Introduction
Over 20 years ago, the melanoma-associated antigens
(MAGEs) were identified by van der Bruggen et al (1). MAGEs belong to the group of
cancer/testis antigens (CTA), which includes multiple proteins, for
example NY-ESO-1, sinovial sarcoma X and G antigen 1 (2). It is known that these proteins are found
in adult male germ cells, fetal keratinocytes, the placenta and a
variety of human malignancies, including head and neck cancer
(3–6).
At present, the large group of MAGE tumor antigens consists of ~60
proteins. Their specific expression in the majority of
malignancies, coupled with their immunogenicity, makes these
proteins promising targets for anticancer therapies (7). However, little is known about the
function of MAGE-A tumor antigens. Studies by Doyle et al
(8) and Yang et al (9) demonstrated the negative effect of MAGE
expression on p53 levels. Notably, studies by Ries et al
(10,11) provided clear evidence that MAGE-A
expression serves as predictor of malignant transformation in oral
leukoplakia. Furthermore, there is evidence that MAGE-A is
expressed in dysplastic leukoplakia and carcinoma in situ,
but not in oral lichen planus, oral ulcers or leukoplakia without
dysplasia (12). Another study
revealed that MAGE-A tumor antigens are the most frequently
expressed CTAs in head and neck cancer (13). Notably, there is no correlation
between MAGE-A expression and clinicopathological characteristics
in head and neck cancer. Recently, Laban et al (14) published data indicating a marked
correlation between poor prognosis in a subset of patients with
head and neck cancer and the expression of MAGE-A antigens. In
addition, a previous study by our group identified a correlation
between MAGE-A5 and -A8 expression and poorer responses to
anti-epidermal growth factor receptor (EGFR) therapy in
vitro (15). Of note, MAGE-A11
expression is also correlated with poorer responses to cisplatin,
5-fluorouracil, docetaxel and paclitaxel (16).
In head and neck cancer, targeted therapy has an
expanding role. This therapeutic approach is largely based on the
EGFR and its associated pathways. Downstream signaling molecules of
the EGFR, mediate the invasion, growth, progression and survival of
tumor cells (17). The majority of
head and neck cancer specimens demonstrate overexpression of the
EGFR, and alterations in the copy number of this receptor are
associated with poor prognosis (18).
In general, EGFR-targeted therapies for head and neck cancer are
performed with cetuximab, a chimeric antibody directed against the
EGFR. Targeting the EGFR by tyrosine kinase inhibitors is another
well-studied field of oncology, particularly in the case of
EGFR-mutated non-small cell lung cancer (NSCLC), in which erlotinib
and gefitinib serve as useful drugs that aid the improvement of
progression-free survival and overall survival (19). Unfortunately, in an unselected cohort
of head and neck cancer patients, erlotinib failed to improve
progression-free survival when used in combination with cisplatin
and radiotherapy (20). The role of
erlotinib in head and neck cancer may be more efficacious in the
field of chemoprevention. Using a combined approach of erlotinib
and sulindac, data from Shin et al (21) demonstrated effective chemoprevention
in preclinical and clinical models. These findings are discussed in
a study recently published by Gross et al (22).
The present study therefore aimed to investigate
whether the expression of MAGE-A tumor antigens was associated with
poor efficacy of erlotinib and gefitinib.
Materials and methods
Cell lines
The cell lines used in the present study (Table I) were established at the Cancer
Institute of the University of Pittsburgh (Pittsburgh, PA, USA)
(23). As described previously
(15,24), the cells were cultured in a humidified
atmosphere of 5% CO2/95% air at 37°C and were fed 2–3
times per week with Dulbecco's Modified Eagle's Medium (DMEM; Life
Technologies, GmbH, Darmstadt, Germany) with low glucose, 10% fetal
calf serum (Life Technologies, GmbH), 1% penicillin/streptomycin
(Life Technologies, GmbH) and 1% L-glutamine (Biochrom KG, Berlin,
Germany).
 | Table I.Name, origin and TNM status of the
five cell lines used in the present study. |
Table I.
