Introduction
Chondrosarcoma is one of the most common bone tumors
and originates from hyaline cartilage. Although this cancer affects
individuals of all ages, chondrosarcoma most frequently occurs in
the elderly. The most notable symptom is pain, but pathological
bone fractures may also occur. The pelvic bone, shoulder and small
bones in the hands and feet are the most commonly affected
(1). In chondrosarcoma, surgical
resection is the best treatment option, as the tumor is resistant
to chemotherapy and radiotherapy. While low-grade tumors spread
locally, high-grade tumors undergo metastasis, most commonly to the
lung. These tumors resemble normal cartilage and produce a dense
cartilaginous extracellular matrix (ECM) (2). No non-invasive option is available for
the treatment of chondrosarcoma at present (3). To treat chondrosarcoma, the inhibition
of matrix metalloproteinases and angiogenic activities are
particularly used (4).
The OUMS-27 cell line produces proteoglycan and type
II, IX and XI collagen and is derived from chondrosarcoma cells,
which produce cartilage (5). This
cell line is a useful model for studies investigating the
association between chondrocytes and the ECM, and the development,
differentiation and treatment of chondrosarcoma (6–9). Studies
performed using these cells have revealed that recombinant
chondromodulin is an anti-angiogenic factor, while vascular
endothelial growth factor (VEGF) and fibroblastic growth factor
(FGF) are angiogenic factors (4).
However, the etiology of chondrosarcoma tumor formation has not
been completely elucidated. The tumor development depends on
several mechanisms, including angiogenesis and remodeling of the
ECM. During the process of local invasion and metastasis of tumors,
the association between the tumor cells and ECM is crucial
(10).
A disintegrin and metalloproteinase with
thrombospondin motifs (ADAMTS) proteases are a group of secreted
proteases that contains 19 members in humans (11). These proteins perform important tasks
in the protection of the structure and function of cells in humans,
particularly in the turnover and remodeling of the ECM. In
addition, these proteins are involved in coagulation, angiogenesis,
inflammation and fertility (12).
ADAMTS proteases are also associated with diseases that include
Alzheimer's disease, arthritis, tumors, atherosclerosis,
tendinopathy, stroke and Ehler-Danlos syndrome (EDS) (13). As a unique ADAMTS family, ADAMTS2,
ADAMTS3 and ADAMTS14 are also termed procollagen N-proteinases, and
are involved in the processing of procollagens to collagen and
maturation of type I collagen (14).
Mutations in the ADAMTS2 gene, located on the long arm of
chromosome 5, causes Ehlers-Danlos Syndrome (EDS) (15). In EDS, there is a dysfunction in the
processing of type I procollagen into collagen. ADAMTS3 is involved
in pathologies of the skin, cartilage, lung and aorta, whereas
ADAMTS2 gene knockdown in mice results in the development of a lung
disease. ADAMTS3 is essential in collagen-I-rich tissues and
cartilage (16,17). ADAMTS14, located on chromosome 10, is
associated with knee osteoarthritis (18).
Insulin, which is produced by the β cells of the
pancreas, regulates carbohydrate and fat metabolism in organisms.
Insulin receptors are present in the majority of tissues in the
body, including chondrocytes. In mouse chondrosarcoma, it was
demonstrated that insulin upregulates the synthesis of the ECM,
which consists of collagen, hyaluronic acid and proteoglycan, in
chondrocytes (19,20). The insulin receptor in these cells
stimulates proteoglycan synthesis, and tunicamycin inhibits this
process by binding to the insulin receptor (21,22).
In order to achieve an improved understanding of the
reasons for metastasis, local invasion, and the resistance to
chemotherapy and radiotherapy in chondrosarcoma, in addition to the
effect of insulin on the cancer cells, a cell culture method was
designed in the present study to determine the effects of insulin
on the procollagen N-proteinases ADAMTS2, ADAMTS3 and ADAMTS14.
Materials and methods
Cell culture
The OUMS-27 chondrosarcoma cells used in the present
study were kindly provided by Dr Kunisada from Okayama University
Graduate School of Medicine and Dentistry (Okayama, Japan).
Dulbecco's modified Eagle's medium (DMEM), containing 10% fetal
bovine serum and 10,000 U/ml penicillin/10,000 µg/ml streptomycin
(Hyclone SV30010; GE Healthcare Life Sciences, Logan, UT, USA), was
used to culture the chondrosarcoma cells at 37°C in a humidified
atmosphere of 5% CO2 in air. The cells were sub-cultured
every 7–10 days with trypsin plus EDTA at split ratios of 1:2–1:4.
