Introduction
Advanced pancreatic cancer has a poor prognosis with
a median survival time of 3–5 months, which has not been changed in
the era of 5-fluorouracil (5-FU) chemotherapy (1). The emergence of the nucleoside analogue
gemcitabine has improved the response rate and clinical benefits
compared with 5-FU monotherapy. However, the median survival time
of affected patients remains at ~6 months (2–4).
As there are few other agents active against
pancreatic cancer, numerous efforts have sought to improve the
efficacy of combination regimens for this dismal disease. In
previous studies, the combination of gemcitabine+irinotecan or
gemcitabine+docetaxel showed an overall response rate in the range
of 10–20%, however, the overall survival (OS) time was not improved
beyond 6 months (5–7). The combination of gemcitabine plus 5-FU
with or without leucovorin showed an overall response rate in the
range of 5–25.9%, however, the OS time was in the range of 6.7–10.3
months (8–10).
In one study, cisplatin therapy produced a response
rate of 21%, with a median duration of response of 4 months
(11). Use of a combination of
gemcitabine and cisplatin produced a response rate of 11–26.4%,
which was better than gemcitabine only, and the median survival
time was 7.1–8.2 months (12–14). Gemcitabine acts synergistically with
5-FU and with cisplatin (15–20). 5-FU acts synergistically with
cisplatin (21). This suggests that
these three agents may exhibit a triple-synergic effect on advanced
pancreatic cancer.
With the expectation of synergism based on these
previous studies, the present study investigated the efficacy and
toxicities of a combination chemotherapy using a gemcitabine, 5-FU
and cisplatin (GFP) regimen for the treatment of patients with
advanced pancreatic cancer.
Patients and methods
Eligibility
The inclusion criteria were as follows: Patients
with unresectable, relapsed or metastatic adenocarcinoma of the
pancreas (American Joint Committee on Cancer stage III or IV)
(22); at least one measurable lesion
(defined as a mass with clearly demarcated dimensions on computed
tomography, routine chest X-ray or physical examination); no prior
chemotherapy or radiotherapy (lesions outside of the prior
radiation port was acceptable); Eastern Cooperative Oncology Group
performance status (ECOG PS) of 2 or better (23); age between 18 and 70 years; no
concurrent uncontrolled medical illness; no other malignancies
(with the exception of squamous cell carcinoma of the skin treated
by surgery); total bilirubin ≤2 times the upper normal limits
(UNL), transaminases ≤3 times the UNL and alkaline phosphatase ≤2.5
times the UNL; adequate bone marrow function (hemoglobin, ≥9 g/dl;
granulocytes, ≥1,800/µl; and platelets, ≥100,000/µl); and no other
serious organ failure. A complete history was taken and a physical
examination was performed on all patients prior to treatment. A
cancer antigen (CA)19-9 test, electrocardiography, chest X-ray and
abdominal computed tomography (CT) scan were performed. Signed
informed consent was obtained from all patients prior to
chemotherapy. The present study was based on the data from an
initial single center study conducted by Inje University Sanggye
Paik Hospital (Seoul, Korea), which was approved by the ethics
committee of Inje University Saggye Paik Hospital.
Treatment methods
For each patient, 800 mg/m2 gemcitabine
was intravenously infused in D5W solution or 100 ml
normal saline (N/S) at 10 mg/m2/min on day 1 (D1) and
day 8 (D8). 5-FU was continuously infused over 24 h at a dose of
800 mg/m2/day in D5W or N/S 1 l on D1 through
D4. Cisplatin (60 mg/m2) was infused for 3 h on D2, with
a 24-h interval after the start of gemcitabine infusion. The
treatment was repeated every 3 weeks.