Name, origin and TNM status of the
five cell lines used in the present study.
| Cell line | Origin | Patient gender | TNM |
|---|
| PCI-1 | Laryngeal carcinoma
of the glottis | male | pT2N00M0G2 |
| PCI-9 | Primary carcinoma at
the base of the tongue | male | pT4N3M0G2 |
| PCI-13 | Oral squamous cell
carcinoma of the retromolar triangle | male | pT4pN1M0G3 |
| PCI-52 | Primary carcinoma of
the aryepiglottic fold | male | pT2N0M0G2 |
| PCI-68 | Primary tongue
carcinoma | male | pT4N0M0G1 |
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis of MAGE-A
The protocol and quantification of MAGE-A expression
by RT-qPCR was conducted as previously described by our group
(15,16).
RNA isolation was executed using the NucleoSpin RNA
II kit (Macherey-Nagel, Düren, Germany), according to the
manufacturer's instructions. Isolated RNA was stored at −80°C prior
to reverse transcription. Complementary (c)DNA was synthesized from
identical quantities of total RNA (1 µg) with the M-MLV RT RNase
H(–) point mutant using the buffer system provided (Promega Corp.,
Mannheim, Germany), according to the manufacturer's
instructions.
MAGE-A expression profiles were quantitatively
analyzed by RT-qPCR using the FastStart DNA Master Plus SYBR-Green
I (Roche Diagnostics GmbH, Mannheim, Germany). Each reaction
mixture (20 µl) was comprised of 0.5 µl cDNA, 1 µl forward primer
(20 µM), 1 µl reverse primer (20 µM) (both from TIB MOLBIOL GmbH,
Berlin, Germany), 4 µl LightCycler DNA Master SYBR-Green I, 1 µl
dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA) and 12.5 µl
water. The cycling conditions for RT-qPCR in the LightCycler 2.0
(Roche Diagnostics, Mannheim, Germany) were as follows: Initial
denaturation at 95°C for 10 min, followed by 45 cycles of
amplification with denaturation at 95°C for 10 sec, primer
annealing at 62–67°C for 3–4 sec and elongation at 68–72°C for 3–4
sec (specific temperature and elongation indicated in Table II). Following completion of this
protocol, a melting range analysis was conducted. The protocol
comprised one cycle at 95°C for 20 sec, followed by one cycle at
60°C for 20 sec with continuously measured fluorescence. The values
measured were analyzed by the LightCycler Relative Quantification
Software 1.0 (Roche Diagnostics GmbH), which provided
efficiency-corrected, calibrator-normalized relative quantification
results. The relative concentrations were normalized to β-actin
messenger RNA levels.
 | Table II.Sequences, base pair lengths,
annealing temperatures and elongation times of the primers
used. |
Table II.
Sequences, base pair lengths,
annealing temperatures and elongation times of the primers
used.
| Gene | Sequence, 5-3 | Base pairs | Annealing,
°C/sec | Elongation,
°C/sec |
|---|
| β-actin | F:
CCAACCGCGAGAAGATGA | 97 | 65/3 | 68/4 |
|
| R:
CCAGAGGCGTACAGGGATAG |
|
|
|
| MAGE-A1 | F:
GGCCGAAGGAACCTGACC | 69 | 67/3 | 72/4 |
|
| R:
GTCCTCTGGGTTGGCCTGT |
|
|
|
| MAGE-A5 | F:
GCCCTAGAGGAGCACCAAAG | 80 | 62/4 | 72/3 |
|
| R:
CGCAACAGGCAGGAGTGT |
|
|
|
| MAGE-A8 | F:
AAAGGTTCGCAGAGAACAGG | 119 | 65/3 | 72/3 |
|
|
|
|
| R:
GTCAGGGCAGCAGGAGAGT |
|
|
|
| MAGE-A9 | F:
GGCCTTGGTCTGAGACAGTG | 97 | 65/3 | 72/3 |
|
| R:
GTCCTCCTGGTTAGCCTGT |
|
|
|
| MAGE-A11 | F:
ACAGGAGTCCCAGGAGAACC | 81 | 67/3 | 72/4 |
|
| R:
CTGTGGGAAATATCTGGGTGA |
|
|
|
| MAGE-A12 | F:
GTCGGTGGAGGGAAGCAG | 104 | 65/3 | 72/3 |
|
|
|
|
| R:
AGGGCAGCAGGTAGGAGTG |
|
|
|
Drug treatment and crystal violet
assay
Cells of each cell line were seeded at 10,000
cells/well, respectively. Erlotinib and gefitinib were purchased
from Selleckchem (distributed by Absource Diagnostics GmbH,
München, Germany) and stored according to the manufacturer's
instructions. The concentrations used in the present study (4.94,
1.65, 0.55, 0.18 and 0.06 µM) were derived from a log 3 dilution
starting at 400 µM (data not shown). The control cells were
cultured in medium as described above (without TKIs). These
concentrations were chosen based on the clinically relevant maximum
serum concentration of the selected tyrosine kinase inhibitors
(TKIs), of ~1 µM (25). Following 24
h of incubation in standard medium, erlotinib or gefitinib was
added, and the cultures were incubated for a further 72 h. Crystal
violet (1 g; Carl Roth GmbH, Karlruhe, Germany) was dissolved in 1
l double-distilled water containing 20% methanol. Following the
removal of the drug-containing medium, 50 µl crystal violet was
added to each well and incubated for 15 min. The 96-well plates
were then washed with distilled water and the optical density (OD)
was measured at 595 nm using a RainBow microplate reader (Tecan,
Maennedorf, Swiss). All experiments were performed in triplicate.
