Introduction
Cholangiocarcinoma is one of the most aggressive
types of malignancy, and is associated with poor patient prognosis
(1,2).
This is due to the fact that at the time of diagnosis, when the
disease becomes clinically evident, the majority of cancers of the
biliary tract will have outgrown the limits of curative resection.
Complete resection represents the only potential curative therapy
for all types of biliary tract neoplasm (3). The morbidity of bile duct cancer varies
according to country, area and ethnicity (4). In China, the survival rate of this kind
of rare carcinoma is decreased compared with in Western countries,
with a tumor-free 5-year survival rate of ~13% (3,5). There are
various underlying causes of cholangiocarcinoma, including
hepatitis virus infection, particularly hepatitis C virus, chronic
biliary infections of intestinal origin, primary desmoplastic
cholangitis, bile duct lithiasis, infection by Clonorchis
sinensis and environmental carcinogens, which represent
potential pathogenesis factors for Chinese patients (3). Advances in biological research,
particularly those regarding mutation-independent activation of the
Hedgehog pathway (6), have aided
understanding of the carcinogenesis and progression underlying this
rare tumor. Furthermore, these results provide an opportunity for
the development of targeted therapies for the treatment of biliary
tract cancer. However, early pathological diagnosis remains
difficult in such highly desmoplastic, submucosal, infiltrating
types of cancer. Currently, sensitivity for the diagnosis of
cholangiocarcinoma is only ~30% for cytology, and 40–70% for
combined brush cytology and biopsy, rendering negative results
virtually useless (2,3). Therefore, novel early diagnostic tools
and therapeutic techniques for this disease are urgently
required.
Pleckstrin homology-like domain family A, member 1
(PHLDA1) encodes a 401-amino acid protein, which comprises a
central pleckstrin homology domain common to proteins involved in
intracellular signaling or as constituents of the cytoskeleton
(7–9),
a central polyglutamine tract and 2C-terminal regions rich in
proline-glutamine and proline-histidine repeats. The PHILDA1 gene
is expressed in a wide range of normal and cancer tissues (10,11).
PHLDA1 function varies with cell type and context, with several
studies reporting proapoptotic (11–14) or
antiproliferative roles (15). PHLDA1
expression is induced by external stresses, for example heat shock
(13,14), and may be modulated by the
insulin-like growth factor I (16)
and extracellular-regulated kinase pathways (16). The expression of PHLDA1 protein was
previously characterized and compared with that of proposed markers
of intestinal stem cells in the human small and large intestine
(17). Sakthianandeswaren et
al (17) found that PHLDA1 was
coexpressed with leucine-rich repeats containing G-protein coupled
receptor 5 (Lgr5) in the previously reported intestinal epithelial
stem cells in murine crypt base cells, and further determined that
PHLDA1 expression was a marker of putative epithelial stem cells
and contributed to intestinal tumorigenesis. PHLDA1 is
overexpressed in human intestinal tumors of all stages, and may be
involved in cell migration, as suggested by the increased staining
and nuclear relocalization of the protein at the invasive front of
intestinal carcinomas. Accordingly, colon cancer cells demonstrate
significantly reduced migratory behavior in response to PHLDA1
suppression (17), and in skin
tumors, PHLDA1 is recognized as a follicular stem cell marker for
differentiation between basal cell carcinomas and trichoblastomas
(18). PHLDA1 protein is
constitutively expressed by nevi in vivo (11), which raises the possibility that
PHLDA1 expression may contribute to the benign nature of these
tumors, maintaining the regulation of growth and apoptotic
sensitivity to the loss of survival signals provided by adjacent
keratinocytes. Therefore, the progressive downregulation of PHLDA1
expression associated with malignant transformation may contribute
to the loss of these characteristics in melanoma. Downregulation of
PHLDA1 protein has also been reported to be a significant predictor
of poor prognosis for breast cancer patients (10). PHLDA1 is a substrate of Aurora A
(19), which directly phosphorylates
PHLDA1, resulting in its degradation. PHLDA1 also negatively
regulates Aurora A, via the promotion of Aurora A degradation,
thereby forming a feedback loop. PHLDA1 upregulation therefore
antagonizes Aurora A-mediated oncogenic pathways, thereby revealing
PHLDA1 degradation as a mechanism by which Aurora A promotes
biliary tract malignancy (20).
Therefore, although the mechanisms underlying the downregulation of
PHLDA1 expression remain to be elucidated, the loss of PHLDA1
expression may contribute to the development of apoptosis
resistance in cholangiocarcinomas. The progressive loss of PHLDA1
expression in cholangiocarinomas may induce dysregulated cell
growth and apoptosis resistance in these tumors. PHLDA1 is a
putative tumor suppressor in cholangiocarcinoma and therefore the
significance of the loss of expression of PHLDA1 in
cholangiocarcinoma requires further investigation.
The present study aimed to identify the possible
role of PHLDA1 protein in the progression of human
cholangiocarcinoma by investigating its expression in 218 samples
of cholangiocarcinoma.
