Introduction
Osteosarcoma is the most common malignant bone
tumor, accounting for 20% of all bone tumors (1,2). Current
therapy involves surgical removal of the malignant lesion, in
association with chemotherapy. Survival rates of 60–80% are
obtainable for cases of osteosarcoma without metastasis (3). In recent years, there have been advances
in gene therapy for osteosarcoma, including immune gene therapy,
antisense gene therapy and suicide gene therapy (4–6). Cancer
gene therapy is a novel treatment approach, and it is a revolution
in cancer treatment. Studies have focused on the involvement of
certain growth factors, which may affect the development of
osteosarcoma. These factors may be of use in the development of
novel medications for the treatment of this disease.
The insulin-like growth factor I (IGF-I) gene
generates three mRNA isoforms during transcription, including IGF-I
Ea, IGF-I Eb and IGF-I Ec. IGF-I Eb in rodents, and IGF-I Ec in
humans, are also termed mechano-growth factor (MGF) (7–9). MGF has
been widely studied in biological and medical fields. It is
established as a stimulator of myoblast and osteoblast
proliferation, and protects neuronal and cardiomyocyte apoptosis,
inhibits osteoblast differentiation and mineralization, and
stimulates mesenchymal stem cell proliferation and migration
(10–13). Furthermore, MGF expression has been
demonstrated to be associated with different diseases, including
those affecting tissue repair and regeneration, and cancer
(14). Compared with healthy tissues,
MGF has been indicated to be overexpressed in neuroblastoma,
prostate cancer and osteosarcoma (15–17).
There is a unique E domain in the C-terminal of MGF
that distinguishes MGF from other IGF-I isoforms in terms of its
peptide sequence and function (12,18,19). The
present study aimed to measure the expression of MGF mRNA in Hos,
MHos and MG-63 cells, and to investigate the actions of the MGF-E
peptide in human MG-63 cells in vitro. It has previously
been demonstrated that cyclinD1 is required for G1/S transition in
cell proliferation (20), caspase-3
is essential for apoptosis (21), and
VEGF is the best characterized regulator of angiogenesis (22). High expression levels of CD147 and
MMP-9 are positively correlated with invasion and metastasis of
various cancers, such as triple-negative breast cancer and
laryngeal carcinoma (23,24). The expression levels of these proteins
in MG-63 cell after MGF-E treatment are detected. The results
indicated that exogenous MGF-E peptide is involved in the
regulation of cell cycle distribution, in addition to the
proliferation, migration and invasion of MG-63 cells. This
indicates that MGF may be a suitable biomarker gene of malignant
osteosarcoma.
Materials and methods
Cell lines and culture
The human osteosarcoma cell lines Hos, MHos and
MG-63 were purchased from CCTCC (Shanghai, China) and all cultured
in MEM medium (GE Healthcare Life Sciences, Logan, UT, USA) with
10% fetal bovine serum (FBS, Merck Millipore, USA) and 1%
Penicillin-Streptomycin (Solarbio, Beijing, China). Cells were
incubated at 37°C in 5% CO2. For cell seeding, the cells
were washed with phosphate-buffered saline (PBS) and digested with
0.25% Trypsin-EDTA (Solarbio, Beijing, China). The MHos and MG-63
cell lines are more malignant than Hos (25).
Cell counting kit-8 (CCK-8) assay for
measurement of MG-63 cell proliferation
The study was approved by the ethics committee of
Department of Orthopedics, Xinqiao Hospital (Chongqing, China).
MG-63 human osteosarcoma cell proliferation activity was assessed
by direct cell counting subsequent to cell seeding. Briefly, MG-63
cells were cultured at a density of 2×103 cells/well in
96-well plates (200 µl/well) and incubated at 37°C for 24 h. Cells
were then exposed to conditioned medium (containing 0, 10, 20, 50
or 100 ng/ml MGF-E; Catalog no. 033–42; Phoenix Pharmaceuticals,
Burlingame, CA, USA) for 0, 24 or 48 h. Cell proliferation was
evaluated using the CCK-8 assay (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
instructions. CCK-8 solution (20 µl) was added to each well of the
96-well plate. Following incubation at 37°C for 2 h, the plates
were analyzed using an ELISA reader at 450 nm. Data are presented
as the mean ± standard deviation from 5 independent
experiments.
Cell cycle assay
Conditioned cultured MG-63 cells were washed with
phosphate-buffered saline (PBS) and digested with 0.25%
Trypsin-EDTA solution (Solarbio). Test cells were immobilized with
75% alcohol and stained with propidium iodide (PI; Sigma-Aldrich
Chemie GmbH, Munich, Germany). A FACSCalibur Flow Cytometry System
(BD Biosciences, Franklin Lakes, NJ, USA) was used for single-cell
analysis.
Scarification test
MG-63 cells were seeded in 6-well plates at a
density of 2×105 cells/well. Following culture for 24 h,
wounds were created in the cell monolayer using a pipette tip. Dead
cells were removed using 0.1 mM PBS. Cells were treated with MGF-E
peptide at various concentrations (0, 10, 20, 50 and 100 ng/ml) and
all the groups were treated with serum-free medium for 24 h. Images
were captured at 0 and 24 h. The migration distance was using
Photoshop version 3.0 (Abode Systems, Inc., San Jose, CA, USA).
Transwell chamber assay
MG-63 cells were seeded at a density of
2×105 cells/well into the top chamber of Transwell-COL
co-culture systems (8.0 µm pore size; Costar, Corning, Shanghai,
China) were starved in serum-free minimum essential medium (MEM)
for 24 h. Cells were then treated with MGF-E peptide at various
concentrations (0, 10, 20, 50 and 100 ng/ml) in 200 µl serum-free
MEM (GE Healthcare Life Sciences). MEM (500 µl) containing 20% FBS
was added to the bottom chamber. The Transwell plates were
incubated at 37°C for 24 h. The upper chamber was removed, and
cells on the upper chamber surface of the basement membrane were
removed using cotton swabs. Cells that had invaded the lower
chamber surface of the basement membrane were stained with crystal
violet (Solarbio). The number of migrated cells in the bottom
chamber was quantified using an Inverse Fluorescent IX73 Microscope
with a micropublisher 5.0 RTV (Olympus Corporation, Tokyo,
Japan).