The medium was changed every other day, with the cells being
cultured in either control media or control media supplemented with
10 µg/ml insulin for 11 days in total. Four groups of cells were
treated with insulin: 2×105 cells for the experiment on
day 1; 1×105 cells for the experiment on day 3;
5×104 cells for the experiment on day 7; and
3×104 cells for the experiment on day 11 were plated in
20-mm dishes and treated with the same concentration of insulin on
the indicated days. Following the experiment, the cells were
harvested and total RNA measurements were performed.
Isolation of total RNA
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) in accordance with the
manufacturer's instructions. In total, 2 µg of mRNA was reverse
transcribed using ReverTra Ace (Thermo Fisher Scientific, Waltham,
MA, USA) with random hexamers (Thermo Fisher Scientific) and random
primers, according to the manufacturer's instructions (Table I). Human GAPDH was amplified to act as
a control for the polymerase chain reaction (PCR). Those samples
lacking reverse transcriptase were amplified as a control for
genomic DNA contamination. RNase-free water was utilized for the
elution of total RNA from each sample. Ultraviolet
spectrophotometry was utilized to quantify and determine the purity
of each sample.
 | Table I.The forward and reverse primers used
in the real-time polymerase chain reaction analyses for ADAMTS2,
ADAMTS3, ADAMTS14, and GAPDH. |
Table I.
The forward and reverse primers used
in the real-time polymerase chain reaction analyses for ADAMTS2,
ADAMTS3, ADAMTS14, and GAPDH.
| Gene | Direction | Primer sequence | Product size, bp |
|---|
| ADAMTS2 | Forward |
ACATCAACGTGGTCCTGGTG | 148 |
|
| Reverse |
TATTCATCGTGGCCCGTGTC |
|
| ADAMTS3 | Forward |
ACCATGATGAGTCCCTCGGA | 128 |
|
| Reverse |
GCGACACACATTCTCCAAGC |
|
| ADAMTS14 | Forward |
TGAGTCCCTGGGGGTTCATA | 180 |
|
| Reverse |
ACAACGTGGTCATGGTGCT |
|
| GAPDH | Forward |
CCTGCACCACCAACTGCTTA | 108 |
|
| Reverse |
TCTTCTGGGTGGCAGTGATG |
|
Quantitative PCR (qPCR)
qPCR was conducted on obtained cDNA samples
(Rotor-Gene Q; Qiagen, Venlo, Limburg, Netherlands) as previously
described (12). The intercalating
dye SYBR green (Maxima SYBR Green/ROX qPCR Mater Mix, Thermo Fisher
Scientific) was used for qPCR of the total RNA in the presence of
primer pairs. The PCR mixture consisted of SYBR Green PCR Master
Mix, which consists of DNA polymerase, SYBR Green I dye,
deoxynucleotide triphosphates, including deoxyuridine triphosphate,
PCR buffer, 10 pmol forward and reverse primers and cDNA of samples
in a total volume of 20 µl. The amplification of the housekeeping
gene GAPDH was utilized for normalizing the efficiency of cDNA
synthesis and the amount of mRNA applied. PCR was performed with an
initial denaturation at 95°C for 5 min, followed by amplification
for 40 cycles, each cycle consisting of denaturation at 95°C for
10s, annealing at 57°C for 30s and polymerization at 72°C for 30s.
The final stage consisted of polymerization at 72°C for 5 min. The
results for the PCR performed for ADAMTS2, ADAMTS3 and ADAMTS14
were represented as graphic charts. The bars and error bars
represent the mean and standard deviation of the mean,
respectively.
