Introduction
Pancreatic cancer (PC) is among the most lethal
malignancies, with a high incidence and rate of metastasis
(1). At the time of diagnosis, the
majority of patients are at an advanced stage of disease, with
multi-organ metastasis, which indicates a poor prognosis for
digestive system tumors (2). It is
challenging to diagnose PC at an early stage, which results in a
low 5-year survival rate; only 4% of patients diagnosed with
pancreatic cancer survive after 5 years in China (3). Therefore, the identification of novel
gene targets, which are differentially expressed in PC and
functionally involved in the development of malignant phenotypes,
is required in order to enable early diagnosis and the development
of effective therapeutic strategies.
Hypoxia is an important characteristic of solid
tumors. As a tumor increases in size, it quickly outgrows its blood
supply, leaving regions of the tumor, in which the oxygen
concentration is significantly lower than that in normal tissues.
In order to support tumor growth and proliferation within hypoxic
environments, the expression of a number of genes is altered, and
changes in metabolism also occur (4).
Recently, a novel class of endogenous small non-coding regulatory
RNAs, termed microRNAs (miRNAs), has received increasing attention.
These small molecules exert their regulatory effects by base
pairing with partially complementary messenger RNAs (mRNAs). They
act via one of two mechanisms: Degradation of target mRNA or
inhibition of its translation. It has been shown that miRNAs are
involved in the development of cancer via alteration of the
expression of oncogenes or tumor suppressor genes. Increasing
evidence, accumulated using microarray technology, has indicated a
number of miRNAs that are differentially expressed in response to
hypoxia. For example, miRNA-210, -155, -372/373 and -10b were shown
to be upregulated, whereas miR-20b and -200b were found to be
downregulated in response to hypoxia (5–8). A recent
study suggested that miRNA-150, a hypoxia-sensitive miRNA, is
involved in cancer metastasis via regulation of its target genes
(9).
The present study investigated the role of hypoxia
in the regulation of miRNA-150 and the expression of its target
gene, C-X-C chemokine receptor type 4 (CXCR4), in primary PC
tissues and PC cells. The findings demonstrated that hypoxia
downregulates the expression of miRNA-150 in PC cells. Furthermore,
it was shown that miRNA-150 directly targets the 3′ untranslated
region (UTR) of CXCR4 mRNA, thereby suppressing its expression.
Downregulation of miRNA-150 by hypoxia also led to a concomitant
increase in CXCR4 expression. The present findings also
demonstrated that miRNA-150 overexpression leads to increased
migration and invasion of hypoxia cultured PC cells. In conclusion,
the present study suggests a novel mechanism underlying the
hypoxia-induced promotion of tumor invasion.
Materials and methods
Clinical samples and cell line
A total of 15 pancreatic tissue samples were
obtained from patients who had undergone pancreatoduodenectomy at
Urumqi General Hospital (Urumqi, China) between 2008 and 2012. None
of these patients received radiotherapy or chemotherapy prior
surgery. Three parts of pancreatic tissues from each of 15 patients
with PC were collected: Tumor tissues, adjacent non-tumor
pancreatic tissues within 2 cm, and tumor free tissues 5 cm
distance from the tumor edge. All PC and normal pancreatic tissue
samples were histologically confirmed. Tissues were snap frozen in
liquid nitrogen following surgical resection, prior to use. Written
informed consent conforming to the tenets of the Declaration of
Helsinki, was obtained from each participant prior to the study.
The institutional review boards of the Chinese PLA Urumqi General
Hospital Ethics Committee (Urumqi, China) approved the current
study.
