Introduction
Lung cancer is a common cause of cancer-associated
mortality in men and women worldwide (1,2). Lung
adenocarcinoma (LAC), which is classified as a non-small cell lung
cancer (NSCLC), is a prevalent subtype, accounting for ~25% of lung
cancers (2,3). Cigarette smoke remains to be a major
etiological risk factor for lung cancer (1,4); a
previous study reported that an increase in the incidence of LAC
was correlated with cigarette smoking (5). In addition, several signaling pathway
abnormalities in lung cancer have been found to be associated with
smoking (6,7). Therefore, further studies that aim to
identify the signals activated by smoking-associated carcinogens
may aid in the development of targeted therapies for lung cancer
patients with a history of smoking.
Notch signaling pathways have been identified to
have an important role in the regulation of cell differentiation,
proliferation and apoptosis. At present, four Notch receptors
(Notch1-4) have been identified in mammals. Of these, a previous
study found that an abnormality in the Notch1 and Notch3 signaling
pathway contributed to the pathogenesis of lung cancer (8).
Certain studies (9–11) have
produced controversial findings concerning the expression of Notch1
protein in NSCLC; Donnem et al (9) and Jiang et al (10) concluded that the overexpression of
Notch1 was associated with a poorer prognosis in patients with
NSCLC. By contrast, a study by Huang et al (11), which focused on LAC, demonstrated
opposing results. With respect to Notch3, Haruki et al
(12) found that the positive
expression rate was ~37% (32/87). However, Zhou et al
(13) and Ye et al (14) identified that the level of Notch3
increased in NSCLC tissues. The deviations in the expression of
Notch1 and Notch3 may be due to the heterogeneity of the lung
cancer samples. Although the study by Huang et al (11), discussed the association between
Notch1 and smoking, this correlation remains uncertain due to the
relatively insufficient number of smokers with LAC who were
recruited to the study. Therefore, a requirement exists to analyze
the effect of cigarette smoke on Notch expression in LAC.
In the present study, the association between Notch1
or Notch3 and smokers with LAC was analyzed by
immunohistochemistry. In addition, cigarette smoke extract (CSE)
was administered to LAC A549 cells and the expression of Notch1 and
Notch 3 were then detected by western blot analysis.
Materials and methods
Ethics statement
Ethical approval for the present study was granted
by the Institutional Ethics Committee of Zhongnan Hospital, Wuhan
University (Wuhan, China) and written informed consent was obtained
from all patients.
Patients and tissue samples
In total, 102 LAC samples were obtained from
patients diagnosed with pathological stage II LAC at Zhongnan
Hospital, Wuhan University between July 2010 and February 2014.
Following surgery (consisting of lobar or sublobar resection),
these patients were interviewed to determine their smoking history.
The tumors were staged according to the seventh edition of the
International Association for the Study of Lung Cancer (15) and the histological subtype was graded
according to guidelines provided by the World Health Organization
(16). The clinical characteristics
of the patients are shown in Table
I.
 | Table I.Characteristics of all patients with
lung adenocarcinoma. |
Table I.
Characteristics of all patients with
lung adenocarcinoma.
| Characteristic | Smokers | Non-smokers |
|---|
| Total, n | 52 | 50 |
| Males/females,
n | 40/12 | 24/26 |
| Mean age, years
(range) | 61.2 (44–74) | 62.4 (45–76) |
| Smoking history,
packs/year | 48.3±5.6 | 0 |
The criteria used for the smoker group was as
follows (17): i) A smoker prior to
the diagnosis of lung cancer; ii) a smoking history of ≥10
packs/year; and iii) a smoking habit of ≥10 cigarettes per day
during recent years. The non-smokers were defined as patients who
had smoked <100 cigarettes during their lifetime and who had
been exposed to passive smoking for <0.5 h everyday. Ex-smokers
were excluded from the present study. All patients had not received
chemotherapy or radiotherapy prior to their surgery.
Notch1 and Notch3
immunohistochemistry
Immunostaining of the tumor samples was performed
using an avidin-biotinylated horseradish peroxidase H complex (ABC
kit; Vector Laboratories, Inc., Burlingame, CA, USA) according to
the manufacturer's instructions. Samples were prepared as follows:
Samples (~100 mm3) were cut from each tissue and
immersed in 10% neutral formalin. Following fixation for 24 h, the
tissue block was washed in dH2O and embedded in
paraffin. Prior to experimentation, the samples were cut using a
microtome to ~5-µm thickness and affixed onto the slides which had
been coated by amino-propyl-tri-ethoxy-silane. The slides were then
incubated the slides at 60°C for 2 h, then placed in a rack and
washed as follows: xylene, 2 × 10 min; 100% ethanol, 2 × 5 min; 95%
ethanol, 5 min; 70% ethanol, 5 min; 50% ethanol, 5 min; and finally
rinsed in cold tap water. Deparaffinized slides were heated at 98°C
in 10 mmol/l citrate buffer (pH 6.0; Boster Biological Technology,
Ltd., Wuhan, China) for 30 min. Next, the sections were immersed
for 15 min in methanol containing 0.3% hydrogen peroxide (Boster
Biological Technology, Ltd.) in order to block endogenous
peroxidase activity. This was followed by 30 min of incubation with
5% blocking horse serum (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China)to reduce non-specific
binding. Goat polyclonal IgG antibodies for human Notch1 (C-20;
catalog no. sc-6014; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA; dilution, 1:200) and Notch3 (M-20; catalog no. sc-7424; Santa
Cruz Biotechnology, Inc.; dilution, 1:200) were then added to the
slides and incubated at 4°C overnight. 3,3′-diaminobenzidine (DAB;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) was used
as a chromogen; 100 µl DAB was added to each section and monitored
under a microscope until the staining developed, which was followed
by immersion slides in dH2O for 2 × 3 min. Slides were
then counterstained with hematoxylin (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.) for 1 min and washed in
dH2O for 2 × 5 min. For the negative controls, primary
antibodies were omitted. Each section was analyzed at five randomly
selected high-power fields. The sections with <5% positive cells
were regarded as negative for protein expression. For the
positively stained sections, the staining intensity (gray level) of
positive granules was assessed using a MIAS-300 image analyzer
(Nanjing Aokang Biotechnology Analytical Co., Ltd., Nanjing,
China).