Statistical analyses
SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA) was
utilized for the statistical assessment of the data, and the
non-parametric Kruskal Wallis test was used. The association
between the variables was assessed using the Mann-Whitney U test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
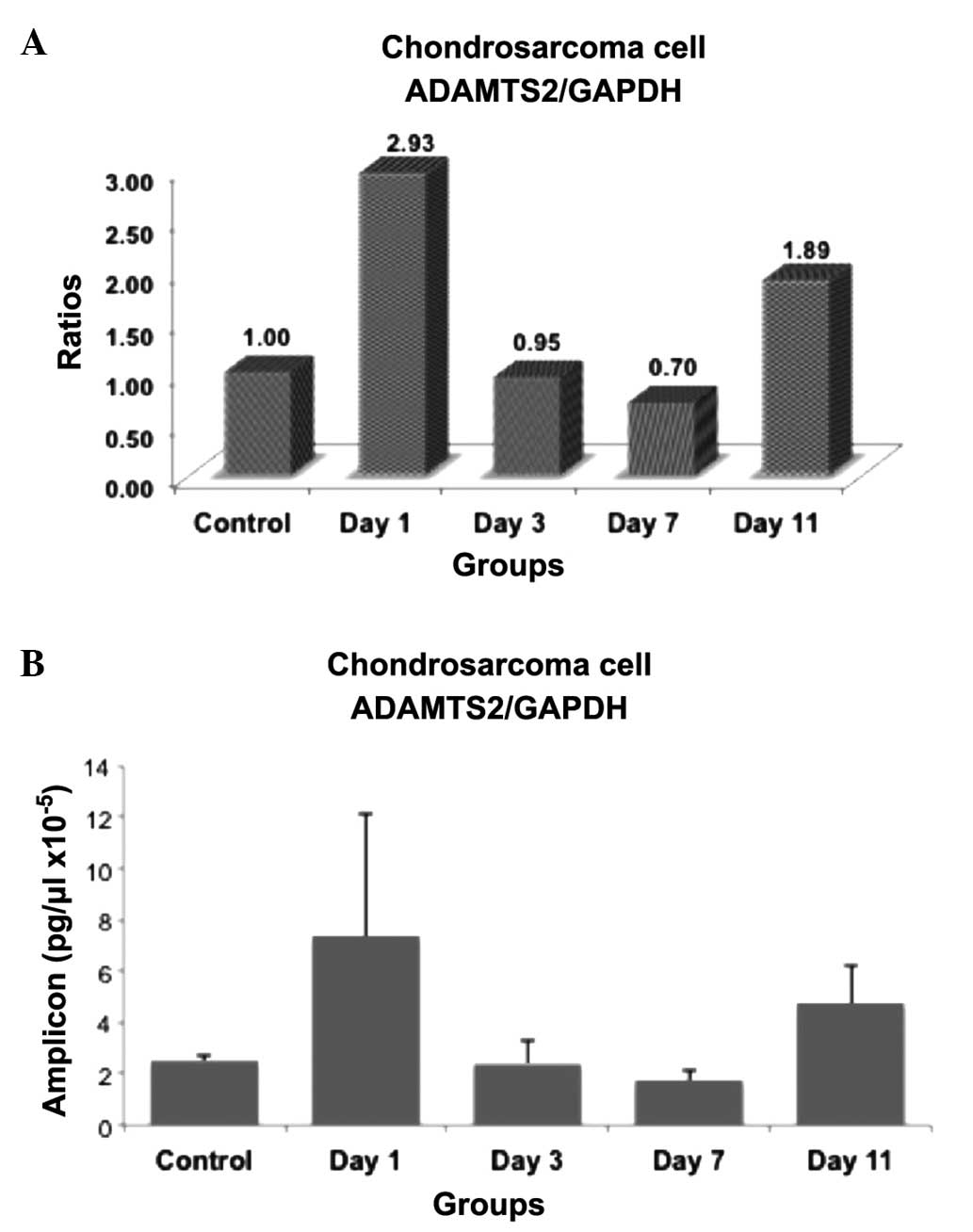
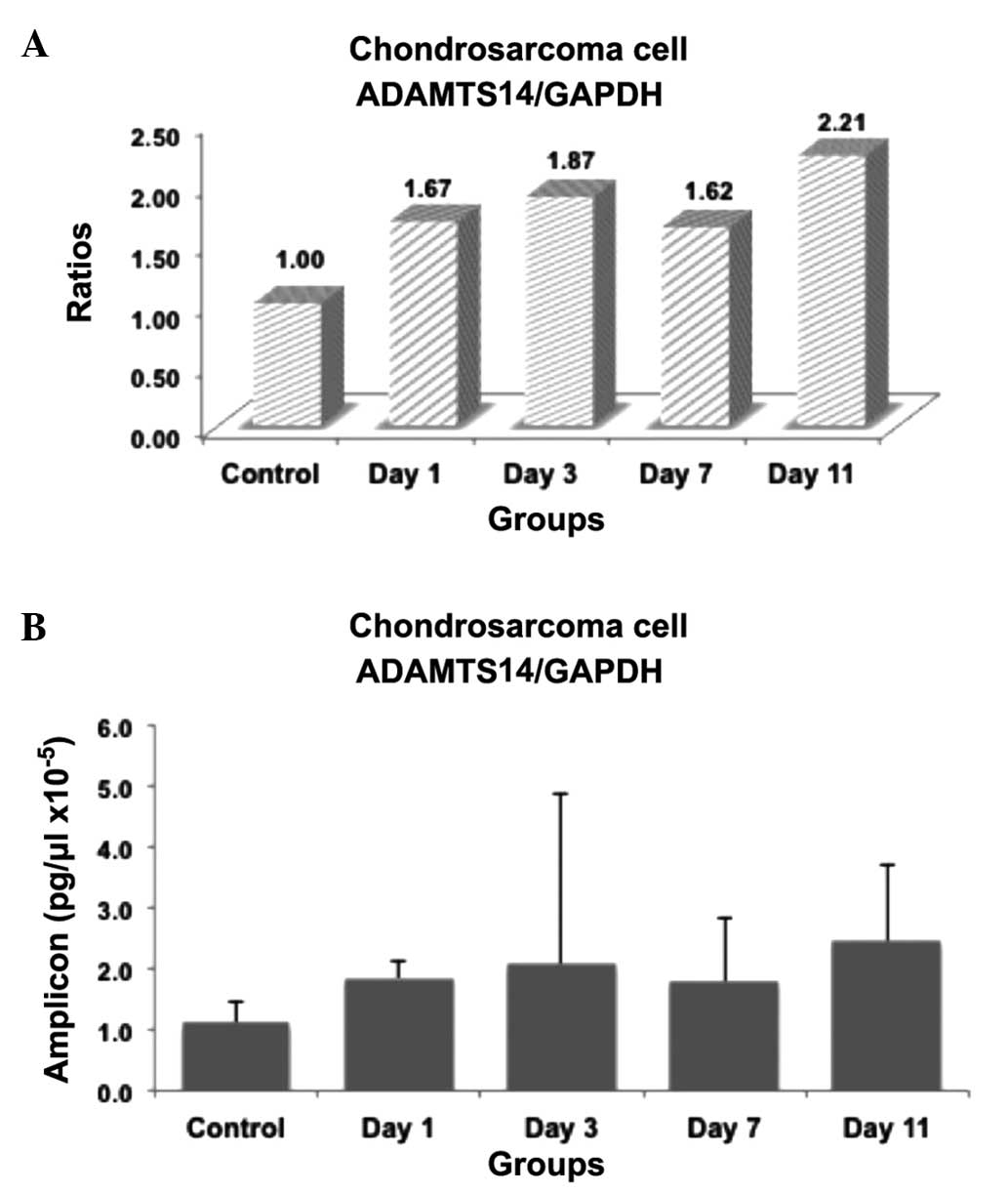
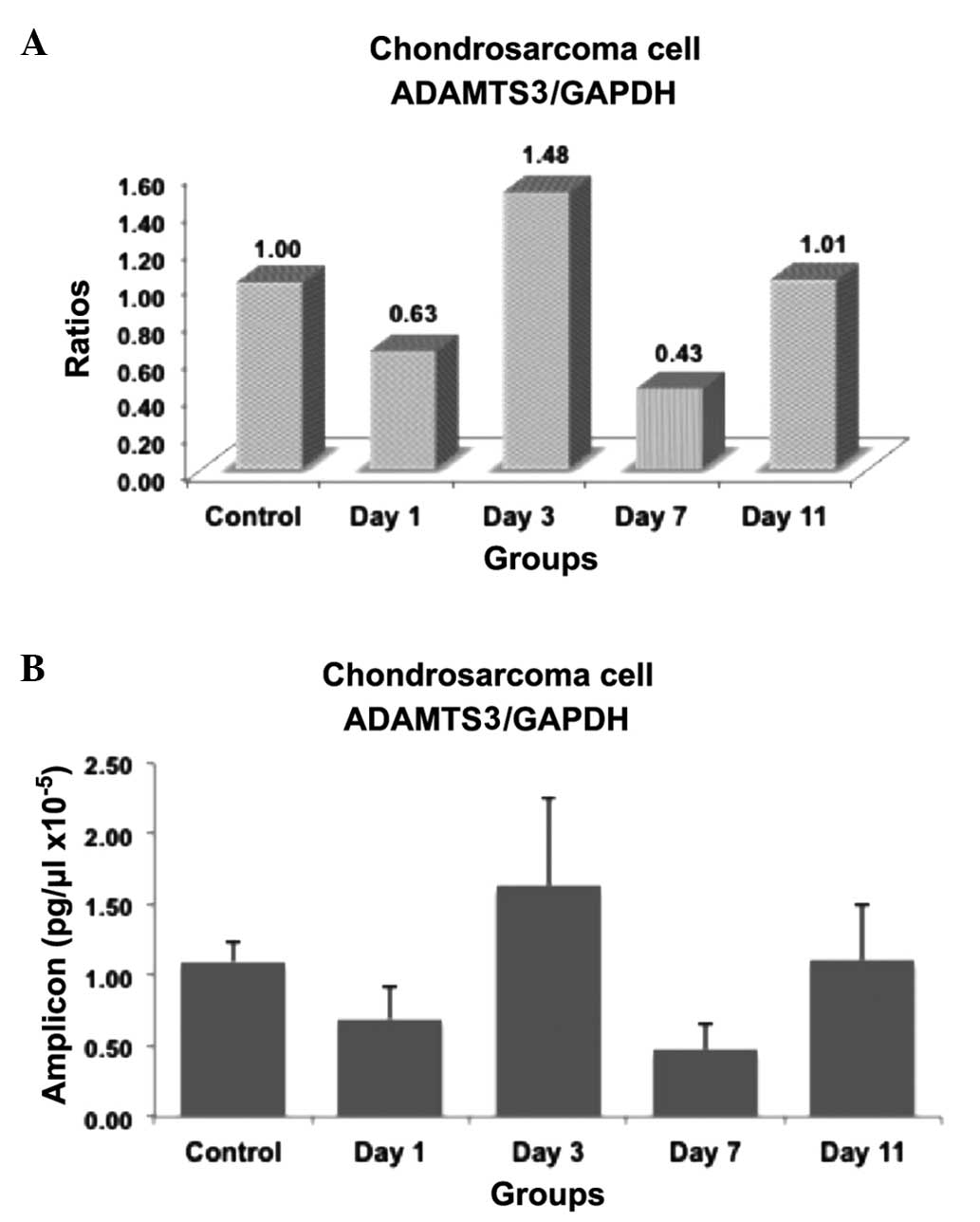
The present study examined whether the expression of
the ADAMTS2, ADAMTS3 and ADAMTS14 genes are upregulated or
downregulated by insulin in chondrosarcoma OUMS-27 cells. The
results were summarized in Figs.
1–3. The ratios and amplicon
concentrations of the insulin-induced cells compared with the
control cells are shown in Figs.
1–3. In these figures, the mRNA
expression levels were expressed as ratios within the groups
(Figs. 1A, 2A and 3A) and
the PCR product concentration was expressed as pg/ml (Figs. 1B, 2B
and 3B). Reverse transcription
(RT)-qPCR analyses demonstrated that ADAMTS2 mRNA expression had
increased compared with the control group on day 1 subsequent to