Materials and methods
Patients and specimens
Two-hundred and eighteen cholangiocarcinoma tissue
samples from a cohort of patients who had undergone surgery for
cholangiocarcinoma, 30 samples of the corresponding para-neoplastic
bile duct tissue and 20 samples of normal bile ducts with
inflammation were retrieved from the archives of the Department of
Pathology, Chinese People's Liberation Army General Hospital
(Beijing, China). All specimens were from patients who had
undergone surgery between July 1998 and December 2006. The
specimens were 10% neutral formalin-fixed, paraffin-embedded and
stored in the archives. Each tissue specimen was histologically
evaluated by at least two experienced pathologists. The carcinoma
patients included 130 males and 88 females aged 17–73 years old
(mean age, 53.6 years; median age, 56.0 years). The tumor locations
were as follows: 53 intrahepatic, 103 perihilar and 62 distal
cholangiocarcinomas. Tumor grading and staging was performed by
applying World Health Organization (2000) and Union for
International Cancer Control (1997) criteria (1). The tumor grading was as follows: 69
cases at grade 1, 91 cases at grade 2 and 58 cases at grade 3; and
staging; 7 cases at stage I, 95 cases at stage II, 88 cases at
stage III and 28 cases at stage IV. Ethical approval for this study
was not required by The Committee of Medical Ethics of Chinese
People's Liberation Army Hospital as the experiments conducted were
not associated with the privacy, impairment or treatment of the
patients.
Immunohistochemical analysis
All reagents were purchased from Zhongshan Golden
Bridge Biotechnology Co., Ltd (Beijing, China) unless otherwise
stated. Paraffin-embedded tissue sections (4-µm thick) were cut,
dewaxed in xylene and rehydrated in a graded ethanol series.
Subsequently, endogenous peroxidase activity was blocked by
immersing the sections in 3% hydrogen peroxide in methanol for 10
min prior to rinsing in running water. Sections were then immersed
in boiling 0.01 M EDTA buffer (pH 8.0) in a pressure cooker, which
was sealed and brought to full pressure for 2 min. The pressure
cooker was then depressurized and cooled under running water, prior
to removal of the lid and flushing out of the hot buffer with cold
water from a running tap. The cooled sections were washed twice in
phosphate-buffered saline (PBS) prior to immunohistochemical
analysis. The sections were incubated at 4°C overnight in a
humidified chamber with monoclonal mouse antibody against human
PHLDA1 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 1:50
dilution and polyclonal rabbit antibody against human CD133 (Abcam
Inc., Cambridge, MA, USA) at 1:100 dilution in blocking solution.
Following exposure to the primary antibodies, sections were
incubated with the polyperoxidase-anti-mouse/rabbit immunoglobulin
G for 20 min using the standard non-biotin PV-6000 Polymer
Detection System (Zymed Laboratories Inc., San Francisco, CA, USA).
The sections were then washed in water, counter-stained with
Mayer's hematoxylin for 1 min at room temperature, dehydrated,
cleaned and mounted. Paraffin blocks of human breast ductal
carcinoma tissues were used as positive controls. Negative controls
were sections treated analogously, but 0.01 M PBS was substituted
for the primary antibodies. For immunohistochemical evaluation of
PHLDA1 and CD133 expression, cytoplasmic and nucleic labeling of
tumor cells was classified as positive. In scoring expression of
PHLDA1 and CD133 protein, both the extent and intensity of staining
were considered, in accordance with a study by Hao et al
(21). The intensity of
immunopositivity was scored as follows: Negative, 0; weak, 1;
moderate, 2; strong, 3. While the extent of positivity was scored
as follows: <5%, 0; >5–25%, 1; >25–50%, 2; >50–75%, 3;
>75%, 4, of cells in the respective lesions. The final score was
obtained by multiplying the intensity and the extent of positivity
scores, yielding a potential range of 0–12. Scores of ≥4 were
defined as a positive expression pattern, while scores of <4
were recorded as negative.
Statistical analysis
Fisher's exact test, Pearson's χ2 test,
Spearman's correlation coefficient test for trends in proportions
and the Kaplan-Meier method with the Log rank test or Cox
regression method for univariate or multivariate overall survival
analysis were used to assess the associations between PHLDA1 or
CD133 expression and pathological indices. P<0.05 was considered
to indicate a statistically significant difference.