The CaPan2 human pancreatic cancer cell line was
obtained from the State Key Laboratory of Cancer Biology at Fourth
Military Medical University (Xi'an, China). The cell line was
maintained in RPMI-1640, supplemented with 10% FBS. For the
induction of hypoxia, cells were incubated in
temperature-controlled hypoxic culture chambers with 1%
O2, 5% CO2 and 94% N2. Hypoxia
induced cells were collected following incubation for 0, 8, 12 and
24 h respectively, and the miRNA-150 expression was detected at
these different time points.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA, including miRNA, was extracted using a
mirVanamiR isolation kit (Life Technology, USA), according
to the manufacturer's instructions. Following extraction, all RNAs
were treated with DNase in order to remove genomic DNA. The first
strand cDNA was synthesized using RT2miRNA First Strand
kit (Qiagen China, Shanghai, China) and specific miRNA-150 primers
(Qiagen China) were used for RT-qPCR. The sequence of the primers
were as follows: F 5′-TCT CCC AAC CCT TGT ACC-3′ and R 5′-CGA GGA
AGA AGA CGG AAG AAT-3′. The PCR cycling conditions used were: 35
cycles of 2 sec at 92°C and 10 s at 70°C. The expression of
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an
internal control, by which to calculate relative target gene
expression levels. Relative expression was calculated using the
comparative Ct method (2−ΔΔCt) (10,11). All
PCR reactions were performed in triplicate.
miRNA-150 mimic or inhibitor
transfection
The miRNA-150 mimic (miRNA-150-agomir) and inhibitor
(miRNA-150-antagomir) were obtained from GenePharma Company
(Shanghai, China). The day prior to transfection, CaPan2 cells were
seeded in antibiotic-free medium. Transfections were conducted
using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer's instructions. In order to
monitor transfection efficiency, fluorescein (FAM) siRNA
(GenePharma Company) was used as a control. Successfully
transfected cells were observed using a fluorescence microscope
(BX51, Olympus Corporation, Tokyo, Japan).
Western blot analysis
CaPan2 cells or tissue samples were lysed in lysis
buffer [50 mmol/l Tris (pH 7.5), 100 mmol/l NaCl, 1 mmol/l EDTA,
0.5% NP-40, 0.5% Triton X-100, 2.5 mmol/l sodium orthovanadate, 10
µl/ml protease inhibitor cocktail and 1 mmol/l PMSF] by incubating
for 20 min at 4°C. Whole cell protein extracts were quantified
using a bicinchoninic acid assay (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Total proteins were concentrated and separated
using 10% sodium dodecyl sulphate polyacrylamide gel
electrophoresis. The proteins were transferred to polyvinylidene
difluoride membranes (AmershamBiosciences, Pittsburgh, PA, USA) and
sequentially incubated with rabbit anti-human antibody to CXCR4
(cat. no. ab1047, 1:500; Abcam, Cambridge, MA, USA) for overnight
at 4°C. Following this incubation the membrane was rinsed and
incubated in a horseradish peroxidase (HRP)-conjugated mouse
anti-rabbit monoclonal antibody (cat. no. ZB5301, 1:1,000;
Zhongshan Goldenbridge, Ltd., China) for 1 h at room temperature. A
HRP-conjugated rabbit anti-GAPDH polyclonal antibody (cat no.
sc-25778 HRP, 1:1,000; SantaCruz Biotechnology, Dallas TX, USA) was
used for the analysis of protein loading. Bands were developed
using enhanced chemiluminescence western blotting detection
reagents (Thermo Fisher, Rockford, IL, USA).
Migration assay
Cell invasion was analyzed with Matrigel-coated
Transwell™ cell culture chambers (8-µm pore size; Millipore,
Billerica, MA, USA). Briefly, differently treated cells
(5×104 cells/well) were serum starved for 24 h at 37°C
and plated in the upper insert of a 24-well chamber in a serum-free
medium. A medium containing 10% serum as a chemoattractant was
added to the wells. Cells were incubated for 24 h. Cells on the
upper side of the filters were mechanically removed by scrubbing
with a cotton swab, following which the membrane was fixed with 4%
formaldehyde for 10 min at room temperature and stained with 0.5%
crystal violet (Sigma-Aldrich, St. Louis, MO, USA) for 10 min.
Finally, invasive cells were counted using a light microscope
(E100T HD, Nikon Corporation, Japan) at x200 magnification in 6
different fields from each filter (12).