The mean was calculated from at least three independent experiments
and used in further analyses.
Statistical analysis
The association between chemosensitivity and MAGE-A
expression status was analyzed using a linear regression model.
This model allows evaluation of the correlation between the viable
fraction of the cell culture and the concentration of the drug, as
well as the expression level of MAGE-A subgroups during drug
treatment. However, since the linear regression model based on 6
MAGE-A subgroups is only able to include 4 possible variables,
MAGE-A1 and -A9 were excluded by SPSS. P≤0.05 was considered to
indicate a statistically significant difference. Statistical
analysis of the data was performed using Microsoft Excel 2010
(Microsoft, Redmond, WA, USA) and SPSS Statistics 22 (IBM SPSS,
Armonk, NY, USA) and was supported by the Department of Statistics,
University of Würzburg. GraphPad Prism 6.04 (GraphPad Software
Inc., La Jolla, CA, USA) was used to generate graphical
illustrations.
Results
MAGE-A expression varies between
squamous cell carcinoma cell lines
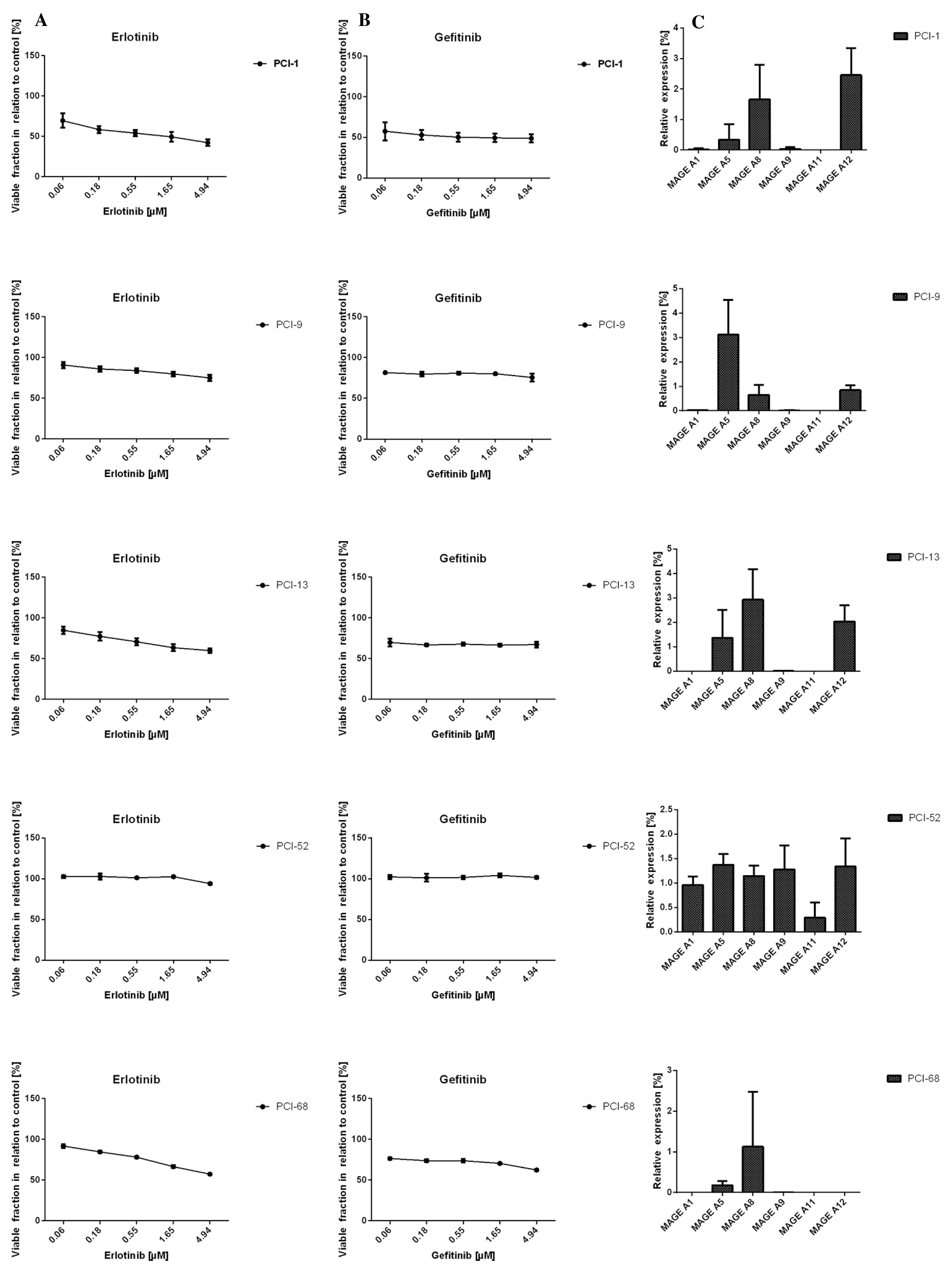
MAGE-A expression was detected in all five of the
cell lines. The minimum number of expressed subgroups was two (in
PCI-68). The lowest quantities of MAGE-A tumor antigens were also
detected in PCI-68. In contrast to PCI-68, all MAGE-A tumor antigen
subgroups examined were expressed by PCI-52. Furthermore, the
highest expression of the subgroups -A1, -A9 and -A11 was also
detected in PCI-52. The highest expression of MAGE-A5 was detected
in PCI-9, while PCI-13 exhibited the highest levels of MAGE-A8, and
the greatest expression of MAGE-A12 was identified in the PCI-1