Complete blood cell counts (CBCs) were checked
between D8-15 and whenever necessary to check the nadir blood cell
counts thereafter. CBCs, and serum calcium, creatinine, liver
enzyme and electrolyte levels were checked prior to commencing each
treatment cycle. The dose and treatment interval were adjusted
based on the following criteria: The chemotherapy was continued
when the D1 granulocyte and platelet count were ≥1,800/µl and
≥100,000/µl, respectively. If not, the chemotherapy was delayed for
1 week. If the chemotherapy was delayed for longer than 2 weeks
prior to hematological recovery, the study was discontinued. In
cases where a nadir granulocyte count of <500/µl or a platelet
count of <25,000/µl was observed during the previous cycle, the
dose of gemcitabine and cisplatin was reduced by 10% in the next
cycle. The reduction was allowed only once unless the patient
experienced serious infection and/or hemorrhage. On each successive
D8, 100% of gemcitabine was administered in cases where
granulocytes recovered to ≥1,000/µl and platelets recovered to
≥100,000/µl. When the D8 granulocyte count was 500–999/µl and/or
the platelet count was 50,000–99,000/µl, the D8 dose of gemcitabine
was reduced to half. Furthermore, if the granulocyte and/or
platelet counts were <500/µl and <50,000/µl, respectively,
the D8 dose of gemcitabine was administered 1 week later when the
above criteria were met; otherwise, the D8 dose of gemcitabine was
skipped. During the chemotherapy, granulocyte/macrophage-colony
stimulating factor (G/M-CSF) use was allowed according to the
clinician's decision, while prophylactic use was not allowed. When
the creatinine clearance was between 30–50 ml/min, the dose of
cisplatin was reduced to half; cisplatin was discontinued if the
creatinine clearance was <30 ml/min, and the study was
discontinued.
For grade 3/4 stomatitis or diarrhea, 5-FU was
reduced by 25%. If the grade 3/4 toxicity was observed again in the
next cycle, the study was discontinued. When grade 3/4
neurotoxicity (intolerable paresthesia and/or marked motor function
loss) occurred, cisplatin was discontinued and the patient was
taken off the protocol.
Response and toxicity evaluation
A physical examination and chest X-ray was performed
every cycle prior to commencing chemotherapy. An abdominal CT scan
was repeated prior to every 2 cycles of chemotherapy or any time
disease progression was suspected. Blood chemistry and CBCs were
also checked every cycle prior to chemotherapy. If the level of
CA19-9 was high prior to treatment, the tumor marker was checked
every cycle prior to the next chemotherapy.
Response and toxicity were evaluated according to
WHO criteria (24). Complete response
(CR) was defined as disappearance of all measurable or evaluable
disease, signs, symptoms, and biochemical change related to tumor
for at least 4 weeks. A partial response (PR) was defined as a
reduction of ≥50% in the sum of the products of two perpendicular
diameters of all measured lesions lasting ≥4 weeks. Stable disease
(SD) was defined as <50% reduction and <25% increase in the
sum of the products of all measurable lesions without appearance of
new lesion. Progressive disease was defined as the appearance of
any new lesion or definite increase in tumor size and a >25%
increase in the sum of the products of all measured lesions.
Treatment was continued until tumor progression or
unacceptable toxicity occurred. Toxicity was evaluated prior to
each cycle of therapy. The duration of response or SD was measured
from the beginning of treatment until the documentation of
progression. The response assessment was performed every 2 cycles
of chemotherapy.
Statistics
The primary objective of the study was to examine
the response rate and toxicity. The secondary objectives were to
examine progression-free survival (PFS) and OS. The response rate
of the gemcitabine/cisplatin combination for advanced pancreatic
cancer patients has been recorded at 10–30% (12–14). If
40% was used as an expected response rate, 20% as a minimal
acceptable response rate and 10% as a drop-out rate, 33 patients
were required as a minimum, according to the Fleming one-stage
procedure (P1-P0=0.20, α=0.05, β=0.2)
(25).