Statistical analysis
All data are presented as the mean ± standard
deviation. Comparisons between two mean values were made using an
unpaired Student two-tailed t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
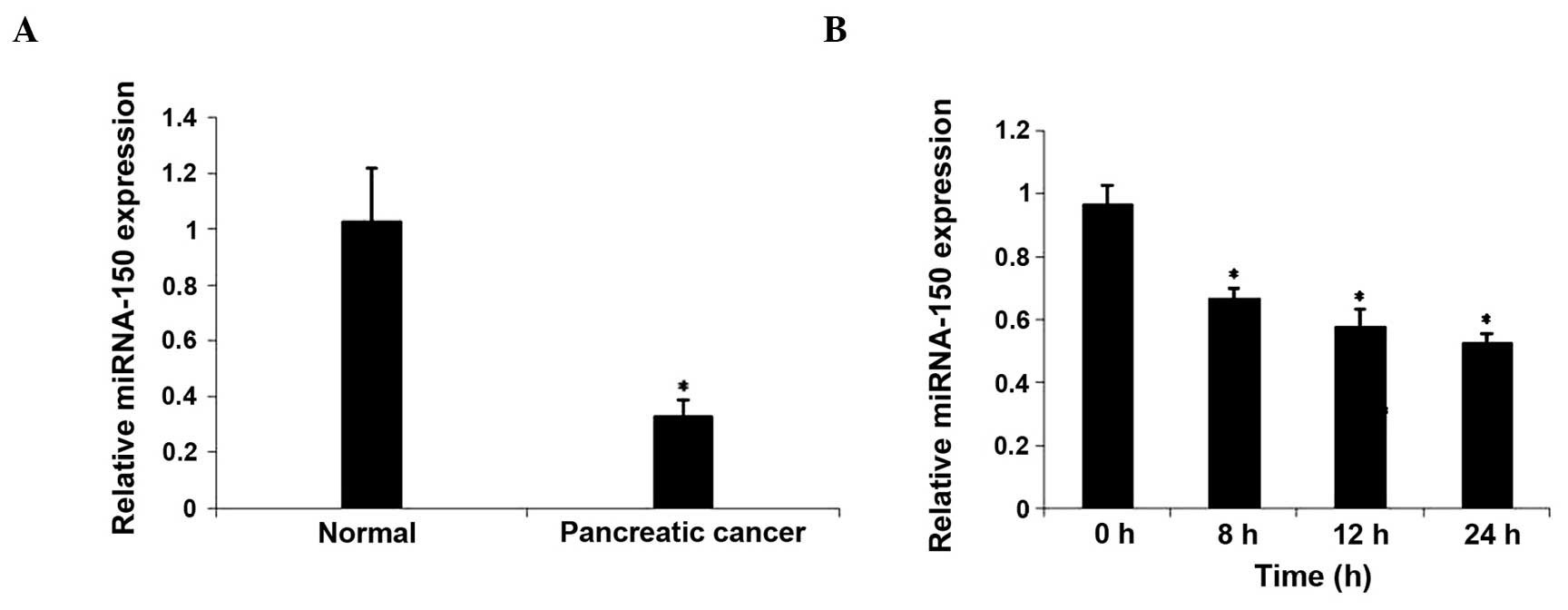
miRNA-150 expression was downregulated
in PC tissues and hypoxia-induced CaPan2 cells
In order to investigate the association between
miRNA-150 and hypoxia, the expression of miRNA-150 was evaluated in
PC samples from 15 patients. As demonstrated by RT-qPCR analysis,
miRNA-150 expression was significantly reduced in PC tissues
compared with tumor free pancreatic tissues (P<0.05). In
addition, there was a trend (P=0.073) towards reduced expression in
adjacent non-tumor tissues compared with tumor free pancreatic
tissues. The expression of miRNA-150 in hypoxia-cultured CaPan2
cells was subsequently examined. The expression of miRNA-150 was
downregulated following culture in hypoxic conditions for 8 h.
These results demonstrated that hypoxia may downregulate the
expression of miRNA-150 (Fig. 1).
