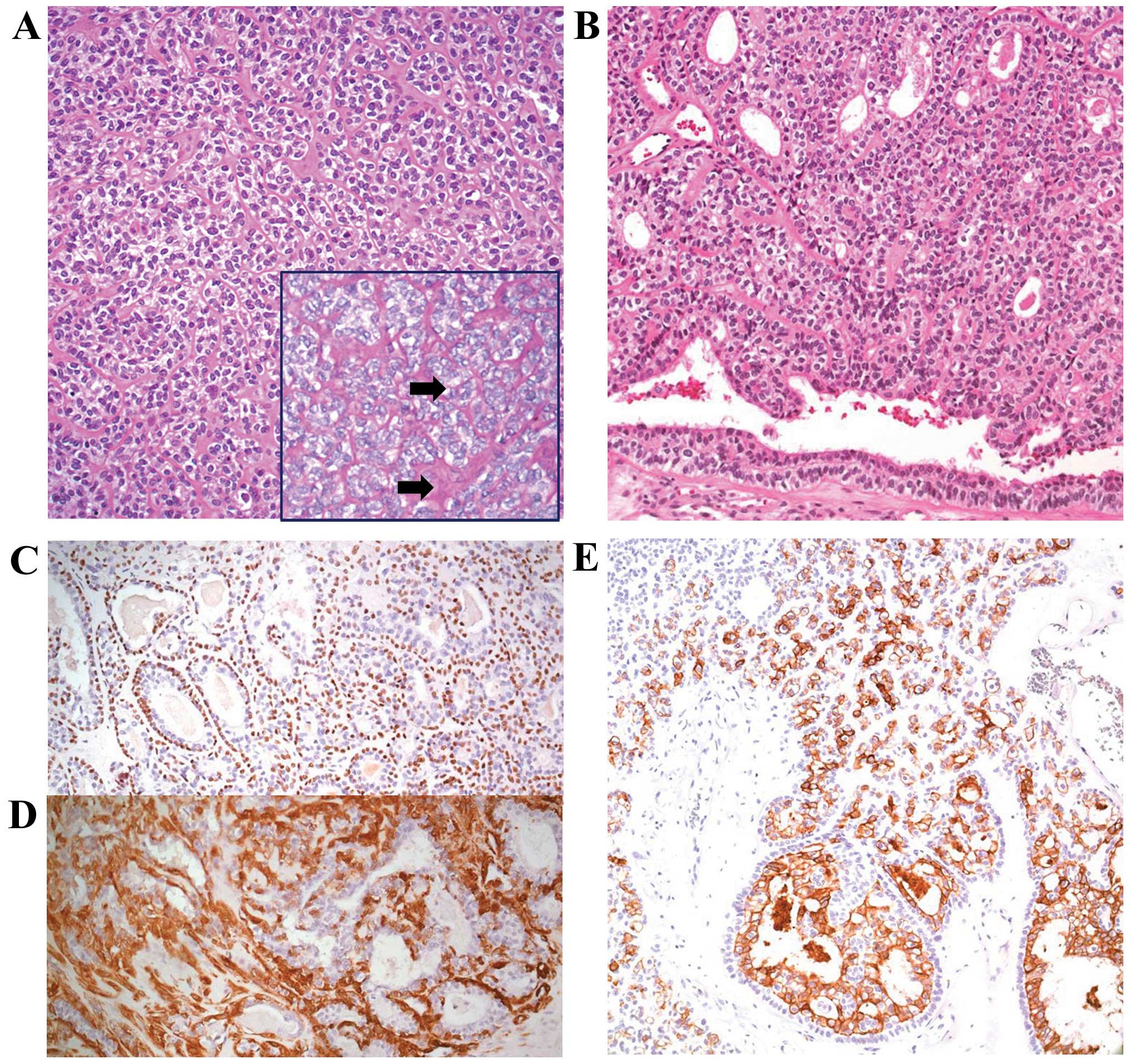
partially of glandular components. Hematoxylin and eosin staining
revealed that a major portion of the tumor was composed of
solid-growing, oval, clear cells, nested within periodic
acid-Schiff (PAS) stain-positive basement membrane-like material
(Fig. 3A). The minor section
demonstrated bi-layered duct-like structures with an inner layer of
a single row of cuboidal cells and an outer layer of a single or
multiple layers of oval cells, a hallmark histological feature of
EMCa (Fig. 3B). Immunohistochemical
staining revealed that the inner cells stained positive for
cytokeratin 7, an epithelial cell marker, whereas the outer oval
cells were positive for P63 (nuclear expression pattern; Fig. 3C) and smooth muscle actin (cytoplasmic
expression pattern; Fig. 3D),
consistent with a myoepithelial phenotype, confirming the diagnosis
of EMCa. There was no nuclear atypia or increased mitotic activity.