RNA isolation
Total RNA was isolated from the conditioned cultured
cells using a High Pure Viral RNA kit (Bioteke Corporation,
Beijing, China). The integrity of RNA was determined by
electrophoresis at 100 V on a 1.5% agarose gel (Beyotime Institute
of Biotechnology, Beijing, China) with Goldview (Solarbio). RNA
quality and quantity were determined using a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated from the conditioned cultured
Hos, MHos and MG-63 cells. Then the RNAs were transcribed to cDNAs
use the PrimeScript RT reagent Kit with gDNA Eraser (Cat#RR047A,
Takara Biotechnology Co., Ltd., Dalian, China)). The expressions of
genes associated with proliferation, migration and invasion were
measured by qPCR with a StepOne Plus thermocycler (Bio-Rad
Laboratories, Hercules, CA, USA). A cDNA template (2 µl) and 12.5
µl 2X SYBR Premix ExTaq II (Takara Biotechnology Co., Ltd.) were
added, to obtain a final volume of 25 µl. The thermal cycles were
performed at 95°C for 30 sec, 40 cycles at 95°C for 5 sec, and 60°C
for 30 sec. The primer sequences were as follows: MGF, F
5′-GCCCCCATCTACCAACAAGAACAC-3′ and R 5′-CGGTGGCATGTCACTCTTCACTC-3′;
GADPH, F 5′-CCTCCTGCACCACCAACTGCTT-3′ and R
5′-GAGGGGCCATCCACAGTCTTCT-3′. Sequences of differentially expressed
genes were obtained from GenBank (http://www.ncbi.nlm.nih.gov/Genbank/index.html).
Primers were designed by Primer 3.0 (http://frodo.wi.mit.edu).
Western blotting
Cells were lysed in radioimmunoprecipitation buffer
(Beyotime Institute of Biotechnology) with protein inhibitor,
phenylmethylsulfonyl fluoride (CWBIO, Beijing, China), to obtain a
final concentration of 1 mM. Samples were maintained on ice for 30
min, then centrifuged at 14,000 × g for 3–5 min at 4°C, and the
supernatant was collected. The concentrations of cyclin D1, CD147,
matrix metalloproteinase 9 (MMP-9) and vascular endothelial growth
factor (VEGF) were detected using an Enhanced BCA Assay kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Proteins were resolved by SDS-PAGE in
a 10% polyacrylamide gel and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes
were blocked in Tris-buffered saline with 0.1% Tween-20 and 5%
non-fat milk (Solarbio) for 1 h at room temperature. The membranes
were immunoblotted with mouse monoclonal anti-human MMP-9 (2C3)
(Cat No. sc-21733), mouse monoclonal anti-human EMMPRIN (F-5) (Cat.
No. sc-374101), mouse monoclonal anti-human cyclin D1 (HD11) (Cat.
No. sc-246), mouse monoclonal anti-human VEGF (JH121) (Cat. No.
sc-57496), mouse monoclonal anti-human caspase-3 (31A1067) (Cat.
No. sc-56053), mouse monoclonal anti-human Actin (C-2) (Cat No.
sc-8432) (1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
overnight at 4°C, and HRP-labeled Goat Anti-Mouse IgG (H+L)(Cat
No.AB503-01A, Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China)for 2 h at RT. Immunoreactive proteins were
visualized using BeyoECL Plus chemiluminescent detection (Beyotime
Institute of Biotechnology) and the band intensity relative to
actin was acquired using Quantity One software version 4.2 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The results were analyzed using SPSS software,
version 19.0 (IBM SPSS, Armonk, NY, USA). Student's t-test was
conducted, and the results are presented as the mean ± standard
error. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of MGF in the Hos, MHos and
MG-63 cell lines
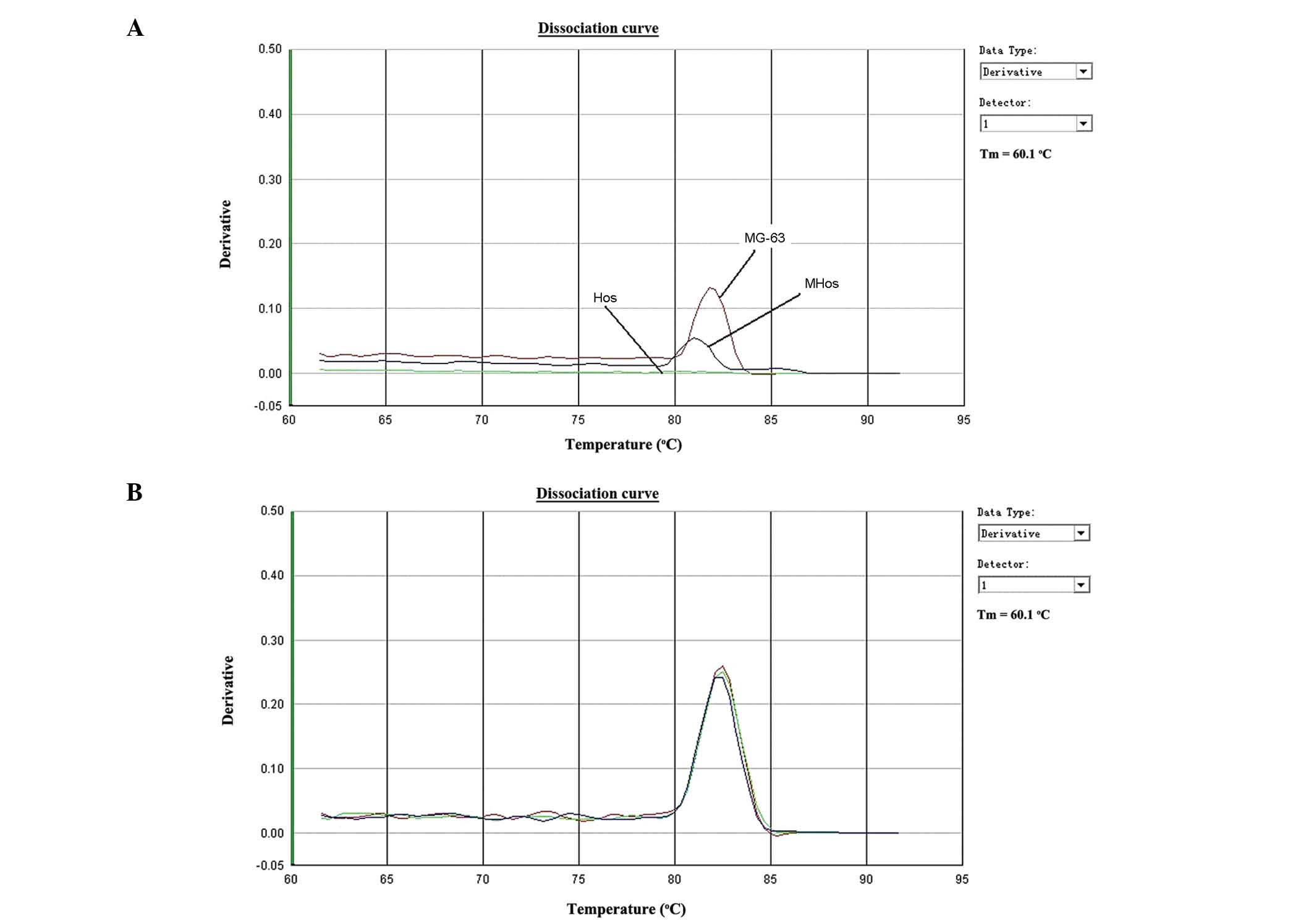
qPCR analysis demonstrated that MHos and MG-63 cells
expressed MGF, while Hos cells did not (Fig. 1A). As presented in Fig. 1, MGF was differentially expressed in
osteosarcoma cells. Normalization was conducted using GAPDH, and
its expression is presented in Fig.
1B. Furthermore, MGF expression was higher in MG-63 cells than
that in MHos cells, indicating that the expression of MGF is
associated with the degree of malignancy in osteosarcoma.
Effect of the MGF-E peptide on the
proliferation capacity of MG-63 cells
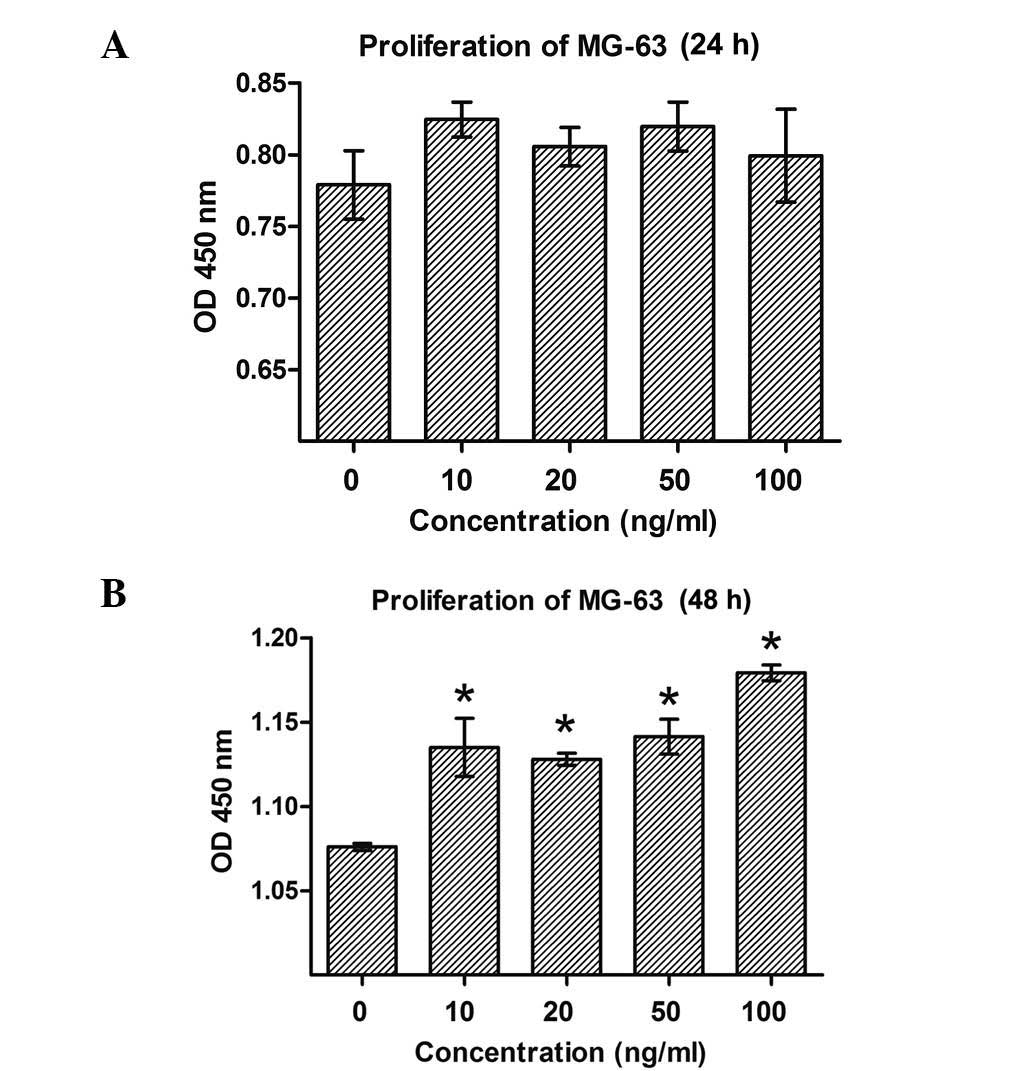
Cell proliferation was evaluated in MG-63 cells
treated with various concentrations of MGF-E peptide (10, 20, 50 or
100 ng/ml), as presented in Fig. 2.