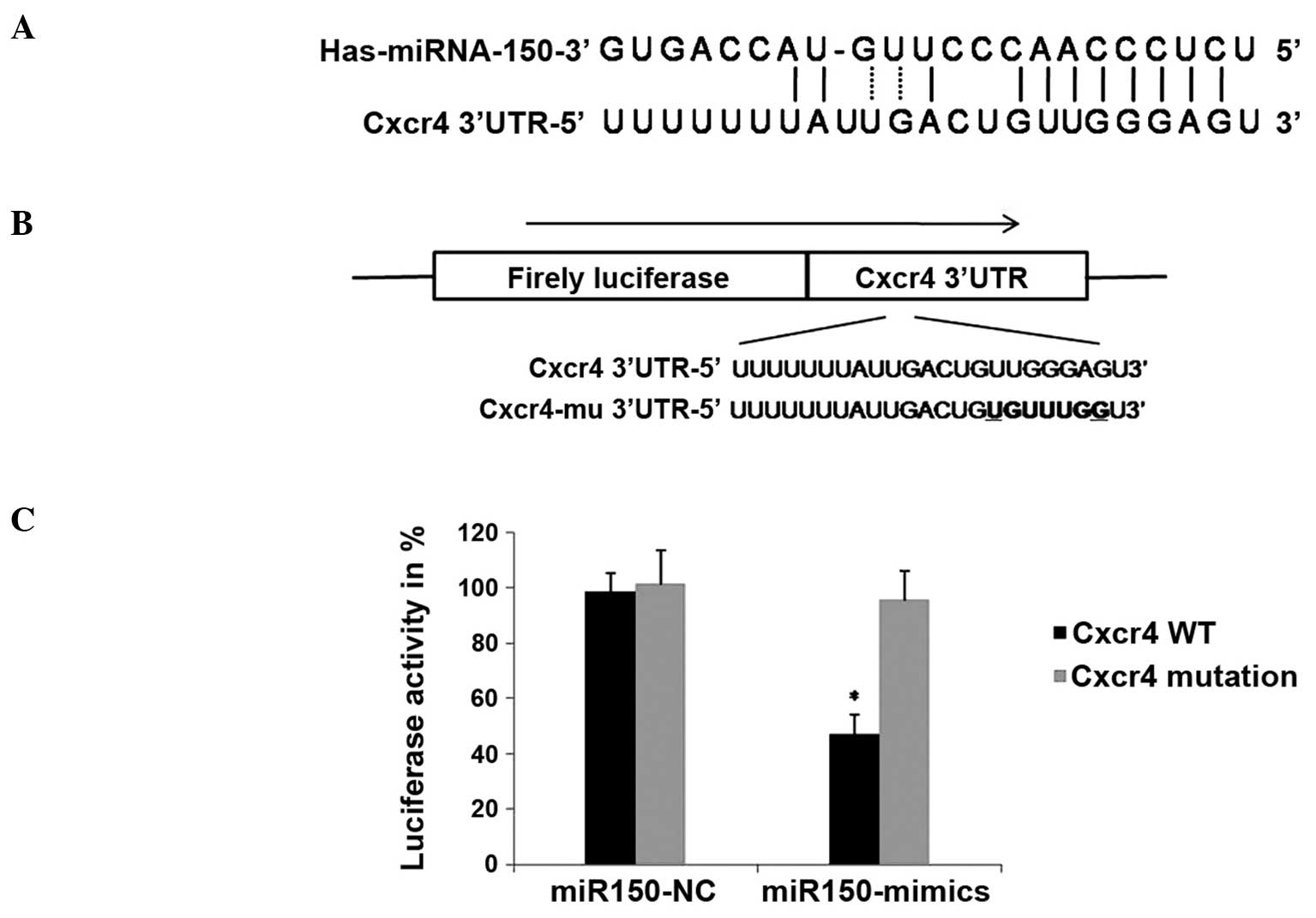
miRNA-150 directly targets the 3′UTR
of CXCR4 mRNA
Bioinformatics was employed to identify potential
targets of miRNA-150, using TargetScan (www.targetscan.org) and miRanda (www.microrna.org). Over 100 genes with sequence
matching for the mature miRNA-150 sequence were identified. Given
that miRNAs frequently target multiple genes
post-transcriptionally, miRNA-150 may exert its effects by
negatively regulating genes involved in cell migration and invasion
(Fig. 2A). Therefore, CXCR4, which is
known to be involved in the migration of a number of types of
cancer (13), was selected as a
candidate.