cell line (Fig. 1).
Efficacy of erlotinib treatment varies
between squamous cell carcinoma cell lines
Four of the five cell lines (PCI-1, PCI-9, PCI-13
and PCI-68) exhibited a concentration-dependent response to 72 h of
erlotinib treatment (Fig. 1). At the
minimum concentration of 0.06 µM, the fraction of viable cells
ranged from 69.51 (PCI-1) to 91.71% (PCI-68), compared with that of
the control. The intermediate concentration (0.55 µM) of erlotinib
resulted in a viable fraction of 54.07% in the PCI-1 cell line,
whereas this concentration resulted in a viable fraction of 83.93%
in PCI-9 cells. The maximum concentration of erlotinib (4.94 µM)
resulted in a viable fraction of 42.15% in PCI-1 cells, while in
PCI-9 cells, this concentration resulted in a viable fraction of
74.90%. By contrast, the PCI-52 cell line demonstrated no
concentration-dependent response to erlotinib. Erlotinib
concentrations of 0.06, 0.18, 0.55 and 1.65 µM yielded no
significant effect on viability (resulting in 102.78, 102.85,
101.14 and 102.60%, respectively). Only the highest concentration
of 4.94 µM erlotinib resulted in a small decrease in viability
compared with that of the control (94.03%).
Erlotinib treatment efficacy is
affected by MAGE-A expression
A linear regression model was produced using
erlotinib concentration and MAGE-A expression levels as independent
variables (Table III), with the
viable fraction compared with the control as the dependent
variable. The model produced an r-value of 0.848, indicating a
notable adaptation. MAGE-A1 and -A9 were excluded as independent
variables. However, potential effects of MAGE-A1 and -A9 may be
determined using a larger regression model with more cell lines.