insulin induction, but the expression was not significantly
different due to the large range of individual results (P=0.095).
On day 3, the ADAMTS2 levels were not significantly different
compared with the control group (P>0.05), whereas a significant
decrease in ADAMTS2 expression was observed on day 7 (P=0.028) and
an increase was also observed on day 11 in the cells induced with
insulin compared with the control group (P=0.016). The increase in
mRNA concentration observed on day 11 was significantly different
from the mRNA concentration on days 3 (P=0.047) and 7 (P=0.008).
ADAMTS3 mRNA expression decreased immediately subsequent to insulin
induction on day 1 compared with the control group (P=0.008). The
most evident decrease in mRNA concentration was observed on day 7
subsequent to insulin induction (P=0.008). No significant
difference was identified between the amplicon concentration in
insulin-induced cells on days 3 and 11 compared with the control
cells. Significant differences were detected between the
concentrations observed in the insulin-induced cells on days 1 and
3 (P=0.016), days 1 and 7 (P=0.047), days 3 and 7 (P=0.008) and
days 7 and 11 (P=0.008). No statistically significant differences
were identified between the ADAMTS14 mRNA concentrations in the
control and insulin-induced groups.
Discussion
There are a limited number of studies in the
literature that assess the effects of insulin on chondrosarcoma
OUMS-27 cells (23,24). In addition, to the best of our
knowledge, no studies have investigated the expression levels of
procollagen N-proteinases in an insulin-induced human
chondrosarcoma cell line, or the role of the procollagen
N-proteinases ADAMTS2, ADAMTS3 and ADAMTS14 in chondrosarcoma.
According to the present study, there were significant differences
in the ADAMTS2 and ADAMTS3 mRNA concentrations between the control
and insulin-induced groups, but no difference in the concentration
of ADAMTS14 mRNA. As a notable finding, ADAMTS2 and ADAMTS3 have
been revealed to be modulated by insulin in a time-dependent
manner.
ADAMTS are secreted enzymes that are involved in ECM
degradation and turnover (25).