Following treatment for 48 h, significant increases in the number
of MG-63 cells were observed at all concentrations of MGF-E ≥10
ng/ml (P<0.05 compared with 0 ng/ml; Fig. 2A). However, no effect on the
proliferation of MG-63 cells was detected at 24 h (Fig. 2B). These results demonstrated that the
MGF-E peptide may promote the proliferation of osteosarcoma
cells.
Cell cycle distribution and
proliferation index of MG-63 cells in response to MGF-E peptide
administration
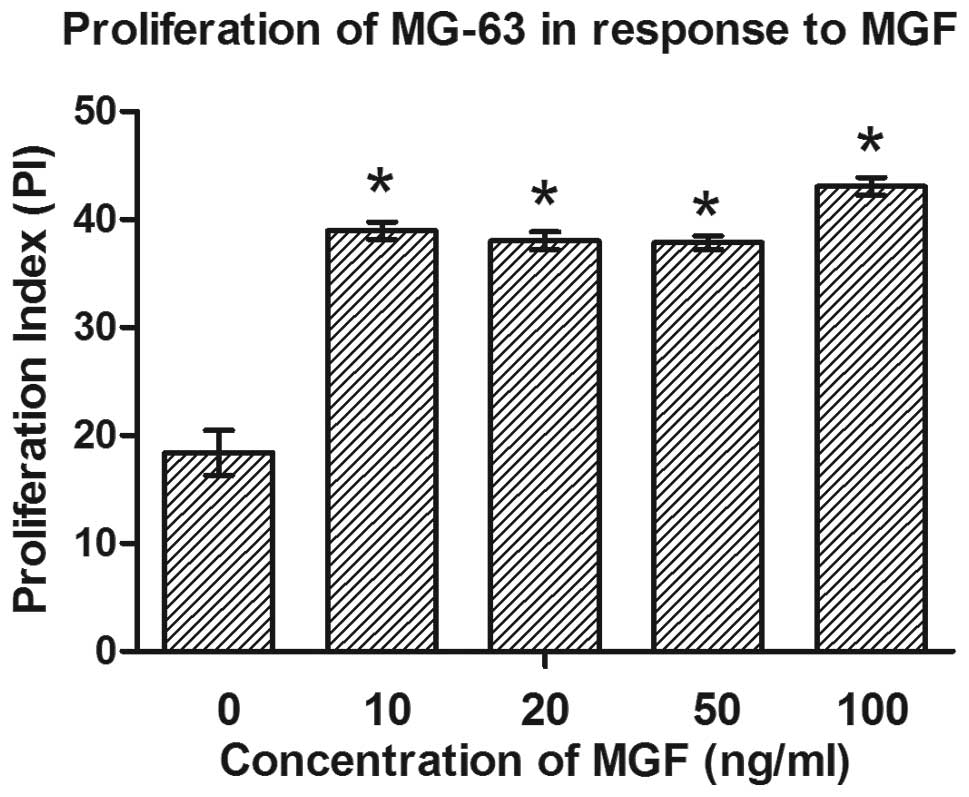
In order to confirm whether the proliferation of
MG-63 cells in response to MGF-E peptide was due to arrest at a
certain cell cycle phase, flow cytometry and PI staining were used
to detect DNA content and the proportion of cells in different
phases of the cell cycle. MGF-E significantly altered cell cycle
distribution in the cells, resulting in increased accumulation of
cells in the G2/M + S phases (from 18.395% in the
control to 43.075% in the 100 ng/ml group; P≤0.05; Table I). Each concentration of MGF used in
the present study exerted an effect on cell cycle progression,
compared with the control group. Cell proliferation activity was
also indicated by the proliferation index (Fig. 3). Following treatment with MGF-E,
proliferation index increased significantly compared with the
control (P<0.05). These results demonstrated that the
pro-proliferation effect of the MGF-E peptide is mediated via an
effect on cell cycle progression in MG-63 cells.
 | Table I.Cell cycle phase and proliferation
index of MG-63 cells exposed to MGF-E for 24 h. |
Table I.
Cell cycle phase and proliferation
index of MG-63 cells exposed to MGF-E for 24 h.