Negative values of the standardized coefficients represent an
inhibitory effect on the fraction of viable cells. By contrast,
positive values of the standardized coefficients indicate a
beneficial effect on the fraction of viable cells. Increasing
concentrations of erlotinib significantly decreased the viability
of cells (P<0.001). MAGE-A12 was also associated with a decrease
in the viable fraction during erlotinib treatment (P=0.009), while
MAGE-A5 (P=0.015) and -A11 (P<0.001) were correlated with a
higher fraction of viable cells following erlotinib treatment.
 | Table III.Linear regression of erlotinib/MAGE-A
subgroup following 72 h of treatment. |
Table III.
Linear regression of erlotinib/MAGE-A
subgroup following 72 h of treatment.
| Independent
variable | Standardized
coefficient | P-value |
|---|
| MAGE-A5 |
0.299 |
0.015 |
| MAGE-A8 |
0.168 |
0.274 |
| MAGE-A11 |
0.574 |
<0.001 |
| MAGE-A12 | −0.398 |
0.009 |
| Erlotinib
concentration | −0.482 |
<0.001 |
Efficacy of gefitinib treatment varies
between squamous cell carcinoma cell lines
Analogously with the results described for erlotinib
treatment, four of the five cell lines (PCI-1, PCI-9, PCI-13 and
PCI-68) exhibited a concentration-dependent response to 72 h of
gefitinib treatment (Fig. 1). The
minimum concentration of 0.06 µM resulted in cell viabilities of
57.56% in PCI-1, 81.57% in PCI-9, 69.77% in PCI-13 and 76.38% in
PCI-68 cells, indicating a markedly greater efficacy of gefitinib
than that of the lowest concentration of erlotinib. At the
intermediate concentration (0.55 µM) of gefitinib, the fraction of
viable cells ranged from 50.34% in PCI-1 cells to 80.87% in PCI-9
cells. Compared with the control, the maximum concentration of 4.94
µM gefitinib resulted in viable fractions of 48.87 and 75.51% in
PCI-1 and PCI-9 cells, respectively. Notably, compared with the
lowest concentration of erlotinib, gefitinib produced greater
responses in the PCI-1, PCI-9, PCI-13 and PCI-68 cell lines.
Conversely, erlotinib treatment at the intermediate and highest
concentrations yielded better results than those of gefitinib. As
previously observed following erlotinib treatment, PCI-52 did not
demonstrate a concentration-dependent response to gefitinib
treatment. Furthermore, even the highest concentration of gefitinib
(4.94 µM) failed to reduce cell viability compared with that of the
control (101.59%).
Gefitinib treatment efficacy is
affected by MAGE-A expression
The linear regression model was constructed using
the gefitinib concentration and MAGE-A expression levels as
independent variables (Table IV),
with the viable fraction compared with the control as the dependent
variable. The model generated an r-value of 0.805. Analogously to
the results obtained in the analysis of erlotinib, negative
standardized coefficient values represented an inhibitory effect on
the fraction of viable cells, while positive values indicated a
beneficial effect on the fraction of viable cells. The increasing
concentration of gefitinib significantly reduced the fraction of
viable cells (P=0.046), demonstrating a concentration-dependent
response of the cell lines to gefitinib treatment (Table IV). MAGE-A12 was also associated with
a decreased number of viable cells following gefitinib treatment
(P=0.027). Cell lines expressing MAGE-A11 demonstrated the lowest
response to gefitinib treatment, as indicated by the large fraction
of viable cells (P<0.001). MAGE-A5 expression was also
correlated with a significantly poorer efficacy of gefitinib
treatment (P=0.028), while MAGE-A8 expression did not significantly
alter the fraction of viable cells (P=0.364). MAGE-A1 and -A9 were
again excluded as independent variables.
 | Table IV.Linear regression of gefitinib/MAGE-A
subgroup following 72 h of treatment. |
Table IV.