Proportions and categorical data were compared using
Fisher's exact test. Continuous data were compared using Student's
t-test or the Mann-Whitney U test. PFS time was calculated from the
date of the initial treatment to the date of disease progression or
any cause of mortality. OS time was calculated from the date of the
initial treatment until mortality. Survival curves were estimated
using the Kaplan-Meier method and compared with the log-rank test
and Breslow test. All statistical analyses were performed using SAS
version 9.1 (Cary, NC, USA). All analyses were two-sided and
statistically significant differences were defined by a P-value of
<0.05.
Results
Patient characteristics
Between April 2002 and March 2009, 37 patients were
enrolled into this study. The median age was 61 years old (range,
40–70 years old). The patient characteristics are shown in Table I. In total, 26 (70.3%) patients showed
an ECOG PS grade of 0–1 and 11 patients (29.7%) showed a grade of
2. A total of 8 patients underwent a percutaneous transhepatic
biliary drainage procedure due to obstructive jaundice prior to
chemotherapy. After the completion of the study, 29 patients were
evaluable and 8 patients dropped out early without the assessment
of response.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| Median age (range),
years | 61 (40–70) |
| Gender, n (%) |
|
| Male | 27 (73.0) |
|
Female | 10 (27.0) |
| Performance status, n
(%) |
|
| 0–1 | 26 (70.3) |
| 2 | 11 (29.7) |
| Disease status, n
(%) |
|
|
Unresectable/metastatic | 5/27 (13.5/73.0) |
|
Recurrent | 5
(13.5) |
| Previous treatment, n
(%) |
|
| No
previous treatment | 28 (75.7) |
|
Palliative surgery | 4
(10.8) |
| Surgery ±
adjuvant chemo- and/or radiotherapy | 5
(13.5) |
| Primary sites, n
(%) |
|
| Head | 16 (43.2) |
| Body and
tail | 21 (56.8) |
| Metastatic site, n
(%) |
|
|
Liver | 20 (54.1) |
| Distant
lymph node | 19 (51.4) |