Cell culture and reagents
The human LAC A549 cell line (American Type Culture
Collection, Manassas, VA, USA) was cultured in RPMI-1640
(Invitrogen Life Technologies, Carlsbad, CA, USA) containing 10%
fetal bovine serum (Invitrogen Life Technologies). The stock
environment was maintained at 37°C in a humidified 5%
CO2 incubator.
Cell treatment and measurement of cell
viability
CSE was prepared as previously described (18). In brief, the smoke obtained from four
full-strength Marlboro cigarettes (Marlboro Red; Phillip Morris
USA, Pittsburgh, PA, USA) with the filters removed was passed
through 100 ml of RPMI-1640 medium. The percentage of CSE was
referred to as to the undiluted solution and was considered to be
100%. Subsequently, CSE was adjusted to pH 7.4, filtered through a
0.22-µm filter (Sigma-Aldrich, St. Louis, MO, USA) and used within
30 min of preparation. In total, three different concentrations (1,
2.5 and 5%) of CSE diluted with the culture medium were used.
Normal RPMI-1640 without CSE was used as a negative control. The
A549 cells were cultured in RPMI 1640 medium containing 10% fetal
bovine serum (Invitrogen Life Technologies) for 12 h at 37°C in a
humidified atmosphere of 5% CO2. The cells were then
exposed to the CSE for 0 h, 8 h, 24 h or 48 h. Subsequently, using
a hemocytometer (catalog no. 3200; Hausser Scientific, Horsham, PA,
USA), the cell viability was assessed using a Trypan blue (Boster
Biological Technology, Ltd.) exclusion test as previously described
(19). Briefly, the hemocytometer was
filled with a suspension of cells (dilution, 1:1) in 0.4% Trypan
blue solution, and incubated for 2 min at room temperature. The
cells were then counted under a microscope (Tps-N-320m; Shanghai
Toposun Industries Co., Ltd., Shanghai, China) to determine the
mean number of viable cells (unstained cells) per 1 × 1 mm
square.
Western blot analysis
The samples of the A549 cells were harvested. Equal
amounts (20 µg) of the proteins were subjected to SDS-PAGE (6%;
Boster Biological Technology, Ltd.) and then transferred to a
polyvinylidene fluoride (PVDF) membrane (Sigma-Aldrich). The PVDF
membrane was then blocked with phosphate-buffered saline containing
0.1% Tween 20 (Sigma-Alrich) and 5% low-fat milk (Boster Biological
Technology, Ltd.) and incubated overnight at 4°C with goat
polyclonal IgG antibodies against human Notch1 (dilution, 1:400),
Notch3 (dilution, 1:400) and GAPDH (I-19; catalog no. sc-48166;
dilution, 1:2,000), which were all purchased from Santa Cruz
Biotechnology, Inc. PVDF membranes were then incubated with a
horseradish peroxidase-conjugated chicken anti-goat IgG secondary
antibody (catalog no. sc-2953; Santa Cruz Biotechnology, Inc.;
dilution, 1:2,000) for 1 h at room temperature. Immunoreactive
bands were visualized using Luminol reagent (Boster Biological
Technology, Ltd.) and a ChemiDoc™ XRS+ System with Image Lab™
Software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Clinical information is expressed as the median
(range) for the morphological data. The group data are expressed as
the mean ± standard deviation. Differences between groups were
analyzed using the χ2 test and a one-way analysis of
variance for the functional data. P<0.05 was considered to
indicate a statistically significant difference between values.
Statistical analyses were performed using GraphPad Prism 5
(GraphPad Software Inc., La Jolla, CA, USA).