Linear regression of gefitinib/MAGE-A
subgroup following 72 h of treatment.
| Independent
variable | Standardized
coefficient | P-value |
|---|
| MAGE-A5 |
0.300 |
0.028 |
| MAGE-A8 |
0.156 |
0.364 |
| MAGE-A11 |
0.661 |
<0.001 |
| MAGE-A12 | −0.371 |
0.027 |
| Gefitinib
concentration | −0.255 |
0.046 |
Discussion
Due to the high rate of recurrence and a poor
overall survival rate of ~50%, the treatment of head and neck
squamous cell carcinoma (HNSCC) remains challenging. Therefore,
improvements to patient selection in terms of non-surgical
treatment approaches may help to enhance clinical outcomes. One
technique for the identification of high-risk patients may be via
the evaluation of MAGE-A expression status. There is an increasing
understanding that the expression of certain CTAs, including
MAGE-A, is associated with markedly poorer survival amongst
patients with head and neck cancer (14). The present study revealed
inhomogeneous expression of MAGE-A1, -A5, -A8, -A9, -A11 and -A12
in the HNSCC cell lines tested. Furthermore, treatment with
erlotinib and gefitinib at clinically relevant concentrations
yielded differential response rates in the various HNSCC cell
lines. While four of the five cell lines (PCI-1, PCI-9, PCI-13 and
PCI-68) exhibited concentration-dependent responses to erlotinib
and gefitinib, TKI treatment of PCI-52 cells was completely
ineffective. The PCI-1 and PCI-52 cell lines were comparable in
terms of their TNM status; however, PCI-52 was the only cell line
to express all of the MAGE-A subgroups investigated, while PCI-1
expressed three out of the six subgroups. Notably, the PCI-1 cell
line was the most responsive to erlotinib and gefitinib treatment,
whereas PCI-52 was entirely resistant to these two agents. These
results highlight an urgent need for the identification of
additional molecular markers, including MAGE-A tumor antigens.
The linear regression models of erlotinib and
gefitinib revealed marked adaptation (r-values, 0.848 and 0.805,
respectively) and significant effects of agent concentration on the
fraction of viable cells, providing evidence for a useful
experimental setup. Correlation analysis of MAGE-A subgroups and
erlotinib treatment revealed MAGE-A5 and -A11 as significant
negative predictors of treatment success. Of note, MAGE-A11 was
previously shown to act as a proto-oncogene in prostate cancer by
serving as a transcriptional activator of the androgen receptor
(26). By contrast, MAGE-A12 was
associated with an improved outcome of erlotinib administration. In
a previous study by our group, MAGE-A5 and -A8 were reported as
negative predictors of anti-EGFR therapy using panitumumab
(15). In agreement with a recent
study by our group, MAGE-A12 was correlated with a greater response
to panitumumab (15). Mollaoglu et
al (27) previously described
MAGE-A12 as a predictor of improved prognosis in oral squamous cell
carcinoma. As described by their group, N0 cervical lymph node
status was found more frequently in MAGE-A12-positive patients than
that of the MAGE-negative controls. Furthermore, MAGA-A11 was
reported to have a negative impact on treatment with cisplatin,
5-fluorouracil, paclitaxel and docetaxel (16). In the same study, MAGE-A5 was
demonstrated to be associated with improved outcomes following
paclitaxel therapy. Statistical analysis revealed that treatment
with gefitnib or erlotinib were comparable in the context of MAGE-A
expression. MAGE-A5 and -A11 were correlated with poorer outcomes
of gefitinib therapy. Similarly to the effects observed following
erlotinib treatment, improved outcomes of gefitinib treatment were
associated with MAGE-A12 expression. A potential reason for the
negative influence of MAGE-A11 on EGFR-directed therapies may be
due to its association with the expression of other transmembrane
receptors. Recently, Hou et al (28) demonstrated a significant correlation
between MAGE-A9 and -A11 expression and Her2/neu expression in
breast cancer. There is a broad consensus that evasion of
EGFR-targeted therapy is facilitated by the activation of
alternative receptors, including Her2/neu, as well as their
downstream signaling pathways (29,30). Even
if TKI treatment combined with cisplatin and radiotherapy failed to
improve progression-free survival in unselected cohorts, erlotinib
and gefitinib may function as chemopreventive drugs (31). One significant consideration in the
treatment of patients with head and neck cancer is the phenomenon
of field cancerization (32).
Therefore, the possibility of reducing the incidence of malignant
transformation to dysplastic oral mucosa may be a valuable tool,
based on the findings regarding the chemoproventative properties of
TKIs (31,32). MAGE-A subgroup analysis may help to
identify high-risk patients, thus aiding the development of
personalized therapies and follow-up.
Acknowledgements
The present study was funded by the
Interdisciplinary Center for Clinical Research of the University of
Würzburg (grant no. Z-3/13). The manuscript was spell-checked by
the American Journal Experts (Durham, NC, USA).