Results
PHLDA1 expression is downregulated in
cholangiocarcinoma tissues
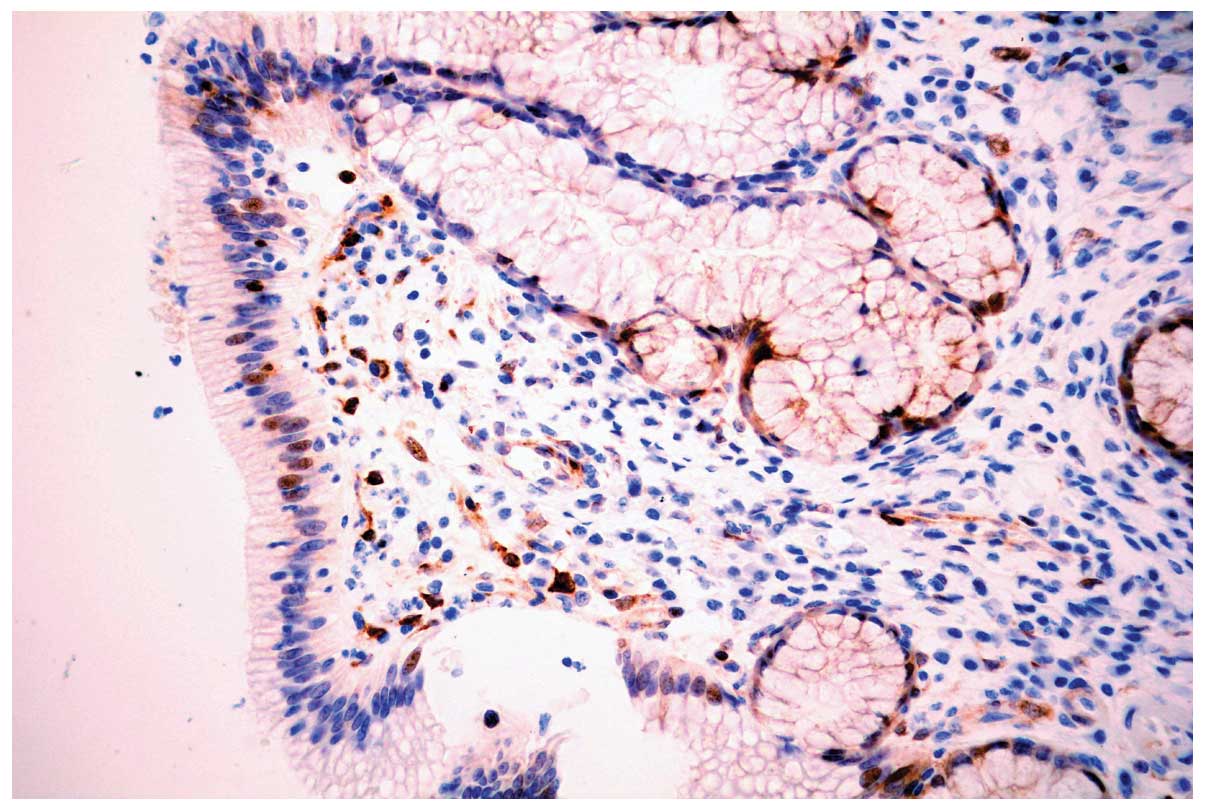
PHLDA1 protein was expressed diffusely in the
cytoplasm and nuclei of discrete cholangiocytes in all 30
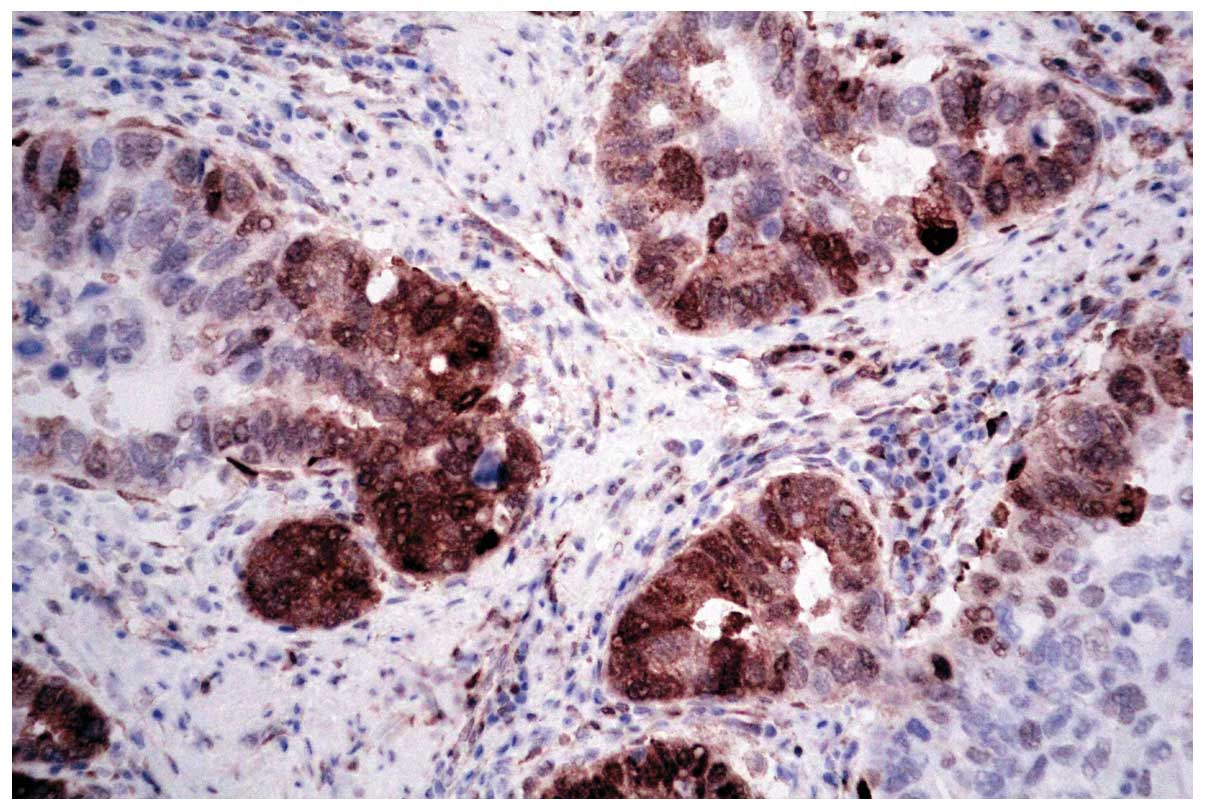
para-neoplastic and 20 normal bile ducts (Fig. 1). In carcinoma, PHLDA1 was expressed
diffusely in the cytoplasm and nuclei of cancer cells in 141 out of
218 cases (64.7%; Fig. 2). The
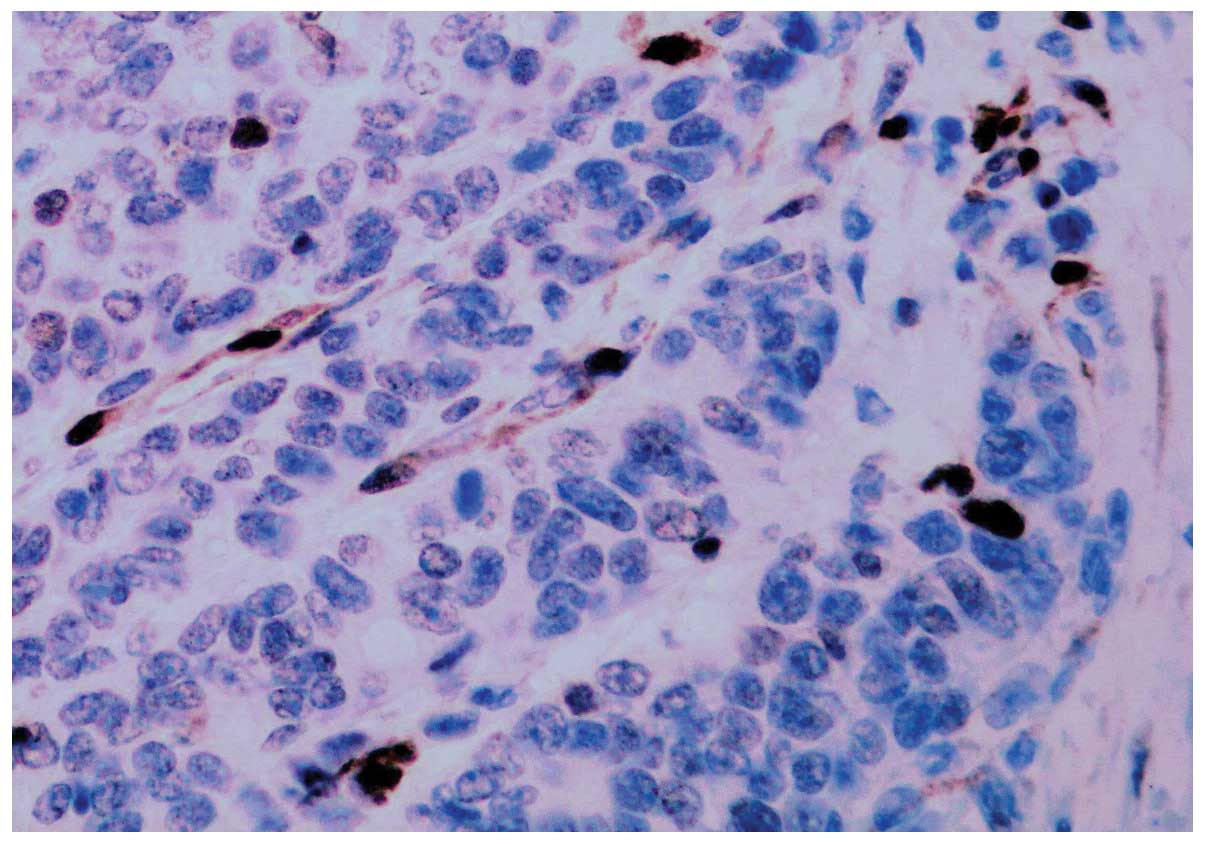
expression of PHLDA1 was low or negative in the remaining 77
(35.3%) cases of carcinoma. The majority of poorly-differentiated
cancer cells were negative for PHLDA1 protein expression (Fig. 3). There was a significant difference
in PHLDA1 immunopositivity between cholangiocarcinomas and
para-neoplastic or normal bile ducts (P<0.0001). In addition,
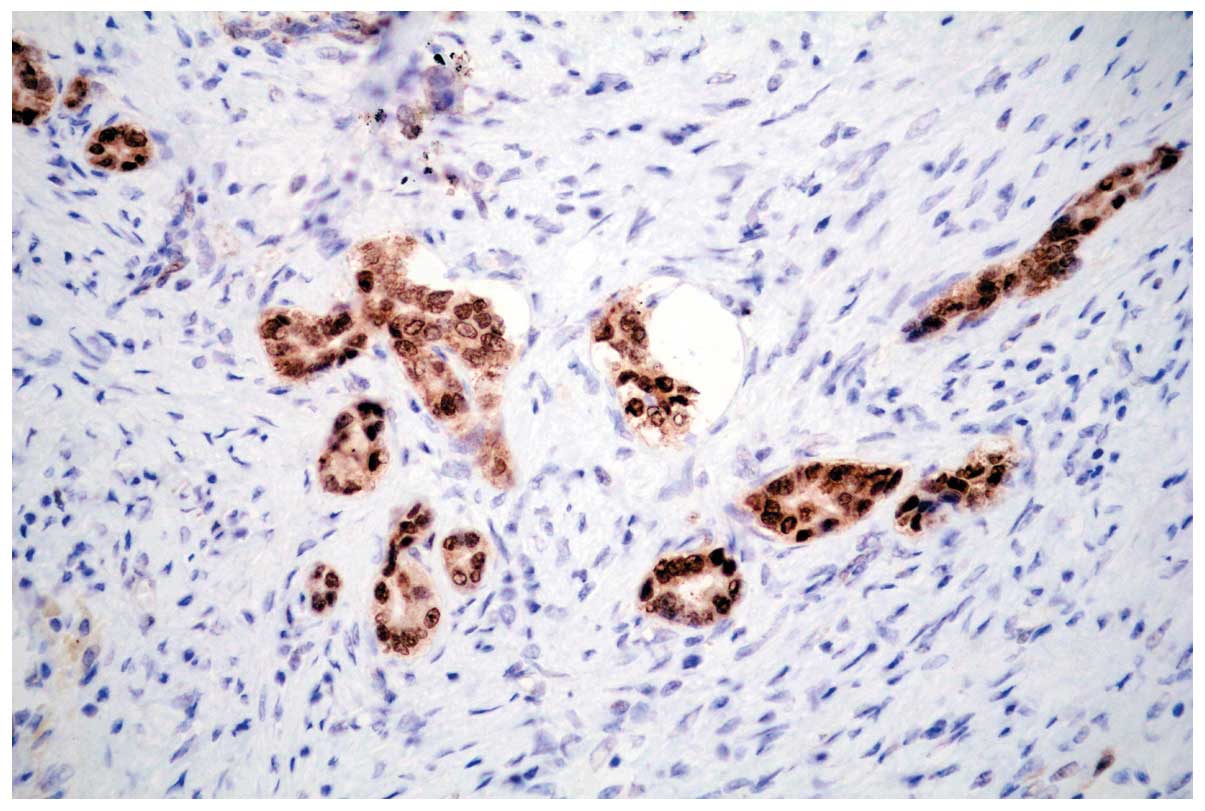
strong PHLDA1 nuclear staining was observed at the invasive margin