Results
Immunostaining of Notch1 and Notch3
proteins in LAC
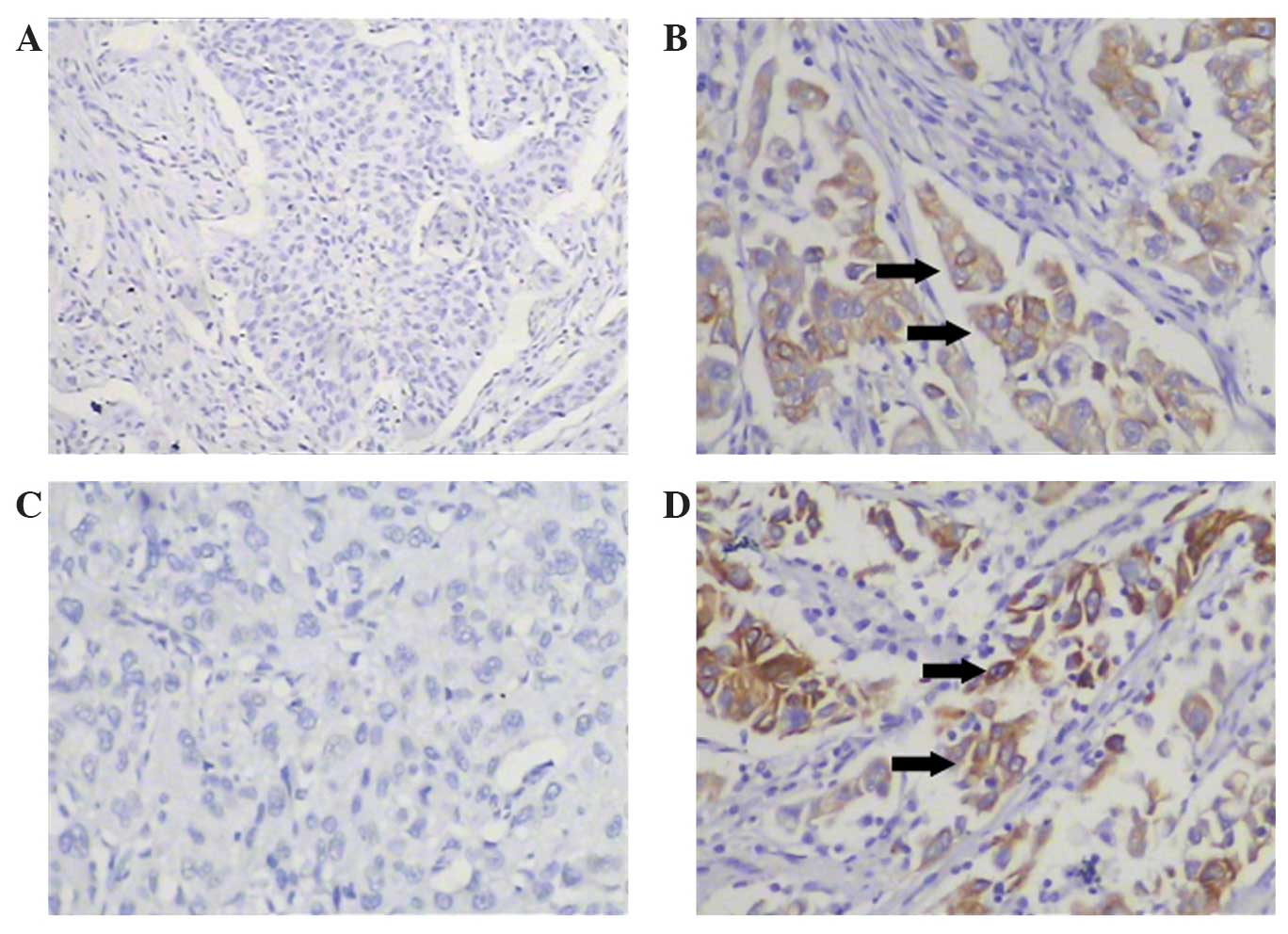
Notch1 and Notch3 were detected by
immunohistochemistry (Fig. 1). The
gray positive granules of Notch1 and Notch3 were predominantly
located in the cell membrane and cytoplasm of the tumor cells. Of
the 102 lung cancer specimens, 42 (41.2%) were positive for Notch1
and 63 (61.8%) were positive for Notch3.
Correlation between Notch1 and Notch3
expression and cigarette smoking
As shown in Table II,
there was no significant difference in the expression of Notch1
(positive rate and staining intensity) between smokers and
non-smokers with LAC. The positive rate of Notch3 expression was
higher in smokers compared with non-smokers. By comparing the
intensities of positive staining, Notch3 was found to be more
highly expressed in the smoking group than in the non-smoking group
(134.7±70.4 vs. 82.6±44.6, respectively; P=0.0012). The effects of
cigarette smoke on the expression of Notch1 and Notch3 according to
gender and histological LAC subtype were not analyzed due to the
limited sample size.
 | Table II.Expression of Notch1 and Notch3 in
LAC tissue samples. |
Table II.
Expression of Notch1 and Notch3 in
LAC tissue samples.