In order to confirm direct targeting of CXCR4 by
miRNA-150, a DNA fragment containing the region of the CXCR4 3′UTR,
in which the miRNA-150 target site is located, was integrated into
a luciferase reporter vector (Fig.
2B), and cotransfected in CaPan2 cells with the miRNA-150 mimic
or miRNA-NC (non-targeting control) (14). As a control, a vector containing a
CXCR4 3′UTR with a mutation in the miRNA-150 target region in order
to disrupt its binding, was generated and cotransfected into CaPan2
cells with the miRNA-150 mimics or miRNA-NC. Luciferase activity
was measured after 24 h of transfection. The results showed that
miRNA-150 overexpression significantly inhibited the expression of
luciferase in the vector containing a wild type miRNA-150 binding
site, compared with the NC group (Fig.
2C). This inhibition was reversed by mutations in the seed
complementary sites of the CXCR4 3′UTR. These results suggested
that miRNA-150 may negatively regulate the expression of CXCR4 by
directly targeting the 3′UTR of CXCR4 mRNA.
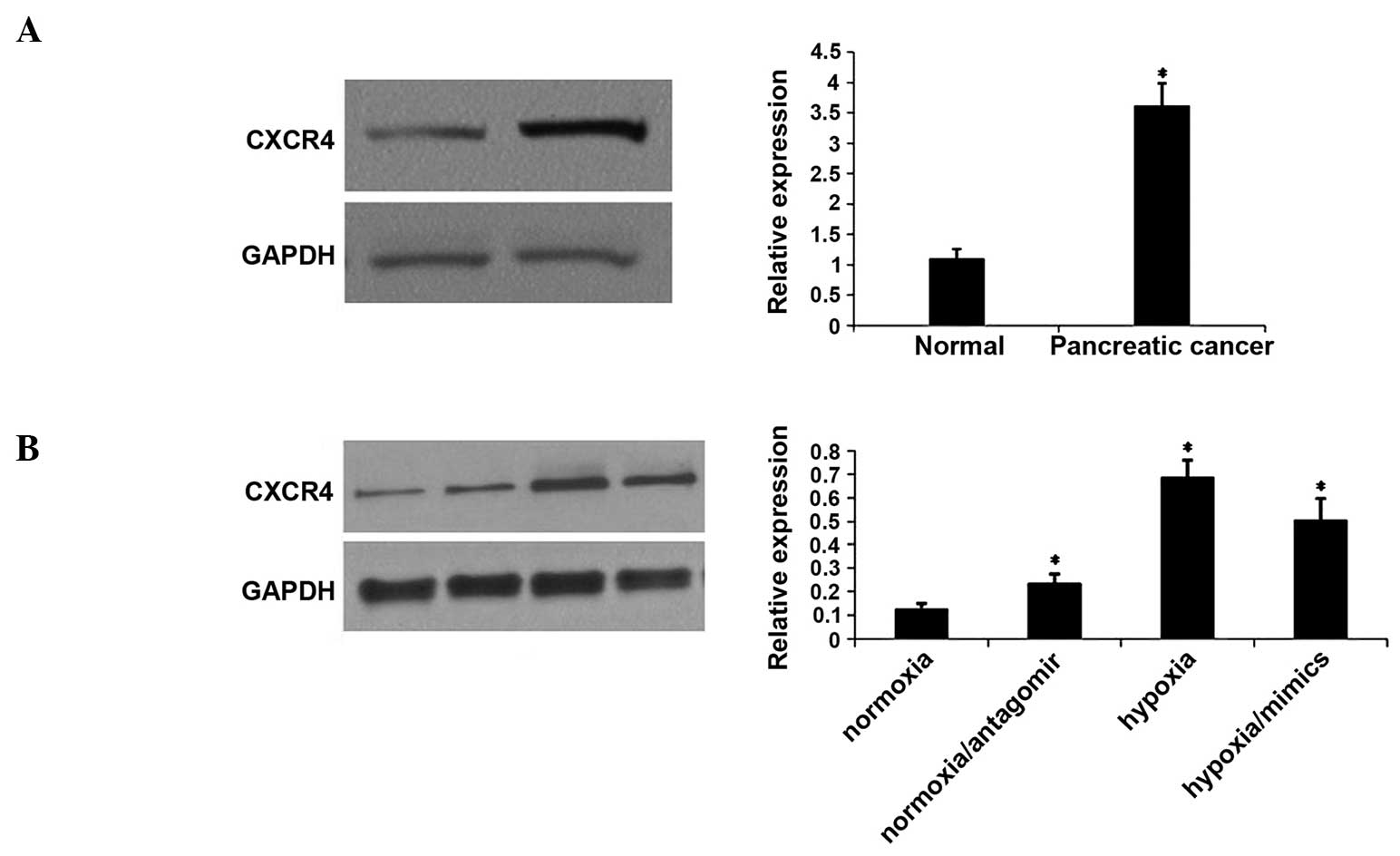
Hypoxia promotes CXCR4 expression