(Fig. 4).
PHLDA1 expression is negatively
correlated with, tumor site and histological grade
In the present group of 218 cholangiocarcinoma
samples, PHLDA1 expression was negatively correlated with tumor
site (P=0.001), grade (P=0.020) and stage (P=0.0001), but not with
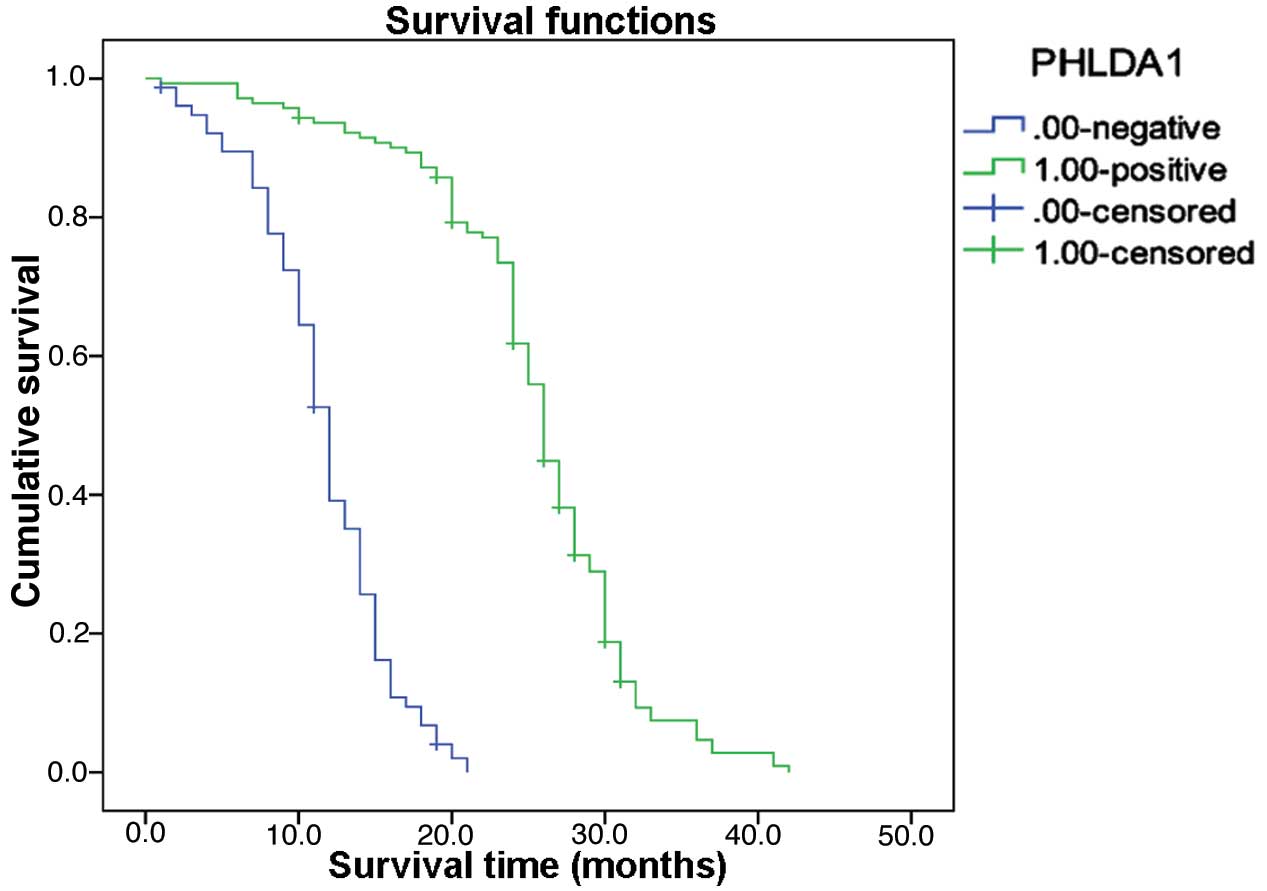
age (P=0.085), gender (P=0.373) or tumor size (P=0.413) (Table I). Follow-up data revealed that there
was a significant difference in overall mean survival time between
patients with PHLDA1-negative carcinomas (11.6 months) and those
with PHLDA1-positive carcinomas (25.4 months; Log rank=193.861;
P=0.0001; Fig. 5). The result of
multivariate analysis by Cox regression indicated that PHLDA1
expression was an independent prognostic factor (P=0.009).
 | Table I.Association between expression of
PHLDA1 or CD133 and clinicopathological features. |
Table I.
Association between expression of