| Patients with
LAC | Positive stain,
n | Negative stain,
n | P-value |
|---|
| Notch1 |
|
| 0.3318 |
|
Smokers | 18 | 34 |
|
|
Non-smokers | 24 | 26 |
|
| Notch3 |
|
| 0.0165 |
|
Smokers | 38 | 14 |
|
|
Non-smokers | 25 | 25 |
|
Cell viability following CSE
treatment
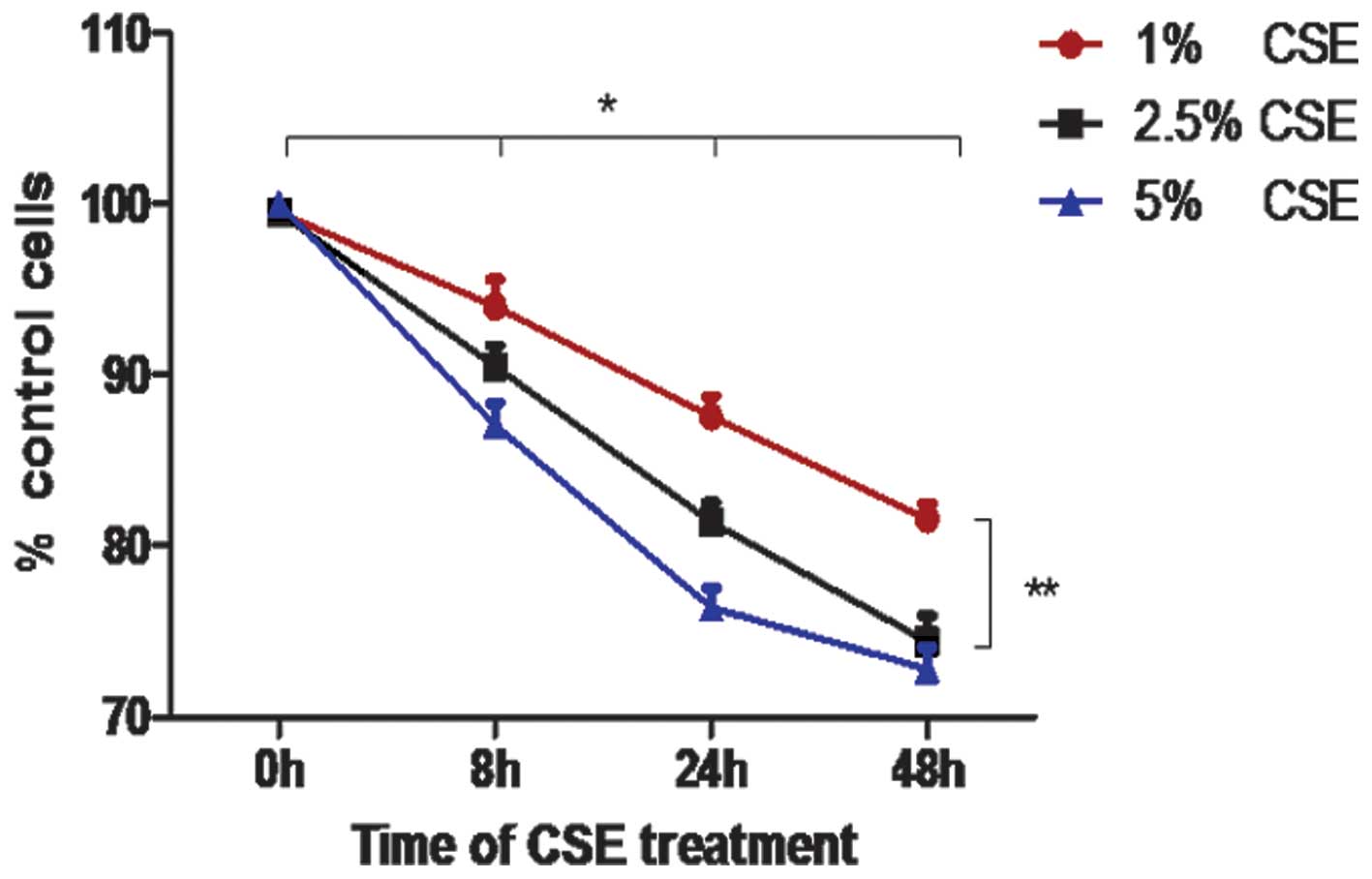
The results of the Trypan blue exclusion test
revealed that CSE significantly reduced the viability of A549 cells
in a time-and dose-dependent manner at concentrations of 1% and
2.5% (Fig. 2; P<0.05). At a
concentration of 5% CSE, the cell viability was markedly reduced at
24 h compared with at 0 and 8 h (P<0.05); however, there was no
difference in the cell viability between 24 h and 48 h following
the administration of 5% CSE (P>0.05).