through downregulation of miRNA-150
In order to investigate the effect of hypoxia on
CXCR4 expression, the level of the CXCR4 protein was measured in
tissue samples and hypoxia-induced CaPan2 cells. Western blotting
demonstrated that CXCR4 protein expression was significantly
increased in PC tissues compared with distant normal tissues
(Fig. 3A). The expression of the
CXCR4 protein was upregulated in hypoxia-induced CaPan2 cells.
However, miRNA-150 mimics reversed the upregulation of CXCR4, which
had been induced by hypoxia. By contrast, the miRNA-150 inhibitor
increased the expression of CXCR4 under normoxic conditions
(Fig. 3B).
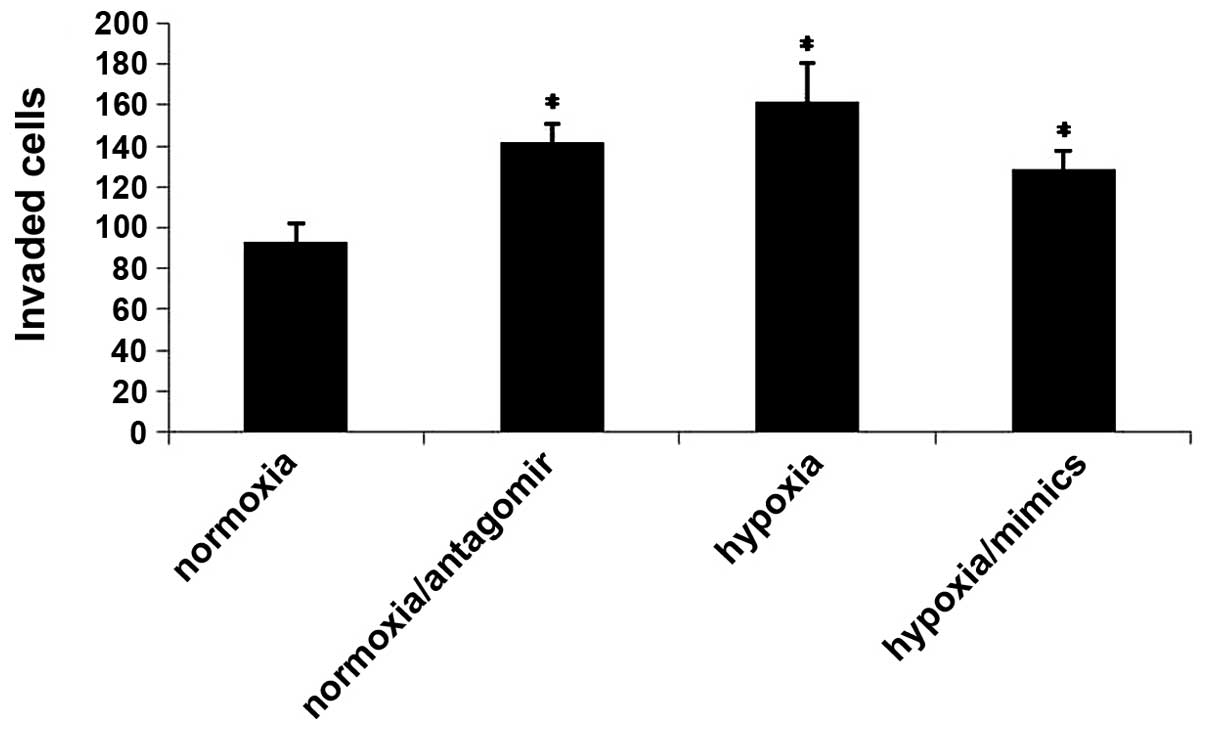
Hypoxia promotes CaPan2 cell invasion
and migration, through its effects on miRNA-150 and CXCR4
The aggressiveness of a cancer cell is determined by
its capacity to invade through the basement membrane. CXCR4 is a
well-established mediator of metastasis in numerous types of cancer
(13,15.16). The
present study investigated whether hypoxia promotes cancer cell
migration and invasion through its effects on miRNA-150 and CXCR4.