|
| Cell cycle phase (%
cells) |
|
|---|
|
|
|
|
|---|
| Sample |
G1/G0 | G2/M +
S | Proliferation index
(%) |
|---|
| Control |
81.605±2.934 |
18.395±2.934 |
18.395±2.934 |
| 10 ng/ml |
61.065±1.039a |
38.960±1.131a |
38.960±1.131a |
| 20 ng/ml |
62.020±1.160a |
38.005±1.181a |
38.005±1.181a |
| 50 ng/ml |
62.140±0.905a |
37.840±0.877a |
37.840±0.877a |
| 100 ng/ml |
56.895±1.138a |
43.075±1.181a |
43.075±1.181a |
Migration of MG-63 cells in response
to the MGF-E peptide
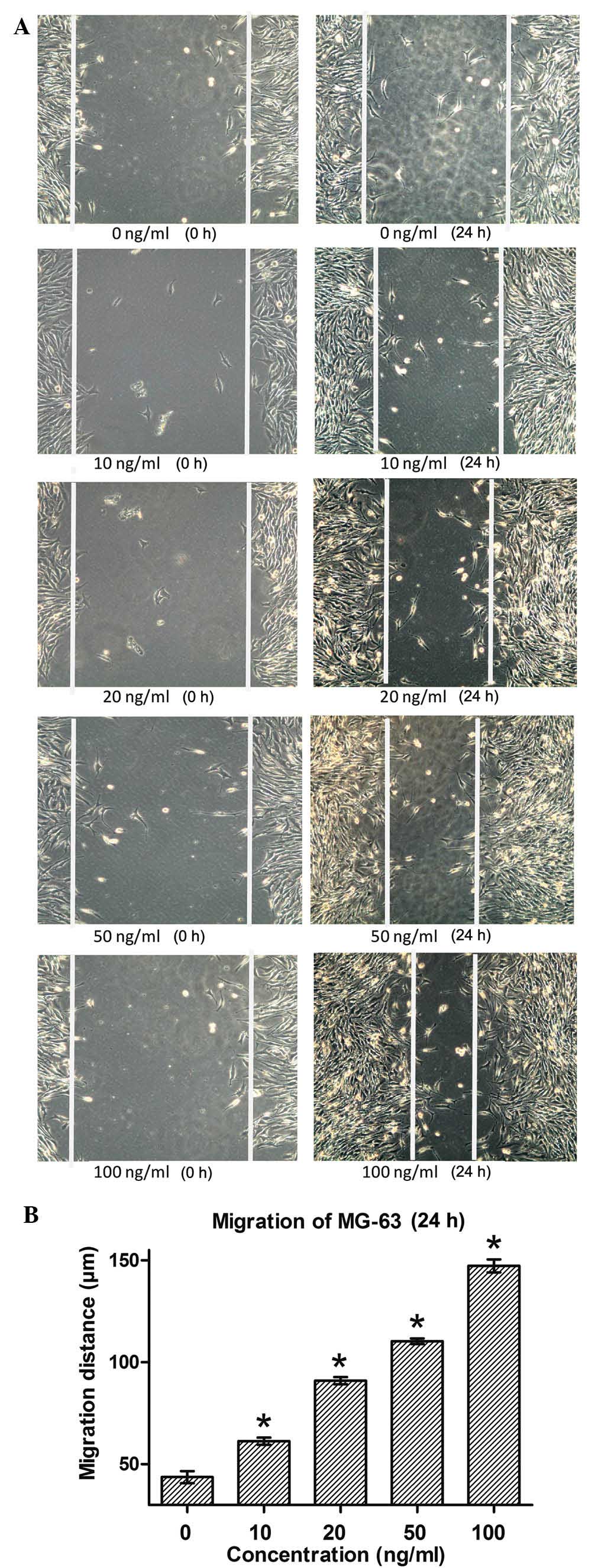
In order to investigate the function of MGF in
osteosarcoma cells, the migration of MG-63 cells in response to
treatment with various concentrations of the MGF-E peptide (0, 10,
20, 50 or 100 ng/ml) was observed. At 24 h after wounds were made,
the migration distances of MG-63 cells were significantly increased
in all MGF-E treatment groups compared with that in the control
group (P<0.05; Fig. 4).
Furthermore, with increasing concentrations of MGF-E, the effect
was more marked. These results demonstrated that MGF-E effectively
promoted the migration of MG-63 cells.
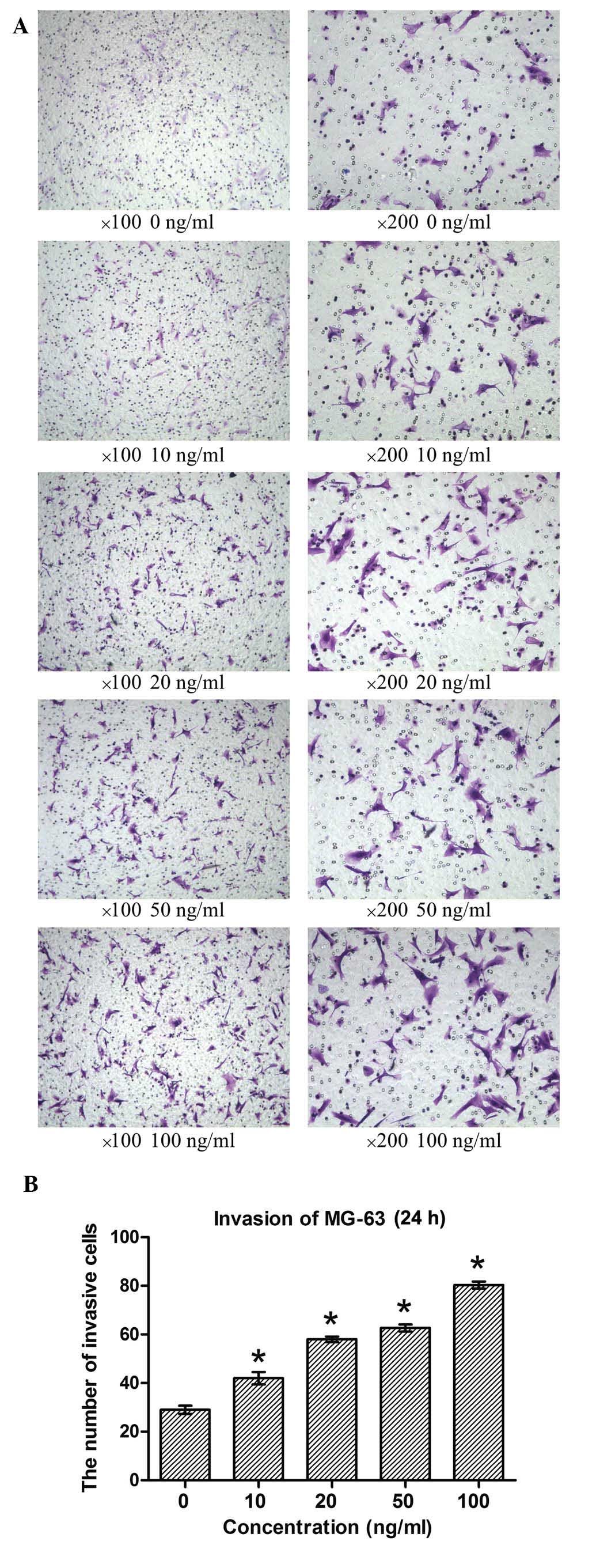
Invasion of MG-63 in response to the
MGF-E peptide
Cell invasion was calculated from the number of
cells observed to have passed through the 8-µm pore of the
polycarbonate membrane coated with collagen, which separated the
upper and lower chambers. Crystal violet staining demonstrated that
the number of MG-63 cells that crossed the basement membrane of the
Transwell system was significantly increased in response to
treatment with MGF-E (P<0.05; Fig.