ADAMTS2, ADAMTS3 and ADAMTS14 are associated with collagen
synthesis. Collagen is synthesized from procollagen by specific
proteinases. These proteinases are bone morphogenetic protein-1
(BMP-1) and ADAMTS2, ADAMTS3 and ADAMTS14, also termed procollagen
N-proteinases, which are a novel family of ECM proteases (26). Procollagen N-proteinases remove
N-terminal peptides from procollagen to synthesize collagen
(27). This event is of considerable
importance for normal and pathological conditions. The degradation
of the ECM occurs normally during development, growth and tissue
repair (28). However, excessive
degradation of the ECM is observed in several pathological
conditions, including osteoarthritis and rheumatoid arthritis
(29). Tumor invasion, metastasis and
tumor angiogenesis require the participation of MMP, the expression
of which increases in association with tumorigenesis (30); however, there is an exception. The
conversion of procollagen to collagen by ADAMTS2, ADAMTS3 and
ADAMTS14 was hypothesized to trigger the effects that protect
tissues from tumor invasion, as it may strengthen the associated
tissues through collagen synthesis (30).
ADAMTS2 is associated with type I and type II
procollagen (28). The ADAMTS2 gene
is located at 5q23-q24 (31), and
mutations in this gene cause EDS and animal dermatosparaxis, which
particularly affects the skin (15,25,32). This
disease results in fragile skin, while other collagen-rich tissues,
including bone and tendons, are not affected (33). Matullo et al (34) revealed that matrix metalloproteinases
and ADAMTS2 influence survival in malignant pleural mesothelioma.
These effects were attributed to the metalloendopeptidase and
metallopeptidase activities of matrix metalloproteinases and
ADAMTS2 (34).
In the present study, ADAMTS2 was upregulated on day
11, which may reflect increased collagen synthesis associated with
tissue repair. Based on our findings, ADAMTS2 has proteolytic
activity and may be involved in cancer progression, similar to
other matrix metalloproteinases. Association between ADAMTS2 and
the ECM causes the invasion and metastasis of cancer cells, and
ADAMTS2 may be involved in this process (35). It has previously been revealed that
ADAMTS1, ADAMTS9, ADAMTS12, ADAMTS15 and ADAMTS18 are associated
with cancer (10). These proteases
possess proteolytic activity and break down the ECM, in addition to
being involved in angiogenesis in cancer (10). ADAMTS2 is a procollagen N-proteinase
that may be involved in cancer development and progression.
ADAMTS3 is located on 4q21 (31) and is also involved in collagen
synthesis. Similar to other procollagen N-proteinases, ADAMTS3
performs an important role during wound healing (36). ADAMTS3 is expressed in bone, cartilage
and musculo-tendinous tissues (25).
In human breast carcinoma, Porter et al (37) revealed that ADAMTS3 was consistently
downregulated, ADAMTS14 was upregulated and ADAMTS2 was not
upregulated or downregulated. In the present study, ADAMTS2 mRNA
expression decreased immediately subsequent to insulin induction on
day 1 and the most evident decrease was observed on day 7. This
finding is similar to that reported in the study by Porter et
al (14) and may reflect the
decrease in ADAMTS2 levels in cancer. However, this finding
requires further investigation by additional studies.
ADAMTS14, first described in 2001, is located in
10q21on (38). Extremely few studies
that investigated ADAMTS14 revealed an association between multiple
sclerosis and osteoarthritis (39,40). Al
Nakouzi et al (41) identified
that the expression of ADAMTS14 was increased in metastatic
prostate cancer. ADAMTS14 has also been revealed to be upregulated
in human breast carcinoma (37). In
the present study, no difference in the mRNA levels of ADAMTS14
mRNA was found in the OUMS-27 cells, compared with the previous
studies, described above.
Extremely little is known about the role of these
enzymes in cancers, particularly in chondrosarcoma. The present
study provides a novel insight into the process of cartilage
metabolism in cancer and the effect of insulin on this complex
process. The present study also indirectly aimed to identify the
effect of diabetes mellitus on the chondrosarcoma-associated
outcome. It was indicated that changes associated with the
application of insulin mediate crucial effects on chondrosarcoma
progression, in which matrix metalloproteinases may play a critical
role. In conclusion, these experiments suggest that the application
of insulin is able to modulate the biosynthesis of ECM
macromolecules altered in diabetes by various pathways and
mechanisms. Additional studies in which other ADAMTS proteins are
investigated in addition to the presently studied ADAMTS proteases
are required in order to achieve more accurate information and data
on the exact role of insulin in ECM metabolism in
chondrosarcoma.
Acknowledgements
This abstract was presented in part at the American
Society for Matrix Biology Biennial Meeting, Oct 12–15, 2014 in
Cleveland, OH, USA.
References
|
1
|
Qasem SA and DeYoung BR: Cartilage-forming
tumors. Semin Diagn Pathol. 31:10–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Söderström M, Böhling T, Ekfors T,
Nelimarkka L, Aro HT and Vuorio E: Molecular profiling of human
chondrosarcomas for matrix production and cancer markers. Int J
Cancer. 100:144–151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto S, Tanaka K, Sakimura R, et al:
Suberoylanilide hydroxamic acid (SAHA) induces apoptosis or
autophagy-associated cell death in chondrosarcoma cell lines.