|
Peritoneum | 14 (37.8) |
| Lung | 6
(16.2) |
Dose intensity of treatment drugs
A total of 153 cycles of treatment were
administered, with a median of 3 cycles per patient (range, 1–12
cycles), and the dose intensity data was available in 147 cycles.
D1 gemcitabine was administered at 97.8% during the 147 cycles of
chemotherapy, D8 gemcitabine was administered at 75.0%, cisplatin
was administered at 96.9% and 5-FU was administered at 97.6%. Among
the 8 patients who could not be evaluated, a total of 10 cycles
were administered, with a median of 1 cycle per patient (range, 1–2
cycles). During the 10 cycles, D8 gemcitabine was omitted in 3
cycles and reduced to the half dose in 2 cycles.
Treatment response and outcomes
Among the 29 patients evaluable for the response, 7
(24.1%) patients exhibited a PR and 13 (44.8%) patients experienced
SD (Table II). The overall response
rate was 24.1% [95% confidence interval (CI), 12.2–42.1) and the
disease control rate was 69.0% (95% CI, 50.8–82.7). The median PFS
and OS times of the evaluable patients were 4.1 months (95% CI,
3.0–5.2) and 6.6 months (95% CI, 4.9–8.2), respectively. The median
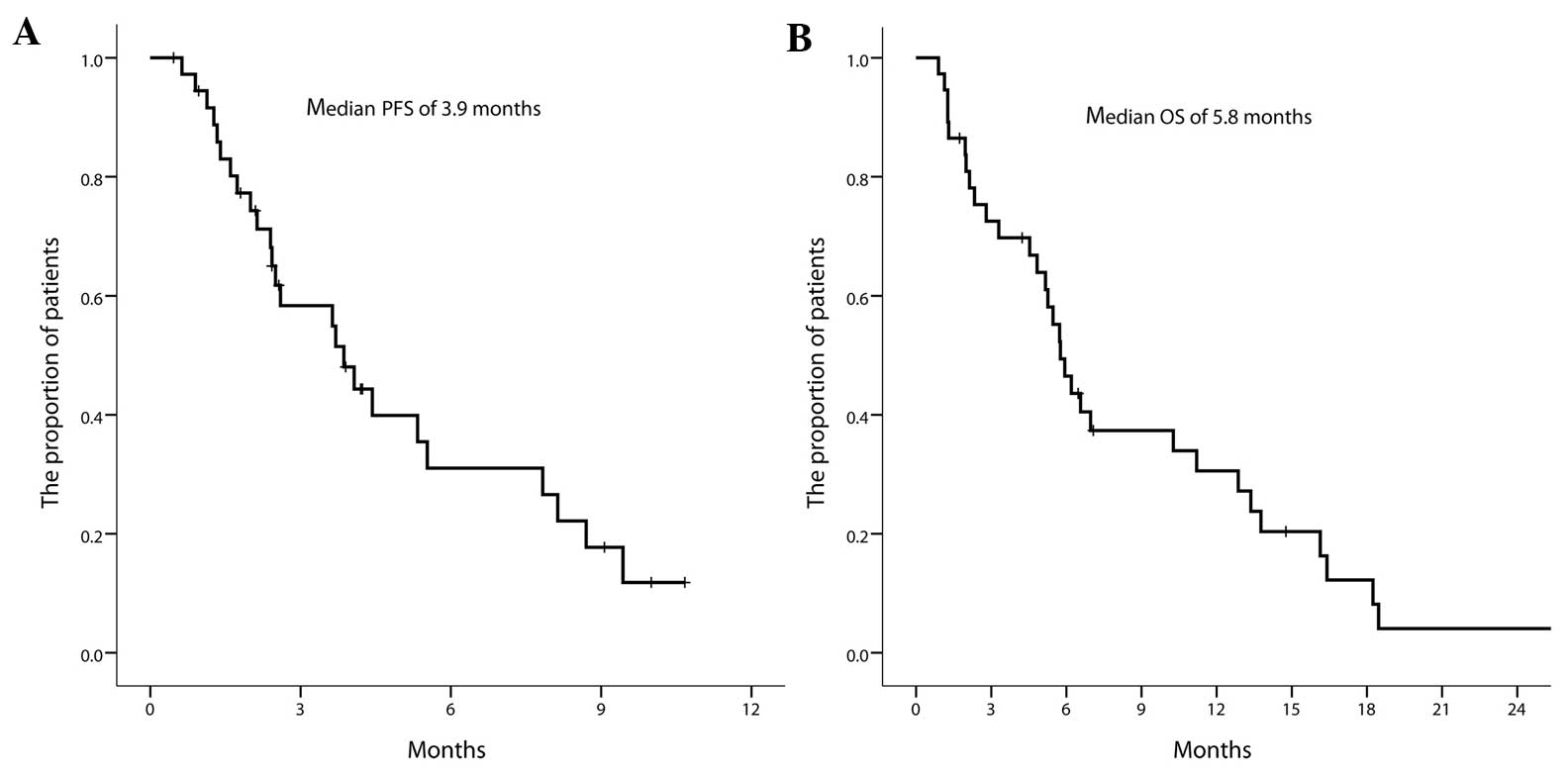
PFS and OS times of all enrolled patients were 3.9 months (95% CI,
2.4–5.4) and 5.8 months (95% CI, 4.8–6.8), respectively (Fig. 1A and B). The PFS rate of all enrolled
patients was 61.5±8.5, 30.9±9.0 and 17.6±7.7% at 3, 6 and 9 months,
respectively. A total of 10 patients underwent second-line therapy
after progression. The most frequently used regimen was an oral
5-FU derivative-based regimen in 9 patients. The OS rate was
46.5±8.4 and 30.6±8.0% at 6 and 12 months, respectively. The
response rate and OS time were not associated with any clinical
factors, including age (<65 vs. ≥65 years) and PS (<2 vs. ≥2)
(both P=1.000).
 | Table II.Overall best response in the 29
evaluable patients. |
Table II.