References
|
1
|
van der Bruggen P, Traversari C, Chomez P,
Lurquin C, De Plaen E, Van den Eynde BJ, Knuth A and Boon T: A gene
encoding an antigen recognized by cytolytic T lymphocytes on a
human melanoma. J Immunol. 178:2617–2621. 2007.PubMed/NCBI
|
|
2
|
Old LJ and Chen YT: New paths in human
cancer serology. J Exp Med. 187:1163–1167. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kienstra MA, Neel HB, Strome SE and Roche
P: Identification of NY-ESO-1, MAGE-1, and MAGE-3 in head and neck
squamous cell carcinoma. Head Neck. 25:457–463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Müller-Richter UD, Dowejko A, Zhou W,
Reichert TE and Driemel O: Different expression of MAGE-A-antigens
in foetal and adult keratinocyte cell lines. Oral Oncol.
44:628–633. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scanlan MJ, Simpson AJ and Old LJ: The
cancer/testis genes: Review, standardization, and commentary.
Cancer Immun. 4:12004.PubMed/NCBI
|
|
6
|
Simpson AJ, Caballero OL, Jungbluth A,
Chen YT and Old LJ: Cancer/testis antigens, gametogenesis and
cancer. Nat Rev Cancer. 5:615–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferris RL: Progress in head and neck
cancer immunotherapy: Can tolerance and immune suppression be
reversed? ORL J Otorhinolaryngol Relat Spec. 66:332–340. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Doyle JM, Gao J, Wang J, Yang M and Potts
PR: MAGE-RING protein complexes comprise a family of E3 ubiquitin
ligases. Mol Cell. 39:963–974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang B, O'Herrin SM, Wu J, Reagan-Shaw S,
Ma Y, Bhat KM, Gravekamp C, Setaluri V, Peters N, Hoffmann FM, et
al: MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1
and suppress p53-dependent apoptosis in MAGE-positive cell lines.
Cancer Res. 67:9954–9962. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ries J, Agaimy A, Vairaktaris E, Gorecki
P, Neukam FW, Strassburg LH and Nkenke E: Detection of MAGE-A
expression predicts malignant transformation of oral leukoplakia.
Cancer Invest. 30:495–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ries J, Agaimy A, Vairaktaris E, Kwon Y,
Neukam FW, Strassburg LH and Nkenke E: Evaluation of MAGE-A
expression and grade of dysplasia for predicting malignant
progression of oral leukoplakia. Int J Oncol. 41:1085–1093.
2012.PubMed/NCBI
|
|
12
|
Krauss E, Rauthe S, Gattenlöhner S,
Reuther T, Kochel M, Kriegebaum U, Kübler AC and Müller-Richter UD:
MAGE-A antigens in lesions of the oral mucosa. Clin Oral Investig.
15:315–320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Piotti KC, Scognamiglio T, Chiu R and Chen
YT: Expression of cancer/testis (CT) antigens in squamous cell
carcinoma of the head and neck: Evaluation as markers of squamous
dysplasia. Pathol Res Pract. 209:721–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laban S, Atanackovic D, Luetkens T, Knecht
R, Busch CJ, Freytag M, Spagnoli G, Ritter G, Hoffmann TK, Knuth A,
et al: Simultaneous cytoplasmic and nuclear protein expression of
melanoma antigen-A family and NY-ESO-1 cancer-testis antigens
represents an independent marker for poor survival in head and neck
cancer. Int J Cancer. 135:1142–1152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hartmann S, Kriegebaum U, Küchler N,
Lessner G, Brands RC, Linz C, Schneider T, Kübler AC and
Müller-Richter UD: Efficacy of cetuximab and panitumumab in oral
squamous cell carcinoma cell lines: Prognostic value of MAGE-A
subgroups for treatment success. J Craniomaxillofac Surg.
41:623–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hartmann S, Kriegebaum U, Küchler N,
Brands RC, Linz C, Kübler AC and Müller-Richter UD: Correlation of
MAGE-A tumor antigens and the efficacy of various chemotherapeutic
agents in head and neck carcinoma cells. Clin Oral Investig.