However, infarct-type necrosis was observed in the excisional
biopsy specimen.
Treatment and outcomes
The patient received concurrent chemoradiation
therapy followed by systemic chemotherapy. Volumetric modulated arc
therapy was administered with a total dose of 66.6 Gy in 37
fractions (180 cGy/fraction, 5 fractions/week). During radiation
therapy, 40 mg/m2 cisplatin was administrated weekly,
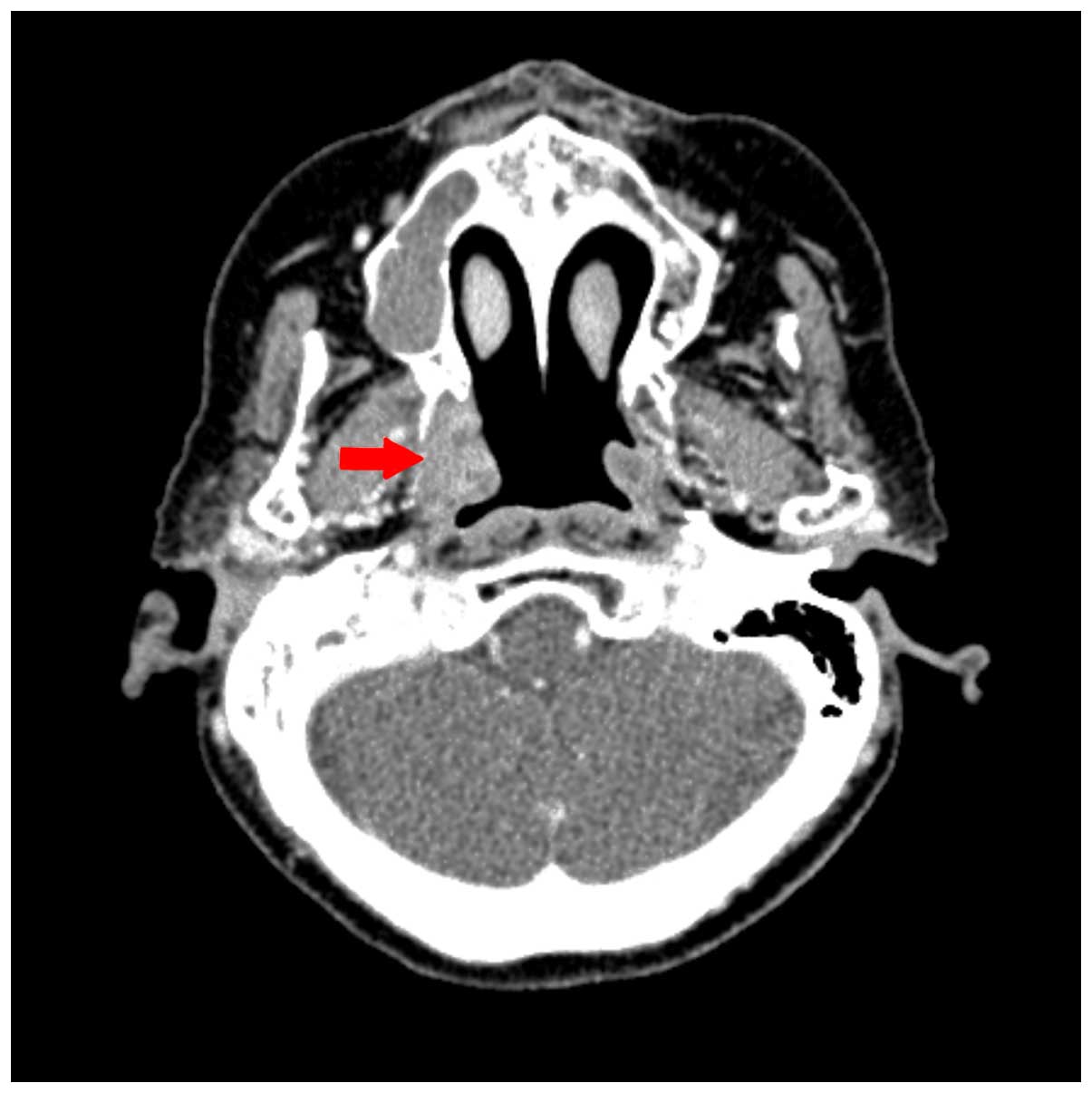
for up to 4 cycles. The patient achieved a partial response, as
observed in a neck CT performed 8 weeks subsequent to the
completion of concurrent chemoradiation therapy (Fig. 4). Subsequently, the patient was
treated with a systemic chemotherapy regimen that included
cisplatin (45 mg/m2, day 1) and 5-fluorouracil (750
mg/m2, days 1–4) at 4-week intervals. Following the
completion of 2 cycles of systemic chemotherapy, no further tumor
shrinkage was observed. Due to the patient's advanced age, poor
performance status and intolerance to chemotherapy toxicity,
systemic chemotherapy was discontinued following completion of 2
cycles. Thereafter, the post-treatment course was uneventful.