Notch1 and Notch3 expression in A549
cells, as detected by western blot analysis
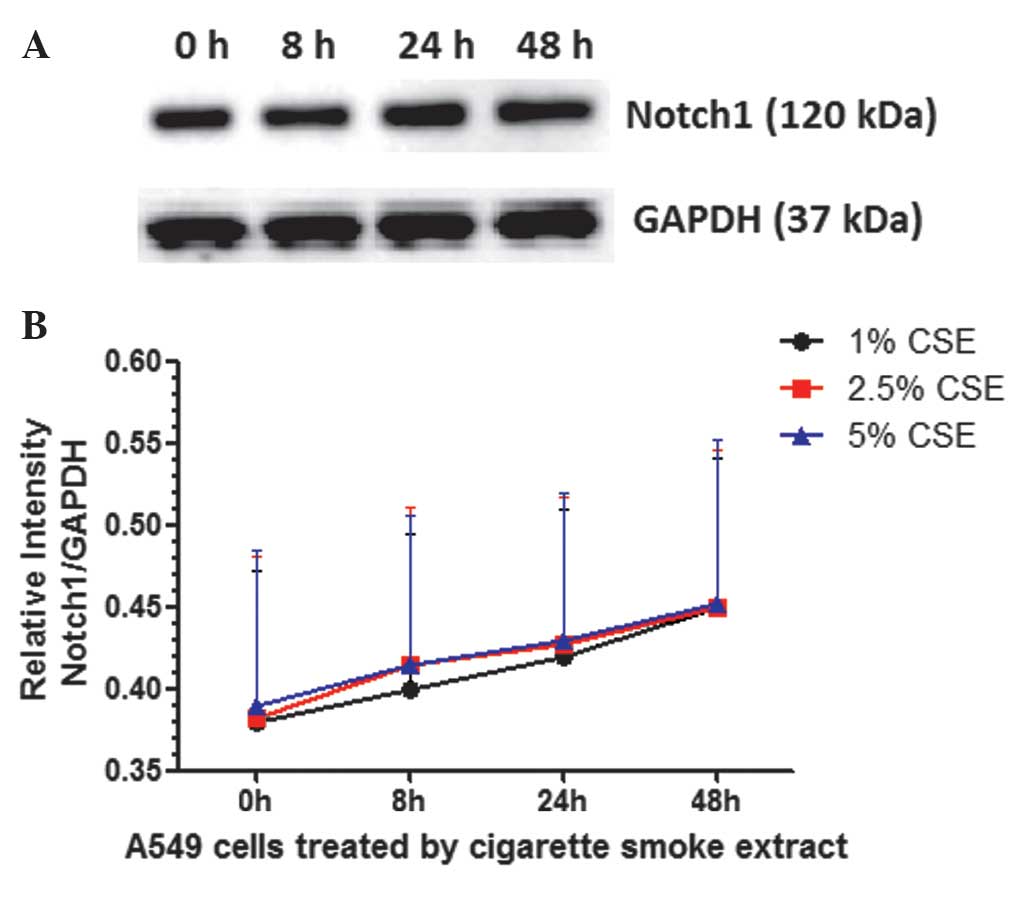
The expression of Notch1 and Notch3 in A549 cells
treated with different concentrations of CSE was analyzed at
continuous time points by western blot analysis. As shown in
Fig. 3, the results revealed that the
expression of Notch1 protein in A549 cells treated with CSE was
relatively stable at different time points (P>0.05) and at
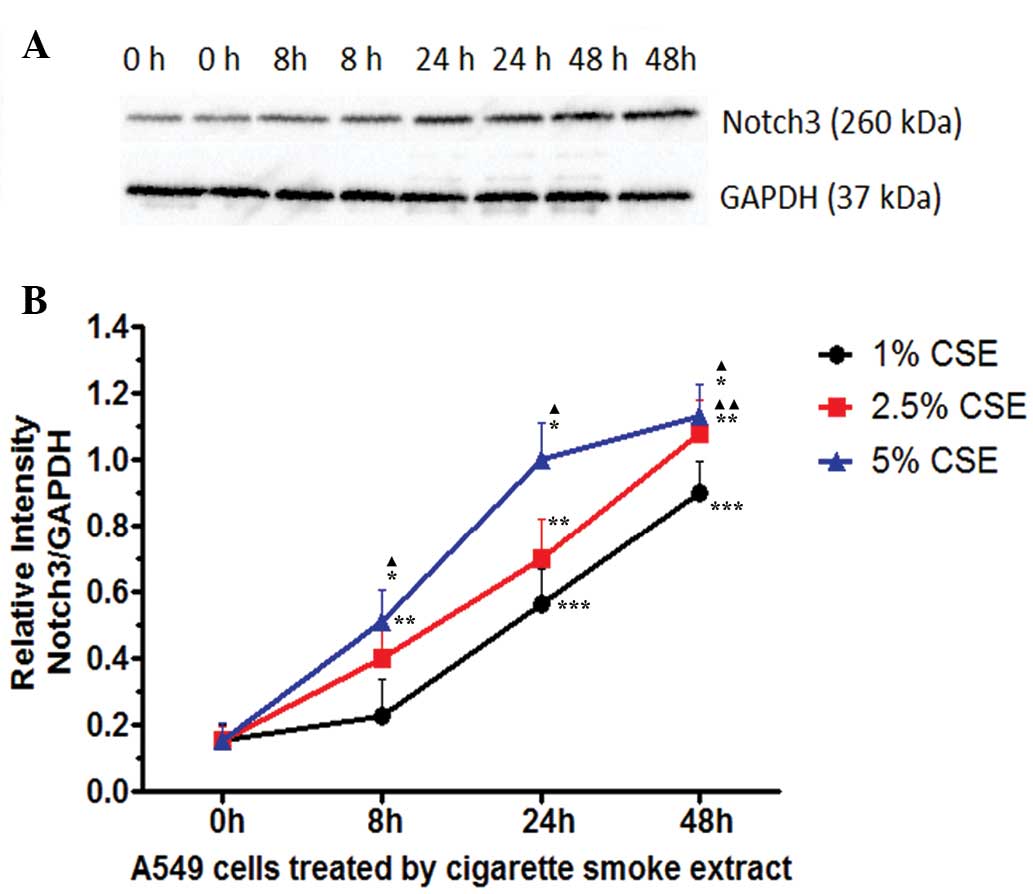
various concentrations (P>0.05). As shown in Fig. 4, the expression of Notch3 in A549
cells increased in a time-and dose-dependent manner following
treatment with CSE at concentrations of 1% and 2.5% (P<0.05).