Overall best response in the 29
evaluable patients.
| Response | Value |
|---|
| Complete response,
n (%) | 0
(0.0) |
| Partial response, n
(%) | 7
(24.1) |
| Stable disease, n
(%) | 13 (44.8) |
| Progressive
disease, n (%) | 9
(31.0) |
| ORR, % (95%
CI) | 24.1
(12.2–42.1) |
| DCR, % (95%
CI) | 69.0
(50.8–82.7) |
Safety
During the 153 cycles of chemotherapy, toxicity was
observed in 147 cycles (Table III).
Grade 3/4 leukopenia, neutropenia and thrombocytopenia were
documented in 18.4, 29.9 and 24.5% of 147 cycles, respectively.
Grade 3/4 oral mucositis was observed in 4.1% of patients and grade
3 nausea/vomiting and diarrhea in 2.0 and 1.4% of 147 cycles,
respectively. The incidence of grade 3/4 neutropenia (the total
number of grade 3/4 neutropenia/total received cycles of each
patient) and grade 3/4 thrombocytopenia (the total number of grade
3/4 thrombocytopenia/total received cycles of each patient) were
associated with PS at diagnosis. The poor PS (PS of 2) was
associated with a higher incidence of grade 3/4 neutropenia
(P=0.009) and grade 3/4 thrombocytopenia (P=0.006) compared with a
PS of 0/1. Although the elderly (≥65 years) more frequently
exhibited a poor PS [PS of 2; 4 out of 9 (44.4%) patients], this
was not statistically significant (P=0.571). Also, the incidence of
neutropenia and thrombocytopenia was not associated with age
(P=0.468 and P=0.906, respectively).
 | Table III.Toxicity profiles observed in 147
cycles. |
Table III.
Toxicity profiles observed in 147
cycles.
|
| Observed cycles, n
(%) | Observed patients,
n (%) |
|---|
|
|
|
|
|---|
| Toxicity | Grade 3 | Grade 4 | Grade 3 | Grade 4 |
|---|
| Hematological |
|
|
|
|
|
Anemia | 13 (8.8) | – | 7 (18.9) |
|
|
Leukopenia | 22 (15.0) | 5 (3.4) | 12 (32.4) | 5
(13.5) |
|
Neutropenia | 26 (17.7) | 18 (12.2) | 9 (24.3) | 14 (37.8) |
|
Thrombocytopenia | 20 (13.6) | 16 (10.9) | 8 (21.6) | 12 (32.4) |
|
Non-hematological |
|
|
|
|
|
Nausea/vomiting | 3 (2.0) | – | 3 (8.1) |
|
|
Mucositis | 4 (2.7) | 2 (1.4) | 3 (8.1) | 2 (5.4) |
|
Diarrhea | 2 (1.4) | – | 1 (2.7) |
|
|
Neuropathy | – | – |
|
|
In the 8 patients whose response could not be
assessed due to early treatment interruption prior to first
response evaluation, 5 of the patients succumbed prior to the first
response evaluation (3 to sepsis, 1 to pneumonia and 1 to
demyelinating disease), and 3 patients were taken off the study due
to adverse events or other diseases (lethargy, cerebral infarct and
pneumonia).