Follow-up at 24 months revealed a stable status with no progression
following the achievement of a partial response.
Discussion
EMCa, first described by Donath et al
(6) in 1972, is a rare and unique
tumor, accounting for ~1% of all epithelial salivary gland
neoplasms (4,7). The mean age at presentation is 60.9
years, with a female predominance (1.5:1) (1). According to the World Health
Organization classification of salivary gland tumors, EMCa is
defined as a distinctive subtype of malignant epithelial tumors,
consisting of varying proportions of two cell types (8).
The diagnosis of EMCa is typically based on
conventional light microscopy findings and confirmed by
immunohistochemistry (8). The tumor
is composed of nests that are partitioned by reticulin fibers. Each
nest is comprised of duct-forming cells bordering a lumen, and
cells surrounding the duct-forming cells. Dark cells with few
organelles form the inner layer of the tubules (similar to those of
the intercalated ducts), and clear cells rich in organelles
(myofilaments, endoplasmic reticulum, pinocytosis vesicles, as well
as glycogen and lipofuscin granules) and glycogen form the outer
layer with myoepithelial differentiation (6). Immunohistochemical studies may be useful
in facilitating the differentiation of EMCa from similar tumors via
identification of the characteristic bi-layered
epithelial-myoepithelial phenotype (1). On immunohistochemical staining, the
epithelial cells are positive for epithelial markers, including
cytokeratins and epithelial membrane antigen, but negative for
myoepithelial cell markers, for example α-smooth muscle actin, P63
and vimentin, thus indicating their epithelial nature (2). By contrast, the cells surrounding these
duct-forming cells exhibit antagonistic characteristics; they are
positive for myoepithelial cell markers but negative for epithelial
cell markers, demonstrating their myoepithelial nature (2,9).
EMCa typically exhibits low-grade malignancy and is
associated with a favorable prognosis. However, aggressive
histological features, local recurrence, regional metastasis and
even mortalities are not uncommon (1,2,4,8). The most
significant univariate factors in the prediction of EMCa recurrence
are margin status, angiolymphatic invasion, tumor necrosis and
myoepithelial anaplasia (1). The EMCa
recurrence rate is 36.3% (1,2). Regional lymph node metastasis and
distant metastasis to the lungs, skin, kidney, bone and brain have
been reported in ~5% of cases (2,3,10). The 5- and 10-year disease-specific
survival rates are 93.5 and 81.8%, respectively (1).
There is currently no consensus regarding the
optimal treatment for this salivary gland neoplasm, largely due to
its rarity (10). Wide surgical
excision with a clear margin has been recommended (2–4,7,10,11). As with other malignant head and neck
tumors, EMCa tumors have a propensity for infiltration and local
recurrence. Therefore, adjuvant radiotherapy has been recommended,
particularly for neoplasms with a diameter of >4 cm, to aid the
prevention of local recurrence (4,10,11). The effect of chemotherapy in the
treatment of EMCa remains unclear (11,12).
In the present case, due to the anatomical location,
the use of radiation therapy was suggested. In addition, the tumor
specimen revealed infarct-type necrosis, a risk factor for
recurrence; hence concurrent chemotherapy was added to the
radiation therapy treatment plan. Cisplatin, 5-fluorouracil,
paclitaxel and cyclophosphamide are frequently used in the
treatment of other histological subtypes of salivary gland
neoplasms; of these, cisplatin was selected for concurrent
chemoradiation therapy to treat the present patient (11,13).