The earliest peak of Notch3 protein expression was observed at 24 h
following treatment with 5% CSE.
Discussion
It is estimated that >300 million people smoke
cigarettes in China (20). Certain
individuals do not stop smoking following the onset of respiratory
symptoms due to an addiction to the tobacco; in addition, numerous
patients continue to smoke cigarettes until a diagnosis of lung
cancer has been established. In the present study, in order to
identify the signaling pathways that are affected by cigarette
smoke, stage II LAC samples were obtained from smokers and
non-smokers; in addition, the effects of CSE on A549 cells were
investigated. A previous study revealed that abnormalities in the
Notch signaling pathway were associated with cigarette smoke in
smokers and patients with chronic obstructive pulmonary disorder
(COPD) (21). Recent studies have
demonstrated that Notch1 and Notch3 have important roles in the
pathogenesis of LAC (9–14). Therefore, the present study chose to
analyze the expression of Notch1 and Notch3 proteins.
Zheng et al (22) reported that overexpression of Notch1
inhibited the growth of A549 cells and interfered with their
ability to form tumors in nude mice. The results of a further study
by Kluk et al (23) revealed
that Notch1 was rarely activated in NSCLC specimens (detailed
clinical data concerning LAC was not provided). Wael et al
(24) demonstrated that blocking
Notch1 in A549 cells resulted in increased cell proliferation.
Furthermore, Huang et al (11)
observed that negative Notch1 expression was significantly
associated with advanced clinical stage and lymph node metastasis
in LAC patients. However, increasing evidence indicates that Notch1
acts as an oncogene in LAC. A number of studies have investigated
the association between the expression of Notch1 and its clinical
significance and found that Notch1 may be used as a predictable
biomarker for poor LAC prognoses (9,10). In
addition, Westhoff et al (25)
established that the activation of Notch1 was correlated with poor
clinical outcomes in NSCLC patients not harboring TP53 mutations.
Microenvironment hypoxia is common in LAC, where it supports cancer
stem cell survival and results in poor responses to anticancer
therapies (26–28). A study by Chen et al (29) demonstrated that hypoxia dramatically
elevated the expression of Notch1 in lung tumor cell lines and that
Notch1 was required for LAC cell survival under hypoxia. It has
been reported that under a hypoxic microenvironment, Notch-1
activates Akt-1 through the inhibition of phosphatase and tensin
homolog expression and the induction of the insulin-like growth
factor 1 receptor (30). Several
studies have revealed that A disintegrin and metalloproteinase
(ADAM)17 (31), ADAM10 (32) and Galectin-1 (33) may contribute to the migration and
invasion of LAC cells via the activation of Notch1. The activation
of the Notch1 signaling pathway also has downstream effects on
protein kinase casein kinase 2α (34), Ras (35)
and tribbles homolog 3 (36). The
activation of Notch1 may contribute to drug resistance in LAC,
since the downregulation of Notch1 has been found to be effective
during treatment with δ-tocotrienol (37–39),
gefitinib (40,41), cisplatin (42) or pterostilbene (43). Blocking Notch1 has been identified to
inhibit the growth of cluster of differentiation 133-positive
cancer cells (44). The results of
the present study demonstrated that there was no significant
difference in the rate and intensity of Notch1 positive expression
between the smokers and non-smokers with LAC. Huang et al
(11) analyzed the expression of
Notch1 in LAC and also did not establish any association between
Notch1 and cigarette smoke; however, only 37 smokers were recruited
in the study. In addition, the present study administered CSE to
the LAC A549 cell line. The results of western blot analysis
revealed that cigarette smoke did not affect the expression of
Notch1 protein in LAC. In summary, Notch1 may be an important
factor in the pathogenesis of LAC; however, in the present study,
it was concluded that cigarette smoke did not affect the expression
of Notch1 protein in LAC.
Notch3 has also been identified to exhibit a
correlation with LAC (45). Haruki
et al (12) reported that the
positive expression rate of Notch3 was ~37% (32/87) in LAC and
indicated that its mechanism of maintaining the neoplastic
phenotype may proceed via the modulation of the epidermal growth
factor pathway. Additional studies have revealed that the protein
expression of Notch3 was higher in NSCLC tissues (13,14).
Konishi et al (46) identified
that the inhibition of Notch3 activation reduced the proliferation
of A549 cells. A further study established that an elevated
expression of Notch3 was present in aldehyde dehydrogenase
(ALDH)-positive tumor cells and that the inhibition of Notch3
decreased the number of ALDH-positive tumor cells (47). However, none of these studies
discussed the effect of cigarette smoke on the expression of Notch3
in LAC. In the present study, the positive staining rate (73.1%)
and the intensity of Notch3 protein in the samples of LAC from
smokers were significantly higher compared with those in the
non-smokers. In addition, it was revealed that CSE was able to
increase the expression of Notch3 protein in A549 cells in a
time-and dose-dependent manner. Therefore it may be hypothesized
that cigarette smoke promotes the pathogenesis of LAC via the
Notch3 pathway.
In conclusion, the present study revealed that
cigarette smoke promoted the expression of Notch3 protein, not
Notch1 protein, in LAC. This differed to results obtained from
patients with COPD and healthy smokers (21). This may be due to the fact that the
Notch signaling pathway has different roles in different diseases.