Anticancer Res. 28:1585–1591. 2008.PubMed/NCBI
|
|
4
|
Clark JC, Dass CR and Choong PF:
Development of chondrosarcoma animal models for assessment of
adjuvant therapy. ANZ J Surg. 79:327–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kunisada T, Miyazaki M, Mihara K, et al: A
new human chondrosarcoma cell line (OUMS-27) that maintains
chondrocytic differentiation. Int J Cancer. 77:854–859. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishida K, Kunisada T, Shen ZN, Kadota Y,
Hashizume K and Ozaki T: Chondrosarcoma and peroxisome
proliferator-activated receptor. PPAR Res. 2008:2505682008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akyol S, Yukselten Y, Cakmak O, et al:
Hydrogen peroxide-induced oxidative damage in human chondrocytes:
The prophylactic effects of Hypericum perforatum Linn
extract on deoxyribonucleic acid damage, apoptosis and matrix
remodeling by a disintegrin-like and metalloproteinase with
thrombospondin motifs proteinases. Arch Rheumatol. 29:203–214.
2014. View Article : Google Scholar
|
|
8
|
Akyol S, Acar M, Unal ZN, et al: The
effects of caffeic acid phenethyl ester (CAPE), royal jelly, and
curcumin on gene expression of ADAMTS −1, −5 and −9 in OUMS −27
chondrosarcoma cells: A preliminary study. Ann Paediatr Rheum.
2:27–37. 2013. View Article : Google Scholar
|
|
9
|
Uysal S, Ünal ZN, Erdoğan S, et al:
Augmentation of ADAMTS9 gene expression by IL-1β is reversed by
NFκB and MAPK inhibitors, but not PI3 kinase inhibitors. Cell
Biochem Funct. 31:539–544. 2013.PubMed/NCBI
|
|
10
|
Przemyslaw L, Boguslaw HA, Elzbieta S and
Malgorzata SM: ADAM and ADAMTS family proteins and their role in
the colorectal cancer etiopathogenesis. BMB Rep. 46:139–150. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demircan K, Cömertoğlu I, Akyol S, et al:
A new biological marker candidate in female reproductive system
diseases: Matrix metalloproteinase with thrombospondin motifs
(ADAMTS). J Turk Ger Gynecol Assoc. 15:250–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Demircan K, Hirohata S, Nishida K, et al:
ADAMTS-9 is synergistically induced by interleukin-1beta and tumor
necrosis factor alpha in OUMS-27 chondrosarcoma cells and in human
chondrocytes. Arthritis Rheum. 52:1451–1460. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo F, Lai Y, Tian Q, Lin EA, Kong L and
Liu C: Granulin-epithelin precursor binds directly to ADAMTS-7 and
ADAMTS-12 and inhibits their degradation of cartilage oligomeric
matrix protein. Arthritis Rheum. 62:2023–2036. 2010.PubMed/NCBI
|
|
14
|
Porter S, Clark IM, Kevorkian L and
Edwards DR: The ADAMTS metalloproteinases. Biochem J. 386:15–27.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colige A, Nuytinck L, Hausser I, et al:
Novel types of mutation responsible for the dermatosparactic type
of Ehlers-Danlos syndrome (Type VIIC) and common polymorphisms in
the ADAMTS2 gene. J Invest Dermatol. 123:656–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le Goff C, Somerville RP, Kesteloot F, et
al: Regulation of procollagen amino-propeptide processing during
mouse embryogenesis by specialization of homologous ADAMTS
proteases: insights on collagen biosynthesis and dermatosparaxis.
Development. 133:1587–1596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernandes RJ, Hirohata S, Engle JM, et al:
Procollagen II amino propeptide processing by ADAMTS-3. Insights on
dermatosparaxis. J Biol Chem. 276:31502–31509. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poonpet T, Honsawek S, Tammachote N,
Kanitnate S and Tammachote R: ADAMTS14 gene polymorphism associated
with knee osteoarthritis in Thai women. Genetics Mol Res.
12:5301–5309. 2013. View Article : Google Scholar
|
|
19
|
Otsu K, Geary ES and Stevens RL: Aberrant
regulation of the metabolism of the insulin receptor in Swarm rat
chondrosarcoma chondrocytes. Biochem J. 254:203–209.