5). This effect occurred in a dose-dependent manner. This
suggested that MGF is involved in inducing the invasion of MG-63
cells, and that this effect is associated with the concentration of
the MGF-E peptide.
Effects of MGF on the expression of
key proteins in MG-63 cells
Western blotting analysis demonstrated that the
expression levels of cyclin D1, CD147, matrix metalloproteinase 9
(MMP-9) and vascular endothelial growth factor (VEGF) were all
significantly increased in response to treatment with the MGF-E
peptide (20 and 50 ng/ml) compared with that in the control group
(0 ng/ml). The expression of caspase 3 was significantly reduced in
response to treatment with the MGF-E peptide (50 ng/ml) compared
with that in the control group (0 ng/ml). These results suggested
that increased MGF expression may upregulate cyclin D1, CD147 and
MMP-9 expression, and suppress that of caspase 3. The effects of
MGF-E on cell cycle distribution and proliferation in osteosarcoma
cells may be due, at least in part, to the upregulation of cyclin
D1 expression. Furthermore, MGF-E upregulated CD147 and MMP-9
expression, which may be responsible for the increased migration
and invasion following treatment with MGF-E.
Discussion
IGF-I is one of the most abundant proteins in bone
and is involved in the process of bone formation (26). In addition, IGFs are associated with
the development of cancer and are involved in events required for
metastasis, including the promotion of cell transformation,
angiogenesis, proliferation and infiltration, and the inhibition of
apoptosis (27,28). MGF is an alternative splicing variant
of IGF-I, which exerts similar effects to IGF-I in numerous
respects, including satellite cell activation, aging and
neuroprotection (29). However, there
have been relatively few studies into the association between MGF
expression and carcinogenesis.
Armakolas et al (16) intitally demosntrated that the IGF-I
gene transcript, MGF, was specifically expressed in PC-3 and LNCaP
prostate cancer cells. However, under the same experimental
conditions, HPrEC normal human prostate epithelial cells did not
express MGF isoforms. Subsequently, Philippou et al
(17) demonstrated that MGF was
expressed in MG-63 osteosarcoma cells. The present study also
indicated that MGF was specifically expressed in malignant MG-63
and MHos cells. This may be an indication that the expression of
MGF is associated with the degree of malignancy of osteosarcoma
cells.
It has been documented that a synthetic MGF-E
peptide, which comprises the final 24 amino acids of the
translation product of the E domain of MGF, can stimulate the
proliferation of prostate and osteosarcoma cancer cells (16,17). The
same proliferative effects of the MGF-E peptide were confirmed in
the current study. To the best of our knowledge, this is the first
study to interpret the effects of MGF on migration and invasion in
cancer cells, in addition to examining the possible molecular
mechanisms underlying its effects on the promotion of
proliferation, migration and invasion.
The present results suggested that MGF significantly
promotes the proliferation, migration and invasion of osteosarcoma
cells. In addition, the promotion of proliferation by MGF was
demonstrated to be a result of an increase in DNA synthesis and
mitosis. During this process, the expression of cyclin D1 was
increased after treatment with MGF-E for 48 h, which indicated that
cell cycle arrest may be due to the upregulation of cyclin D1
expression. The invasion and metastasis-associated molecular
pathways which MGF may affect were also investigated. A previous
study indicated that MMP-9 expression is closely associated with
tumor cell invasion and metastasis (30). However, to the best of our knowledge,
there have been no studies demonstrating that CD147 and MMP-9 are
regulated by MGF. Therefore, the present study sought to determine
the effects of MGF-E on CD147 and MMP-9 expression in MG-63 cells.
The expression levels of CD147 and MMP-9 were significantly higher
following treatment with MGF-E than that in the control cells. This
suggests that MGF may promote MG-63 cell invasion by increasing the
expression of CD147 and MMP-9.