The results demonstrated that hypoxia leads to increased cell
migration and invasion. However, transfection of the miRNA-150
mimic reversed this prometastatic effect. Furthermore, the
miRNA-150 inhibitor promoted cell migration and invasion under
normoxic conditions (Fig. 4). These
results suggested that hypoxia may promote migration and invasion
via its effects on miRNA-150 and CXCR4.
Discussion
In order to adapt to hypoxia, tumors develop
alterations in a number of functions, such as metabolism,
migration, progress through the cell cycle and gene expression. The
expression of certain genes is involved in various biological
processes, including survival, migration and apoptosis. There is
accumulating evidence that dysregulation of the expression of
particular miRNAs occurs following exposure to hypoxia. These
miRNAs have been implicated in a broad range of biological
processes including cell proliferation, apoptosis, differentiation,
metabolism, migration and invasion (17,18). A
previous study confirmed that miR-210 is induced by hypoxia in
hepatocellular carcinoma cells and may promote cancer cell
metastasis (19). Vacuole membrane
protein 1 (VMP1) has been identified as a direct target of miR-210,
and downregulation of VMP1 by hypoxia has been shown to increase
cancer cell migration (19,20). A separate study suggested that
miRNA-103, miRNA-107, miRNA-372 and miRNA-373 may be upregulated in
hypoxic conditions, through the transcriptional regulation of
hypoxia inducible factor-1α. These miRNAs affect tumor behavior by
decreasing the expression of their respective target genes
(2). However, certain miRNAs, such as
miRNA-20b and miRNA-200b, have also been shown to be downregulated
in response to hypoxia (4).
The present study measured the expression of
miRNA-150 in pancreatic cancer tissues and hypoxia-induced CaPan2
cells. The results indicated that miRNA-150 was significantly
downregulated in primary tumors and hypoxia-induced cell lines.
This suggests that hypoxia may downregulate miRNA-150 in PC
cells.
Using bioinformatics, the results of the current
study demonstrated that CXCR4 is a potential target gene of
miRNA-150. miRNA-150 binds the 3′UTR of CXCR4 mRNA though partial
complementary elements. The regulation of the expression of CXCR4
by miRNA-150, was confirmed using a luciferase assay and
transfection with an miRNA-150 mimic. Over recent years,
upregulation of CXCR4 and its unique ligand, stromal
cell-derived-factor-1 (SDF-1), has been reported as an independent
prognostic factor for disease relapse and survival in patients with
PC (13,16). In order to further examine the
mechanism underlying the effect of miRNA-150 in PC cells, the
expression of CXCR4 in PC tissue samples and cultured cell lines
was measured. The results demonstrated that CXCR4 was overexpressed
in PC tissues and hypoxia-induced cells. By contrast, this increase
in the expression of the CXCR4 protein was reversed when cells were
transfected with the miRNA-150 mimics. These results suggested that
hypoxia may promote CXCR4 expression via the downregulation of
miRNA-150.
In conclusion, the present results suggest that
hypoxia may promote CXCR4 expression and PC cancer cell migration
via the downregulation of miRNA-150 expression. To the best of our
knowledge, the current study identified, for the first time, a
hypoxia/miRNA-150/CXCR4/SDF-1 axis in human pancreatic cells that
may be responsible for cancer cell migration and invasion.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no's. 81300596 and
81201000).