1988.PubMed/NCBI
|
|
20
|
Stevens RL and Hascall VC:
Characterization of proteoglycans synthesized by rat chondrosarcoma
chondrocytes treated with multiplication-stimulating activity and
insulin. J Biol Chem. 256:2053–2058. 1981.PubMed/NCBI
|
|
21
|
Foley TP Jr, Nissley SP, Stevens RL, et
al: Demonstration of receptors for insulin and insulin-like growth
factors on Swarm rat chondrosarcoma chondrocytes. Evidence that
insulin stimulates proteoglycan synthesis through the insulin
receptor. J Biol Chem. 257:663–669. 1982.PubMed/NCBI
|
|
22
|
Stevens RL, Schwartz LB, Austen KF,
Lohmander LS and Kimura JH: Effect of tunicamycin on insulin
binding and on proteoglycan synthesis and distribution in Swarm rat
chondrosarcoma cell cultures. J Biol Chem. 257:5745–5750.
1982.PubMed/NCBI
|
|
23
|
Uğurcu V, Akyol S, Altuntaş A, Firat R, et
al: The effects of insulin on the expression levels of ADAMTS6
& 19 in OUMS-27 cells. Dicle Med J. 41:451–456. 2014.
View Article : Google Scholar
|
|
24
|
Firat R, Akyol S, Kurşunlu SF, et al:
ADAMTS13 expression in human chondrosarcoma cells induced by
insulin. J Clin Exp Invest. 5:226–232. 2014. View Article : Google Scholar
|
|
25
|
Le Goff C and Cormier-Daire V: The
ADAMTS(L) family and human genetic disorders. Hum Mol Genet.
20:R163–R167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hartigan N, Garrigue-Antar L and Kadler
KE: Bone morphogenetic protein-1 (BMP-1). Identification of the
minimal domain structure for procollagen C-proteinase activity. J
Biol Chem. 278:18045–18049. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nusgens BV, Goebels Y, Shinkai H and
Lapière CM: Procollagen type III N-terminal endopeptidase in
fibroblast culture. Biochem J. 191:699–706. 1980.PubMed/NCBI
|
|
28
|
Siefert SA and Sarkar R: Matrix
metalloproteinases in vascular physiology and disease. Vascular.
20:210–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng WJ, Yan JW, Wan YN, et al: Matrix
metalloproteinases: A review of their structure and role in
systemic sclerosis. J Clin Immunol. 32:1409–1414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang BL: ADAMTS: A novel family of
extracellular matrix proteases. Int J Biochem Cell Biol. 33:33–44.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou H, Hickford JG and Fang Q: A
premature stop codon in the ADAMTS2 gene is likely to be
responsible for dermatosparaxis in Dorper sheep. Anim Genet.
43:471–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Colige A, Vandenberghe I, Thiry M, et al:
Cloning and characterization of ADAMTS-14, a novel ADAMTS
displaying high homology with ADAMTS-2 and ADAMTS-3. J Biol Chem.
277:5756–5766. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matullo G, Guarrera S, Betti M, et al:
Genetic variants associated with increased risk of malignant
pleural mesothelioma: a genome-wide association study. PLoS One.
8:e612532013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carinci F, Lo Muzio L, Piattelli A, et al:
Potential markers of tongue tumor progression selected by cDNA
microarray. Int J Immunopathol Pharmacol. 18:513–524.
2005.PubMed/NCBI
|
|
36
|
Lee CW, Hwang I, Park CS, et al:
Expression of ADAMTS −2, −3, −13 and −14 in culprit coronary
lesions in patients with acute myocardial infarction or stable
angina. J Thromb Thrombolysis. 33:362–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Porter S, Scott SD, Sassoon EM, et al:
Dysregulated expression of adamalysin-thrombospondin genes in human
breast carcinoma. Clin Cancer Res. 10:2429–2440. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bolz H, Ramírez A, von Brederlow B and
Kubisch C: Characterization of ADAMTS14, a novel member of the
ADAMTS metalloproteinase family. Biochim Biophys Acta.
1522:221–225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goertsches R, Comabella M, Navarro A,
Perkal H and Montalban X: Genetic association between polymorphisms
in the ADAMTS14 gene and multiple sclerosis. J Neuroimmunol.
164:140–147. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rodriguez-Lopez J, Pombo-Suarez M,
Loughlin J, et al: Association of a nsSNP in ADAMTS14 to some
osteoarthritis phenotypes. Osteoarthritis Cartilage. 17:321–327.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Al Nakouzi N, Bawa O, Le Pape A, et al:
The IGR-CaP1 xenograft model recapitulates mixed osteolytic/blastic
bone lesions observed in metastatic prostate cancer. Neoplasia.
14:376–387. 2012. View Article : Google Scholar : PubMed/NCBI
|