Thereafter, subsequent systemic chemotherapy with cisplatin and
5-fluorouracil at 4-week intervals was planned. Although there are
few studies on the treatment of EMCa with a combination of
radiation therapy and chemotherapy, two reports of EMCas
originating from the tongue base demonstrated a positive treatment
response (complete response) with sequential chemoradiation and
radiation therapy, respectively (13,14). In
addition, in one case report of an EMCa of the submandibular gland
with lung metastasis, treatment with cisplatin combined with
5-fluorouracil, and followed by paclitaxel resulted in disease
stabilization and relief of pulmonary symptoms (11). Following treatment, the partial
response has been maintained for 24 months. However, we cannot
conclusively determine whether the long-term response was due to
the low-grade malignant potency of the tumor, the effect of
concurrent chemoradiation therapy or systemic chemotherapy.
To the best of our knowledge, except for a case
report of EMCa in the nasopharynx, which was treated by surgical
resection (3), the present study
reports the only case of EMCa in the nasopharynx treated with
concurrent chemoradiation therapy followed by systemic
chemotherapy, in which a partial response was achieved. Although
the optimal treatment strategy for EMCa remains poorly defined due
to its rarity, concurrent chemoradiation therapy followed by
systemic chemotherapy may offer an alternative or mainstay of
treatment for EMCa as squamous cell carcinoma or a variant of the
nasopharynx.
References
|
1
|
Seethala RR, Barnes EL and Hunt JL:
Epithelial-myoepithelial carcinoma: A review of the
clinicopathologic spectrum and immunophenotypic characteristics in
61 tumors of the salivary glands and upper aerodigestive tract. Am
J Surg Pathol. 31:44–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamanegi K, Uwa N, Hirokawa M, Ohyama H,
Hata M, Yamada N, Ogino K, Toh K, Terada T, Tanaka A, et al:
Epithelial-myoepithelial carcinoma arising in the nasal cavity.
Auris Nasus Larynx. 35:408–413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Imate Y, Yamashita H, Endo S, Okami K,
Kamada T, Takahashi M and Kawano H: Epithelial-myoepithelial
carcinoma of the nasopharynx. ORL J Otorhinolaryngol Relat Spec.
62:282–285. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JO, Jung CK, Sun DI and Kim MS: An
unusual presentation of aggressive epithelial-myoepithelial
carcinoma of the nasal cavity with high-grade histology. J Laryngol
Otol. 125:1286–1289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho SH, Park SD, Ko TY, Lee HY and Kim JI:
Primary epithelial myoepithelial lung carcinoma. Korean J Thorac
Cardiovasc Surg. 47:59–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Donath K, Seifert G and Schmitz R:
Diagnosis and ultrastructure of the tubular carcinoma of salivary
gland ducts. Epithelial-myoepithelial carcinoma of the intercalated
ducts. Virchows Arch A Pathol Pathol Anat. 356:16–31. 1972.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pradhan SA, Khannan R, Hazarika B and
Desai M: Sinonasal epithelial-myoepithelial carcinoma - a rare
entity. Indian J Otolaryngol Head Neck Surg. 59:168–170. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barnes L, Eveson JW, Reichart P and
Sidransky D: Pathology and Genetics of Head and Neck TumoursWorld
Health Organization Classification of Tumours. 9. IARC Press; Lyon:
2005
|
|
9
|
Lavanya N, Joshua E and Ranganathan K:
Epithelial myoepithelial carcinoma. J Oral Maxillofac Pathol.
16:473–474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee HM, Kim AR and Lee SH:
Epithelial-myoepithelial carcinoma of the nasal cavity. Eur Arch
Otorhinolaryngol. 257:376–378. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pierard S, Gregoire V, Weynand B and
Machiels JP: Epithelial-myoepithelial carcinoma of the
submandibular gland with symptomatic lung metastases treated with
chemotherapy. Eur Arch Otorhinolaryngol. 263:1158–1160. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuran G, Sagit M, Akin I, Hucumenoglu S,
Ocal BG and Celik SY: Bilateral epithelial-myoepithelial carcinoma:
An extraordinary tumor of the paranasal sinuses. Skull Base.
18:145–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Puri T, Singh K, Sharma DN and Khurana N:
Epithelial-myoepithelial carcinoma of the base of tongue: Pathology
and management. Indian J Cancer. 41:138–140. 2004.PubMed/NCBI
|
|
14
|
Peters P, Repanos C, Earnshaw J, Stark P,
Burmeister B, McGuire L, Jeavons S and Coman A M WB:
Epithelial-myoepithelial carcinoma of the tongue base: A case for
the case-report and review of the literature. Head Neck Oncol.
2:42010. View Article : Google Scholar : PubMed/NCBI
|