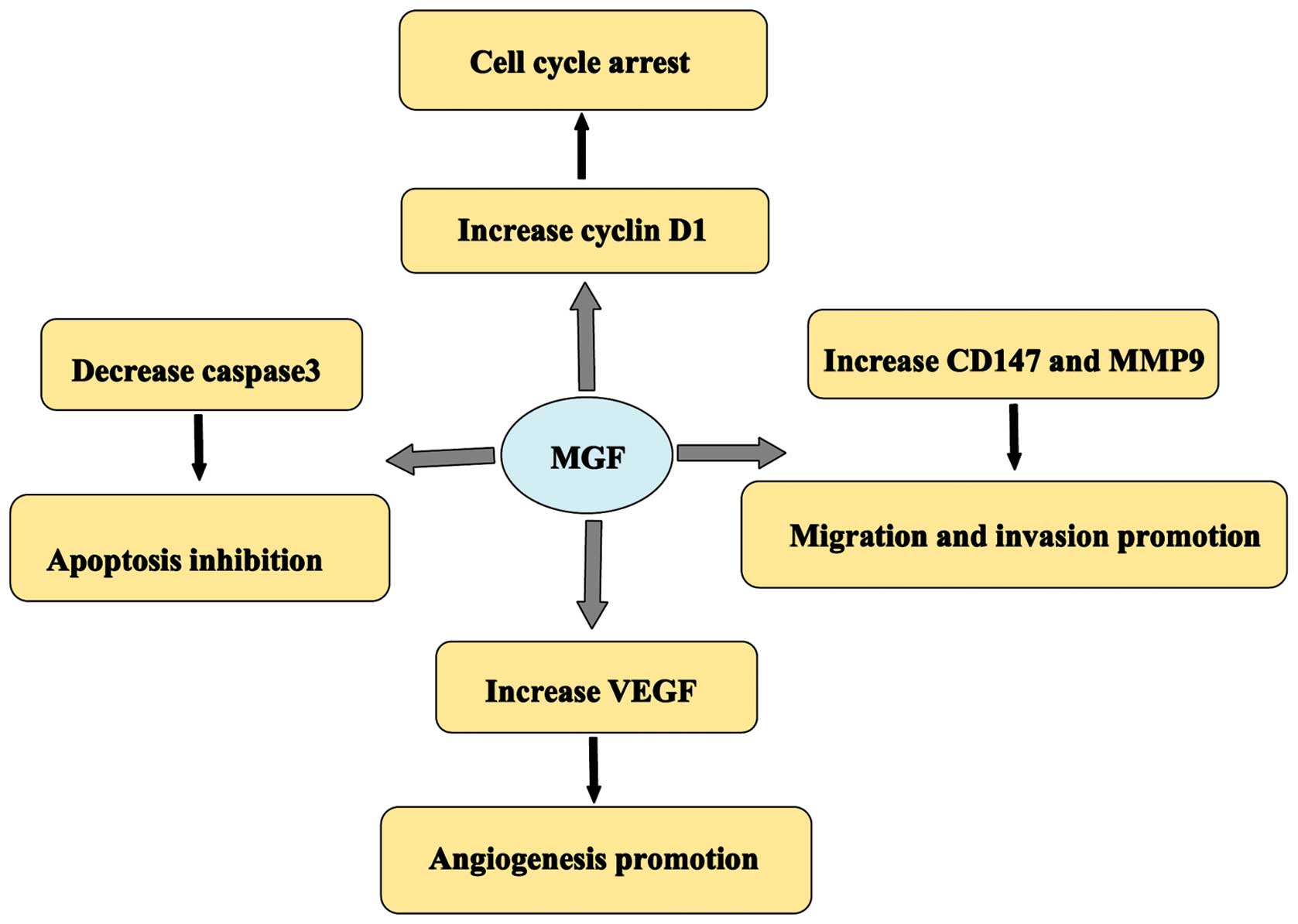
The effects of MGF in osteosarcoma are hypothesized
to act via a number of possible molecular mechanisms (Fig. 7). The upregulation of MGF in the
current study led to cell cycle arrest. Furthermore, cyclin D1
expression was increased in response to treatment with MGF. The
results suggested that MGF may regulate progression through the
cell cycle, by increasing the expression of cyclin D1, which is
required for G1/S transition. CD147 and MMP-9 are
involved in the invasion and metastasis of numerous types of human
malignancy (31,32). The present study demonstrated that MGF
may promote the expression of CD147 and MMP-9, and that this may
underlie the promotion of cell migration and invasion by MGF.
Furthermore, MGF influenced apoptosis and angiogenesis in
osteosarcoma cells by regulating the expression of caspase-3 and
VEGF.
The pathogenesis of cancer is a complicated process,
in which different factors are involved at each stage. However,
cancer cells share certain characteristics, including uncontrolled
growth and the capacity to invade surrounding tissues or to
metastasize to distant tissues. Therefore, the identification of
universal biomarkers, independent of cancer-type is important.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of China (grant nos. 81271982 and 81071498).
References
|
1
|
Marulanda GA, Henderson ER, Johnson DA,
Letson GD and Cheong D: Orthopedic surgery options for the
treatment of primary osteosarcoma. Cancer Control. 15:13–20.
2008.PubMed/NCBI
|
|
2
|
Vander Griend RA: Osteosarcoma and its
variants. Orthop Clin North Am. 27:575–581. 1996.PubMed/NCBI
|
|
3
|
Kim SY and Helman LJ: Strategies to
explore new approaches in the investigation and treatment of
osteosarcoma. Cancer Treat Res. 152:517–528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nardin A, Lefebvre ML, Labroquère K, Faure
O and Abastado JP: Liposomal muramyl tripeptide
phosphatidylethanolamine: Targeting and activating macrophages for
adjuvant treatment of osteosarcoma. Curr Cancer Drug Targets.
6:123–133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie XK, Yang DS, Ye ZM and Tao HM:
Enhancement effect of adenovirus-mediated antisense c-myc and
caffeine on the cytotoxicity of cisplatin in osteosarcoma cell
lines. Chemotherapy. 55:433–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oosterhoff D, Witlox MA, van Beusechem VW,
Haisma HJ, Schaap GR, Bras J, Kruyt FA, Molenaar B, Boven E,
Wuisman PI, et al: Gene-directed enzyme prodrug therapy for
osteosarcoma: Sensitization to CPT-11 in vitro and in vivo by
adenoviral delivery of a gene encoding secreted carboxylesterase-2.
Mol Cancer Ther. 2:765–771. 2003.PubMed/NCBI
|
|
7
|
Shavlakadze T, Winn N, Rosenthal N and
Grounds MD: Reconciling data from transgenic mice that overexpress
IGF-I specifically in skeletal muscle. Growth Horm IGF Res.