PHLDA1 or CD133 and clinicopathological features.
|
| PHLDA1 |
| CD133 |
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
feature | – | + | P-value | – | + | P-value |
|---|
| Age, years |
|
| 0.085 |
|
| 0.395 |
| ≥60 | 33 | 44 |
| 33 | 44 |
|
| <
60 | 44 | 97 |
| 70 | 71 |
|
| Gender |
|
| 0.373 |
|
| 0.407 |
| Male | 49 | 81 |
| 58 | 72 |
|
|
Female | 28 | 60 |
| 45 | 43 |
|
| Tumor site |
|
| 0.001 |
|
| 0.068 |
|
Intrahepatic | 29 | 24 |
| 22 | 31 |
|
|
Perihilar | 35 | 68 |
| 44 | 59 |
|
|
Distal | 13 | 49 |
| 37 | 25 |
|
| Tumor size, cm |
|
| 0.413 |
|
| 0.003 |
| ≤2 | 33 | 70 |
| 60 | 43 |
|
| >2
≤5 | 35 | 56 |
| 37 | 54 |
|
|
>2 | 9 | 15 |
| 6 | 18 |
|
| Grade |
|
| 0.020 |
|
| <0.001 |
| 1 | 16 | 53 |
| 45 | 24 |
|
| 2 | 34 | 57 |
| 42 | 49 |
|
| 3 | 27 | 31 |
| 16 | 42 |
|
| TNM |
|
|
<0.001a |
|
|
<0.001a |
| I | 2 | 5 |
| 5 | 2 |
|
| II | 22 | 73 |
| 57 | 38 |
|
|
III | 33 | 55 |
| 35 | 53 |
|
| IV | 20 | 8 |
| 6 | 22 |
|
| Mean survival,
months | 11.6 | 25.4 | <0.001 | 31.2 | 16.9 | <0.001 |
Association between PHLDA1 and CD133
expression
In the present group of 218 cholangiocarcinoma
samples, 52.3% of cases were CD133 positive. CD133 expression was
correlated with tumor size (P=0.003), grade (P=0.0001), stage
(P=0.0001) and overall mean survival time (P=0.0001), but not with
tumor site (P=0.068), age (P=0.395) or gender (P=0.407; Table I). Frequently, when PHLDA1 expression
was negative, CD133 was found to be positive in the cancerous
glands of cholangiocarcinoma. Of the cholangiocarcinomas with high
expression of CD133, PHLDA1 expression was only detected in 58.2%
of cases (67/115), whereas of the carcinomas with low expression of
CD133, PHLDA1 was expressed in 71.8% of cases (74/103). A
significant inverse association was detected between the expression
of PHDA1 and CD133 (γ=-0.142; P=0.036).