18:189–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yarden Y and Shilo BZ: SnapShot: EGFR
signaling pathway. Cell. 131:10182007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Temam S, Kawaguchi H, El-Naggar AK,
Jelinek J, Tang H, Liu DD, Lang W, Issa JP, Lee JJ and Mao L:
Epidermal growth factor receptor copy number alterations correlate
with poor clinical outcome in patients with head and neck squamous
cancer. J Clin Oncol. 25:2164–2170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asami K and Atagi S: Epidermal growth
factor receptor tyrosine kinase inhibitors for non-small cell lung
cancer. World J Clin Oncol. 5:646–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martins RG, Parvathaneni U, Bauman JE,
Sharma AK, Raez LE, Papagikos MA, Yunus F, Kurland BF, Eaton KD,
Liao JJ, et al: Cisplatin and radiotherapy with or without
erlotinib in locally advanced squamous cell carcinoma of the head
and neck: A randomized phase II trial. J Clin Oncol. 31:1415–1421.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shin DM, Zhang H, Saba NF, Chen AY,
Nannapaneni S, Amin AR, Müller S, Lewis M, Sica G, Kono S, et al:
Chemoprevention of head and neck cancer by simultaneous blocking of
epidermal growth factor receptor and cyclooxygenase-2 signaling
pathways: Preclinical and clinical studies. Clin Cancer Res.
19:1244–1256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gross ND, Bauman JE, Gooding WE, Denq W,
Thomas SM, Wang L, Chiosea S, Hood BL, Flint MS, Sun M, et al:
Erlotinib, erlotinib-sulindac versus placebo: A randomized,
double-blind, placebo-controlled window trial in operable head and
neck cancer. Clin Cancer Res. 20:3289–3298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heo DS, Snyderman C, Gollin SM, Pan S,
Walker E, Deka R, Barnes EL, Johnson JT, Herberman RB and Whiteside
TL: Biology, cytogenetics, and sensitivity to immunological
effector cells of new head and neck squamous cell carcinoma lines.
Cancer Res. 49:5167–5175. 1989.PubMed/NCBI
|
|
24
|
Hartmann S, Seher A, Brands RC, Linz C,
Lessner G, Böhm H, Kübler AC and Müller-Richter UD: Influence of
epidermal growth factor receptor expression on the cetuximab and
panitumumab response rates of head and neck carcinoma cells. J
Craniomaxillofac Surg. 42:1322–1328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mukohara T, Engelman JA, Hanna NH, Yeap
BY, Kobayashi S, Lindeman N, Halmos B, Pearlberg J, Tsuchihashi Z,
Cantley LC, et al: Differential effects of gefitinib and cetuximab
on non-small-cell lung cancers bearing epidermal growth factor
receptor mutations. J Natl Cancer Inst. 97:1185–1194. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su S, Minges JT, Grossman G, Blackwelder
AJ, Mohler JL and Wilson EM: Proto-oncogene activity of melanoma
antigen-A11 (MAGE-A11) regulates retinoblastoma-related p107 and
E2F1 proteins. J Biol Chem. 288:24809–24824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mollaoglu N, Vairaktaris E, Nkenke E,
Neukam FW and Ries J: Expression of MAGE-A12 in oral squamous cell
carcinoma. Dis Markers. 24:27–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou SY, Sang MX, Geng CZ, Liu WH, Lü WH,
Xu YY and Shan BE: Expressions of MAGE-A9 and MAGE-A11 in breast
cancer and their expression mechanism. Arch Med Res. 45:44–51.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Quesnelle KM and Grandis JR: Dual kinase
inhibition of EGFR and HER2 overcomes resistance to cetuximab in a
novel in vivo model of acquired cetuximab resistance. Clin Cancer
Res. 17:5935–5944. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yonesaka K, Zejnullahu K, Okamoto I, Satoh
T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda
M, et al: Activation of ERBB2 signaling causes resistance to the
EGFR-directed therapeutic antibody cetuximab. Sci Transl Med.
3:99ra862011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rosenthal EL, Chung TK, Carroll WR,
Clemons L, Desmond R and Nabell L: Assessment of erlotinib as
adjuvant chemoprevention in high-risk head and neck cancer
patients. Ann Surg Oncol. 21:4263–4269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jaiswal G, Jaiswal S, Kumar R and Sharma
A: Field cancerization: Concept and clinical implications in head
and neck squamous cell carcinoma. J Exp Ther Oncol. 10:209–214.
2013.PubMed/NCBI
|