15:4–18. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang S, Alnaqeeb M, Simpson H and
Goldspink G: Cloning and characterization of an IGF-1 isoform
expressed in skeletal muscle subjected to stretch. J Muscle Res
Cell Motil. 17:487–495. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldspink G: Mechanical signals, IGF-I
gene splicing, and muscle adaptation. Physiology (Bethesda).
20:232–238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carro E, Trejo JL, Núñez A and
Torres-Aleman I: Brain repair and neuroprotection by serum
insulin-like growth factor I. Mol Neurobiol. 27:153–162. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang LL, Xian CY and Wang YL: The MGF
expression of osteoblasts in response to mechanical overload. Arch
Oral Biol. 51:1080–1085. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mills P, Lafrenière JF, Benabdallah BF, El
Fahime M and Tremblay JP: A new pro-migratory activity on human
myogenic precursor cells for a synthetic peptide within the E
domain of the mechano growth factor. Exp Cell Res. 313:527–537.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collins JM, Goldspink PH and Russell B:
Migration and proliferation of human mesenchymal stem cells is
stimulated by different regions of the mechano-growth factor
prohormone. J Mol Cell Cardiol. 49:1042–1045. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matheny RW Jr, Nindl BC and Adamo ML:
Minireview: Mechano-growth factor: A putative product of IGF-I gene
expression involved in tissue repair and regeneration.
Endocrinology. 151:865–875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuo YH and Chen TT: Novel activities of
pro-IGF-I E peptides: Regulation of morphological differentiation
and anchorage-independent growth in human neuroblastoma cells. Exp
Cell Res. 280:75–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Armakolas A, Philippou A, Panteleakou Z,
Nezos A, Sourla A, Petraki C and Koutsilieris M: Preferential
expression of IGF-1Ec (MGF) transcript in cancerous tissues of
human prostate: Evidence for a novel and autonomous growth factor
activity of MGF E peptide in human prostate cancer cells. Prostate.
70:1233–1242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Philippou A, Armakolas A, Panteleakou Z,
Pissimissis N, Nezos A, Theos A, Kaparelou M, Armakolas N,
Pneumaticos SG and Koutsilieris M: IGF1Ec expression in MG-63 human
osteoblast-like osteosarcoma cells. Anticancer Res. 31:4259–4265.
2011.PubMed/NCBI
|
|
18
|
Ates K, Yang SY, Orrell RW, Sinanan AC,
Simons P, Solomon A, Beech S, Goldspink G and Lewis MP: The IGF-I
splice variant MGF increases progenitor cells in ALS, dystrophic,
and normal muscle. FEBS Lett. 581:2727–2732. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quesada A, Micevych P and Handforth A:
C-terminal mechano growth factor protects dopamine neurons: A novel
peptide that induces heme oxygenase-1. Exp Neurol. 220:255–266.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Li Z, Fu M, Bouras T and Pestell
RG: Signal transduction mediated by cyclin D1: From mitogens to
cell proliferation: A molecular target with therapeutic potential.
Cancer Treat Res. 119:217–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harry LE and Paleolog EM: From the cradle
to the clinic: VEGF in developmental, physiological, and
pathological angiogenesis. Birth Defects Res C Embryo Today.
69:363–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao S, Ma W, Zhang M, Tang D, Shi Q, Xu
S, Zhang X, Liu Y, Song Y, Liu L and Zhang Q: High expression of
CD147 and MMP-9 is correlated with poor prognosis of
triple-negative breast cancer (TNBC) patients. Med Oncol.
30:3352013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gou X, Chen H, Jin F, Wu W, Li Y, Long J,
Gong X, Luo M, Bi T, Li Z and He Q: Expressions of CD147, MMP-2 and
MMP-9 in laryngeal carcinoma and its correlation with poor
prognosis. Pathol Oncol Res. 20:475–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Daft PG, Yuan K, Warram JM, et al:
Alpha-CaMKII plays a critical role in determining the aggressive
behavior of human osteosarcoma. Mol Cancer Res. 11:349–359. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Crane JL and Cao X: Function of matrix
IGF-1 in coupling bone resorption and formation. J Mol Med.
92:107–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Durai R, Davies M, Yang W, Yang SY,
Seifalian A, Goldspink G and Winslet M: Biology of insulin-like
growth factor binding protein-4 and its role in cancer (review).
Int J Oncol. 28:1317–1325. 2006.PubMed/NCBI
|
|
28
|
van Golen CM, Schwab TS, Kim B, Soules ME,
Su Oh S, Fung K, van Golen KL and Feldman EL: Insulin-like growth
factor-I receptor expression regulates neuroblastoma metastasis to
bone. Cancer Res. 66:6570–6578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matheny RW Jr, Nindl BC and Adamo ML:
Minireview: Mechano-growth factor: A putative product of IGF-I gene
expression involved in tissue repair and regeneration.
Endocrinology. 151:865–875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang ZY, Liu Y, Liu LX, Ding XY, Zhang H
and Fang LQ: RNAi-mediated MMP-9 silencing inhibits mouse melanoma
cell invasion and migration in vitro and in vivo. Cell Biol Int.
37:849–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Z, Ren Y, Wu QC, Lin SX, Liang YJ and
Liang HZ: Macrophage migration inhibitory factor enhances
neoplastic cell invasion by inducing the expression of matrix
metalloproteinase 9 and interleukin-8 in nasopharyngeal carcinoma
cell lines. Chin Med J (Engl). 117:107–114. 2004.PubMed/NCBI
|
|
32
|
Yu W, Liu J, Xiong X, Ai Y and Wang H:
Expression of MMP9 and CD147 in invasive squamous cell carcinoma of
the uterine cervix and their implication. Pathol Res Pract.
205:709–715. 2009. View Article : Google Scholar : PubMed/NCBI
|