Discussion
Sakthianandeswaren et al (17) identified PHLDA1 as a putative marker
of epithelial stem cells in the human adult small and large
intestine. In the colon and rectum, PHLDA1 protein was expressed in
undifferentiated columnar cells limited to the crypt base. This
distribution of PHLDA1-expressing cells in the human intestine
closely resembles that of Lgr5 in the mouse intestine (17). PHLDA1 was constitutively expressed in
human intestinal adenomas and the majority of carcinomas. A total
of 218 cases of cholangiocarcinoma with follow-up data were
analyzed in the present study and the results indicated that PHLDA1
was positively expressed in 64.7%, and negatively expressed in
35.3% of carcinomas. Furthermore, the loss of PHLDA1 expression was
correlated with the histological degree (P=0.0001) and clinical
stage (P=0.0001). This was in contrast to the previous results in
colorectal cancer by Sakthianandeswaren et al (17), in which no correlation between PHLDA1
staining and adenocarcinoma grade or clinical stage was observed,
suggesting that PHLDA1 may have a significant role in the evolution
and development of cholangiocarcinoma. Enhanced staining and
nuclear relocalization of the PHLDA1 protein was also observed at
the invasive front of the cholangiocarcinomas, similarly to the
results in colorectal cancer reported by Sakthianandeswaren et
al (17), which also suggested a
novel role for PHLDA1 in cell migration. However, the mechanisms
underlying the differential functions of nuclear and cytoplasmic
expression of PHLDA1 remain to be elucidated. Notably, the
polyglutamine tract in PHLDA1 is a feature common to several
transcription factors, suggesting a potential role as a
transcription factor or coactivator (22). Follow-up data revealed a significant
difference in overall mean survival time between the
PHLDA1-negative (11.6 months) and PHLDA1-positive
cholangiocarcinomas (25.4 months) (Log rank=193.861; P=0.0001). The
result of multivariate analysis by Cox regression indicated that
PHLDA1 expression was an independent prognostic factor (P=0.009).
The results of the present study also correspond with those of
previously published results in breast cancer (10), melanoma (11) and oral squamous cell carcinomas
(23). It has been suggested that
PHLDA1 expression may be a potential prognostic factor in
cholangiocarcinoma.
CD133 is an important marker in a variety of tumor
stem cells (24–26). In the present study, CD133 expression
was positive in 52.3% of cholangiocarcinoma cases. Investigation of
the expression of CD133 protein in the 218 cholangiocarcinoma with
follow-up data, indicated that CD133 expression level was
positively correlated with histological degree (P=0.0001) and
clinical stage (P=0.0001). Furthermore, expression of the CD133
protein was significantly associated with overall survival
(P=0.0001), suggesting that CD133 expression may be a potential
prognostic factor for poor prognosis in cholangiocarcinoma. The
results above correspond with those of previous reports regarding
cholangiocarcinoma from Japan (27),
Thailand (28) and a research group
from Xian, China (29), in which the
positive rates of CD133 in cholangiocarcinoma were 48.3, 67.6 and
74.0%, respectively, and CD133 expression was considered to be a
potential prognostic indicator. Subsequently, the potential
association between PHLDA1 and CD133 was investigated. In the
present study, PHLDA1 expression was demonstrated to be inversely
associated with clinicopathological features, including clinical
stage, histological grade and poor prognosis in cholangiocarcinoma.
These results also indicated that PHLDA1 may be inversely
correlated with CD133 (P=0.0001), implying loss of PHLDA1 function
may occur in high CD133-expressing cancer cells. However, the
association between these two genes identified is descriptive
rather than causative, and determination of a clear functional
association required further investigation.
In conclusion, PHLDA1 expression may be
downregulated in malignant phenotypes of cholangiocarcinoma. The
detection of PHLDA1 and CD133 expression may, to some extent,
reflect the biological behavior of cholangiocarcinoma cells, aiding
the selection of appropriate chemotherapy and molecular targeting
therapies.
Acknowledgements
The authors would like to thank their colleagues in
the Department of Pathology, Chinese People's Liberation Army
General Hospital (Beijing, China) for their technical
assistance.
References
|
1
|
Khan SA, Davidson BR, Goldin R, et al:
Consensus document. Gut. 51 (Suppl 6):VI1–VI9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gores GJ: A spotlight on
cholangiocarcinoma. Gastroenterology. 125:1536–1538. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao P, Lu Y, Zhong M, Liu L and Li B:
Inverse correlation of aberrant expression of fragile histidine
triad (FHIT) protein with cyclin D1 protein and prognosis in
Chinese patients with cholangiocarcinoma. Acta Oncol. 47:1557–1563.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heron DE, Stein DE, Eschelman DJ, et al:
Cholangiocarcinoma: The impact of tumor location and treatment
strategy on outcome. Am J Clin Oncol. 26:422–428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu ZF, Wu XY, Zhu N, et al: Prognosis
after resection for hepatitis B virus-associated intrahepatic
cholangiocarcinoma. World J Gastroenterol. 21:935–943.