Further studies should be conducted in order to validate these
results. Cigarettes contain >60 chemicals that have been
identified as carcinogens (48,49);
therefore, studies that aim to identify the chemicals in cigarettes
that may affect Notch3 are required. In addition, specific
inhibitors of the Notch3 pathway may be investigated in future
studies in order to clarify the effects of cigarette smoke on
Notch3 expression.
Acknowledgements
This study was supported, in part, by a research
grant from the National Natural Science Foundation of China (no.
81300031).
References
|
1
|
Stanley KE: Lung cancer and tobacco - a
global problem. Cancer Detect Prev. 9:83–89. 1986.PubMed/NCBI
|
|
2
|
Kadara H, Kabbout M and Wistuba II:
Pulmonary adenocarcinoma: a renewed entity in 2011. Respirology.
17:50–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harris CC: The epidemiology of different
histologic types of bronchogenic carcinoma. Cancer Chemother Rep.
4:59–61. 1973.
|
|
4
|
Allen SS: Cigarette smoking among women:
how can we help? Minn Med. 97:41–3. 2014.PubMed/NCBI
|
|
5
|
Stellman SD, Muscat JE, Hoffmann D and
Wynder EL: Impact of filter cigarette smoking on lung cancer
histology. Prev Med. 26:451–456. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hecht SS: Lung carcinogenesis by tobacco
smoke. Int J Cancer. 131:2724–2732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wen J, Fu JH, Zhang W and Guo M: Lung
carcinoma signaling pathways activated by smoking. Chin J Cancer.
30:551–558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galluzzo P and Bocchetta M: Notch
signaling in lung cancer. Expert Rev Anticancer Ther. 11:533–540.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Donnem T, Andersen S, Al-Shibli K, Al-Saad
S, Busund LT and Bremnes RM: Prognostic impact of Notch ligands and
receptors in nonsmall cell lung cancer: Coexpression of Notch-1 and
vascular endothelial growth factor-A predicts poor survival.
Cancer. 116:5676–5685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang X, Zhou JH, Deng ZH, Qu XH, Jiang HY
and Liu Y: Expression and significance of Notch1, Jagged1 and VEGF
in human non-small cell lung cancer. Zhong Nan Da Xue Xue Bao Yi
Xue Ban. 32:1031–1036. 2007.(In Chinese). PubMed/NCBI
|
|
11
|
Huang J, Song H, Liu B, et al: Expression
of Notch-1 and its clinical significance in different histological
subtypes of human lung adenocarcinoma. J Exp Clin Cancer Res.
32:842013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haruki N, Kawaguchi KS, Eichenberger S, et
al: Dominant-negative Notch3 receptor inhibits mitogen-activated
protein kinase pathway and the growth of human lung cancers. Cancer
Res. 65:3555–3561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou M, Jin WY, Fan ZW and Han RC:
Analysis of the expression of the Notch3 receptor protein in adult
lung cancer. Oncol Lett. 5:499–504. 2013.PubMed/NCBI
|
|
14
|
Ye YZ, Zhang ZH, Fan XY, et al: Notch3
overexpression associates with poor prognosis in human
non-small-cell lung cancer. Med Oncol. 30:5952013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shepherd FA, Crowley J, Van Houtte P, et
al International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions: The
International Association for the Study of Lung Cancer lung cancer
staging project: proposals regarding the clinical staging of small
cell lung cancer in the forthcoming (seventh) edition of the tumor,
node, metastasis classification for lung cancer. J Thorac Oncol.
2:1067–1077. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Travis WD, Garg K, Franklin WA, et al:
Bronchioloalveolar carcinoma and lung adenocarcinoma: the clinical
importance and research relevance of the 2004 World Health
Organization pathologic criteria. J Thorac Oncol. 1:S13–S19. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Cheng Z, Liu W and Wu K:
Expression of interleukin (IL)-10, IL-17A and IL-22 in serum and
sputum of stable chronic obstructive pulmonary disease patients.
COPD. 10:459–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin Y, Hou G, Li E, Wang Q and Kang J:
PPARγ agonists regulate tobacco smoke-induced Toll like receptor 4
expression in alveolar macrophages. Respir Res. 15:282014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol. Appendix 3: Appendix 3B. 2001.
View Article : Google Scholar
|
|
20
|
Au WW, Su D and Yuan J: Cigarette smoking
in China: Public health, science and policy. Rev Environ Health.
27:43–49. 2012.PubMed/NCBI
|
|
21
|
Tilley AE, Harvey BG, Heguy A, et al:
Down-regulation of the notch pathway in human airway epithelium in
association with smoking and chronic obstructive pulmonary disease.
Am J Respir Crit Care Med. 179:457–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng Q, Qin H, Zhang H, et al: Notch
signaling inhibits growth of the human lung adenocarcinoma cell
line A549. Oncol Rep. 17:847–852. 2007.PubMed/NCBI
|
|
23
|
Kluk MJ, Ashworth T, Wang H, et al:
Gauging NOTCH1 activation in cancer using immunohistochemistry.
PLoS One. 8:e673062013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wael H, Yoshida R, Kudoh S, Hasegawa K,
Niimori-Kita K and Ito T: Notch1 signaling controls cell
proliferation, apoptosis and differentiation in lung carcinoma.