References
|
1
|
Olson SH and Kurtz RC: Epidemiology of
pancreatic cancer and the role of family history. J Surg Oncol.
107:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li D and Abbruzzese JL: New strategies in
pancreatic cancer: Emerging epidemiologic and therapeutic concepts.
Clin Cancer Res. 16:4313–4318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li L, Li B, Chen D, Liu L, Huang C, Lu Z,
Lun L and Wan X: miR-139 and miR-200c regulate pancreatic cancer
endothelial cell migration and angiogenesis. Oncol Rep. 2015.(Epub
ahead of print).
|
|
4
|
Esencay M, Sarfraz Y and Zagzag D: CXCR7
is induced by hypoxia and mediates glioma cell migration towards
SDF-1α. BMC Cancer. 13:3472013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen G, Li X, Jia YF, Piazza GA and Xi Y:
Hypoxia-regulated microRNAs in human cancer. Acta Pharmacol Sin.
34:336–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noman MZ, Buart S, Romero P, et al:
Hypoxia-inducible miR-210 regulates the susceptibility of tumor
cells to lysis by cytotoxic T cells. Cancer Res. 72:4629–4641.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pocock R: Invited review: Decoding the
microRNA response to hypoxia. Pflugers Arch. 461:307–315. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bruning U, Cerone L, Neufeld Z, et al:
MicroRNA-155 promotes resolution of hypoxia-inducible factor 1
alpha activity during prolonged hypoxia. Mol Cell Biol.
31:4087–4096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu ZY, Bai YN, Luo LX, Wu H and Zeng Y:
Expression of microRNA-150 targeting vascular endothelial growth
factor-A is downregulated under hypoxia during liver regeneration.
Mol Med Rep. 8:287–293. 2013.PubMed/NCBI
|
|
10
|
Chen C, Ridzon DA, Broomer AJ, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hurley J, Roberts D, Bond A, Keys D and
Chen C: Stem-loop RT-qPCR for microRNA expression profiling.
Methods Mol Biol. 822:33–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: The down-regulation of Notch1 inhibits the invasion and
migration of hepatocellular carcinoma cells by inactivating the
cyclooxygenase-2/Snail/E-cadherin pathway in vitro. Dig Dis Sci.
58:1016–1025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oh YS, Kim HY, Song IC, Yun HJ, Jo DY, Kim
S and Lee HJ: Hypoxia induces CXCR4 expression and biological
activity in gastric cancer cells through activation of
hypoxia-inducible factor-1α. Oncol Rep. 28:2239–2246.
2012.PubMed/NCBI
|
|
14
|
Srivastava SK, Bhardwaj A, Singh S, et al:
MicroRNA-150 directly targets MUC4 and suppresses growth and
malignant behavior of pancreatic cancer cells. Carcinogenesis.
32:1832–1839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sikand K, Slaibi JE, Singh R, Slane SD and
Shukla GC: miR 488* inhibits androgen receptor expression in
prostate carcinoma cells. Int J Cancer. 129:810–819. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sachdeva M and Mo YY: MicroRNA-145
suppresses cell invasion and metastasis by directly targeting mucin
1. Cancer Res. 70:378–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Fei M, Xue G, et al: Elevated
levels of hypoxia-inducible microRNA-210 in pre-eclampsia: New
insights into molecular mechanisms for the disease. J Cell Mol Med.
16:249–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Biswas S, Roy S, Banerjee J, et al:
Hypoxia inducible microRNA 210 attenuates keratinocyte
proliferation and impairs closure in a murine model of ischemic
wounds. Proc Natl Acad Sci USA. 107:6976–6981. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ying Q, Liang L, Guo W, et al:
Hypoxia-inducible microRNA-210 augments the metastatic potential of
tumor cells by targeting vacuole membrane protein 1 in
hepatocellular carcinoma. Hepatology. 54:2064–2075. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu T, Zhao L, Chen W, Li Z, Hou H, Ding L
and Li X: Inactivation of von Hippel-Lindau increases ovarian
cancer cell aggressiveness through the HIF1α/miR-210/VMP1 signaling
pathway. Int J Mol Med. 33:1236–1242. 2014.PubMed/NCBI
|