2015.PubMed/NCBI
|
|
6
|
Berman DM, Karhadkar SS, Maitra A, et al:
Widespread requirement for Hedgehog ligand stimulation in growth of
digestive tract tumors. Nature. 425:846–851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haslam RJ, Koide HB and Hemmings BA:
Pleckstrin domain homology. Nature. 363:309–310. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ingley E and Hemmings BA: Pleckstrin
homology (PH) domains in signal transduction. J Cell Biochem.
56:436–443. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saraste M and Hyvonen M: Pleckstrin
homology domains: A fact file. Curr Opin Struct Biol. 5:403–408.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagai MA, Fregnani JH, Netto MM, et al:
Down-regulation of PHLDA1 gene expression is associated with breast
cancer progression. Breast Cancer Res Treat. 106:49–56. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neef R, Kuske MA, Pröls E and Johnson JP:
Identification of the human PHLDA1/TDAG51 gene: Down-regulation in
metastatic melanoma contributes to apoptosis resistance and growth
deregulation. Cancer Res. 62:5920–5929. 2002.PubMed/NCBI
|
|
12
|
Park CG, Lee SY, Kandala G, Lee SY and
Choi Y: A novel gene product that couples TCR signaling to Fas
(CD95) expression in activation-induced cell death. Immunity.
4:583–591. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hossain GS, van Thienen JV, Werstuck GH,
et al: TDAG51 is induced by homocysteine, promotes
detachment-mediated programmed cell death and contributes to the
development of atherosclerosis in hyperhomocysteinemia. J Biol
Chem. 278:30317–30327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashida N, Inouye S, Fujimoto M, et al:
A novel HSF1-mediated death pathway that is suppressed by heat
shock proteins. EMBO J. 25:4773–4783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oberst MD, Beberman SJ, Zhao L, et al:
TDAG51 is an ERK signaling target that opposes ERK-mediated HME16C
mammary epithelial cell transformation. BMC Cancer. 8:1892008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toyoshima Y, Karas M, Yakar S, Dupont J,
et al: TDAG51 mediates the effects of insulin-like growth factor I
(IGF-I) on cell survival. J Biol Chem. 279:25898–25904. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakthianandeswaren A, Christie M,
D'Andreti C, et al: PHLDA1 expression marks the putative epithelial
stem cells and contributes to intestinal tumorigenesis. Cancer Res.
71:3709–3719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sellheyer K and Nelson P: Follicular stem
cell marker PHLDA1 (TDAG51) is superior to cytokeratin-20 in
differentiating between trichoepithelioma and basal cell carcinoma
in small biopsy specimens. J Cutan Pathol. 38:542–550. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson EO, Chang KH, Pablo Y, et al:
PHLDA1 is a crucial negative regulator and effector of aurora A
kinase in breast cancer. J Cell Sci. 124:2711–2722. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen YC, Hu FC, Jeng YM, et al: Nuclear
overexpression of mitotic regulatory proteins in biliary tract
cancer: Correlation with clinicopathologic features and patient
survival. Cancer Epidemiol Biomarkers Prev. 18:417–423. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hao XP, Willis JE, Pretlow TG, et al: Loss
of fragile histidine triad expression in colorectal carcinomas and
premalignant lesions. Cancer Res. 60:18–21. 2000.PubMed/NCBI
|
|
22
|
Alba MM, Santibáñez-Koref MF and Hancock
JM: The comparative genomics of polyglutamine repeats: Extreme
differences in the codon organization of repeat-encoding regions
between mammals and Drosophila. J Mol Evol. 52:249–259.
2001.PubMed/NCBI
|
|
23
|
Coutinho-Camillo CM, Lourenço SV, Nonogaki
S, Vartanian JG, Nagai MA, Kowalski LP and Soares FA: Expression of
PAR-4 and PHLDA1 is prognostic for overall and disease-free
survival in oral squamous cell carcinomas. Virchows Arch.
463:31–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miraglia S, Godfrey W, Yin AH, Atkins K,
Warnke R, Holden JT, Bray RA, Waller EK and Buck DW: A novel
five-transmembrane hematopoietic stem cell antigen: Isolation,
characterization and molecular cloning. Blood. 90:5013–5021.
1997.PubMed/NCBI
|
|
25
|
Yin AH, Miraglia S, Zanjani ED, et al:
AC133, a novel marker for human hematopoietic stem and progenitor
cells. Blood. 90:5002–5012. 1997.PubMed/NCBI
|
|
26
|
Shmelkov SV, St Clair R, Lyden D and Rafii
S: AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 37:715–719.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimada M, Sugimoto K, Iwahashi S, et al:
CD133 expression is a potential prognostic indicator in
intrahepatic cholangiocarcinoma. J Gastroenterol. 45:896–902. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leelawat K, Thongtawee T, Narong S, et al:
Strong expression of CD133 is associated with increased
cholangiocarcinoma progression. World J Gastroenterol.
17:1192–1198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan L, He F, Liu H, et al: CD133: A
potential indicator for differentiation and prognosis of human
cholangiocarcinoma. BMC Cancer. 11:3202011. View Article : Google Scholar : PubMed/NCBI
|