Lung Cancer. 85:131–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Westhoff B, Colaluca IN, D'Ario G, et al:
Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci
USA. 106:22293–22298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Foster JG, Wong SC and Sharp TV: The
hypoxic tumor microenvironment: driving the tumorigenesis of
non-small-cell lung cancer. Future Oncol. 10:2659–2674. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Graves EE, Maity A and Le QT: The tumor
microenvironment in non-small-cell lung cancer. Semin Radiat Oncol.
20:156–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maity A and Koumenis C: Location,
location, location - makes all the difference for hypoxia in lung
tumors. Clin Cancer Res. 16:4685–4687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, De Marco MA, Graziani I, et al:
Oxygen concentration determines the biological effects of NOTCH-1
signaling in adenocarcinoma of the lung. Cancer Res. 67:7954–7959.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eliasz S, Liang S, Chen Y, et al: Notch-1
stimulates survival of lung adenocarcinoma cells during hypoxia by
activating the IGF-1R pathway. Oncogene. 29:2488–2498. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baumgart A, Seidl S, Vlachou P, et al:
ADAM17 regulates epidermal growth factor receptor expression
through the activation of Notch1 in non-small cell lung cancer.
Cancer Res. 70:5368–5378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo J, He L, Yuan P, et al: ADAM10
overexpression in human non-small cell lung cancer correlates with
cell migration and invasion through the activation of the Notch1
signaling pathway. Oncol Rep. 28:1709–1718. 2012.PubMed/NCBI
|
|
33
|
Hsu YL, Wu CY, Hung JY, Lin YS, Huang MS
and Kuo PL: Galectin-1 promotes lung cancer tumor metastasis by
potentiating integrin α6β4 and Notch1/Jagged2 signaling pathway.
Carcinogenesis. 34:1370–1381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Long H, Yang YL, et al:
Inhibition of CK2α down-regulates Notch1 signaling in lung cancer
cells. J Cell Mol Med. 17:854–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allen TD, Rodriguez EM, Jones KD and
Bishop JM: Activated Notch1 induces lung adenomas in mice and
cooperates with Myc in the generation of lung adenocarcinoma.
Cancer Res. 71:6010–6018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou H, Luo Y, Chen JH, et al: Knockdown
of TRB3 induces apoptosis in human lung adenocarcinoma cells
through regulation of Notch1 expression. Mol Med Rep. 8:47–52.
2013.PubMed/NCBI
|
|
37
|
Ji X, Wang Z, Geamanu A, Sarkar FH and
Gupta SV: Inhibition of cell growth and induction of apoptosis in
non-small cell lung cancer cells by delta-tocotrienol is associated
with notch-1 down-regulation. J Cell Biochem. 112:2773–2783. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ji X, Wang Z, Geamanu A, Goja A, Sarkar FH
and Gupta SV: Delta-tocotrienol suppresses Notch-1 pathway by
upregulating miR-34a in nonsmall cell lung cancer cells. Int J
Cancer. 131:2668–2677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ji X, Wang Z, Sarkar FH and Gupta SV:
Delta-tocotrienol augments cisplatin-induced suppression of
non-small cell lung cancer cells via inhibition of the Notch-1
pathway. Anticancer Res. 32:2647–2655. 2012.PubMed/NCBI
|
|
40
|
Xie M, He CS, Wei SH and Zhang L: Notch-1
contributes to epidermal growth factor receptor tyrosine kinase
inhibitor acquired resistance in non-small cell lung cancer in
vitro and in vivo. Eur J Cancer. 49:3559–3572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie M, Zhang L, He CS, et al: Activation
of Notch-1 enhances epithelial-mesenchymal transition in
gefitinib-acquired resistant lung cancer cells. J Cell Biochem.
113:1501–1513. 2012.PubMed/NCBI
|
|
42
|
Liu YP, Yang CJ, Huang MS, et al:
Cisplatin selects for multidrug-resistant CD133+ cells in lung
adenocarcinoma by activating Notch signaling. Cancer Res.
73:406–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang Y, Yan X, Duan W, et al:
Pterostilbene exerts antitumor activity via the Notch1 signaling
pathway in human lung adenocarcinoma cells. PLoS One. 8:e626522013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu J and Mao Z, Huang J, Xie S, Liu T and
Mao Z: Blocking the NOTCH pathway can inhibit the growth of
CD133-positive A549 cells and sensitize to chemotherapy. Biochem
Biophys Res Commun. 444:670–675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dang TP, Gazdar AF, Virmani AK, et al:
Chromosome 19 translocation, overexpression of Notch3 and human
lung cancer. J Natl Cancer Inst. 92:1355–1357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Konishi J, Kawaguchi KS, Vo H, et al:
Gamma-secretase inhibitor prevents Notch3 activation and reduces
proliferation in human lung cancers. Cancer Res. 67:8051–8057.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sullivan JP, Spinola M, Dodge M, et al:
Aldehyde dehydrogenase activity selects for lung adenocarcinoma
stem cells dependent on notch signaling. Cancer Res. 70:9937–9948.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Weiss W: Cigarette smoke as a carcinogen.
Am Rev Respir Dis. 108:364–366. 1973.PubMed/NCBI
|
|
49
|
Reif AE: Effect of cigarette smoking on
susceptibility to lung cancer. Oncology. 38:76–85. 1981. View Article : Google Scholar : PubMed/NCBI
|