Clinical characteristics associated
with early discontinuation
Early discontinuation (≤3 cycles of chemotherapy
without evident disease progression) occurred in 10 patients. Among
those 10 patients, 8 dropped out early prior to first response
assessment and 2 patients showed SD at first assessment and
withdrew their consent for participation. The median age of the 10
patients who dropped out of the study was 64.5 years (range, 56–69
years), while the median age of the other 27 patients was 60.0
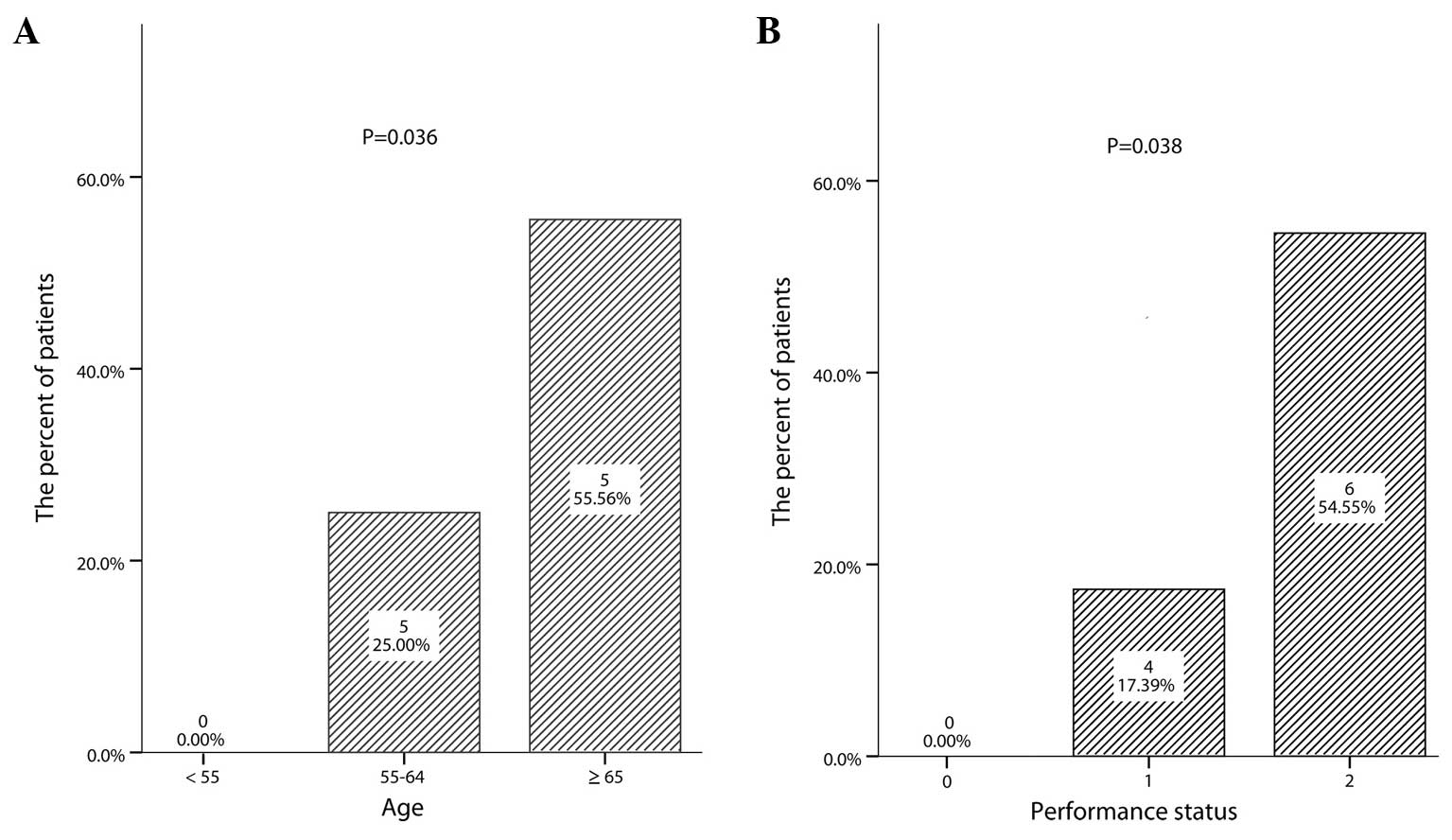
years (range, 40–70 years). The incidence of early discontinuation
was associated with old age (P=0.036; Fig. 2A) and a poor PS (P=0.038; Fig. 2B). A poor PS was observed in 6 (60.0%)
of the 10 early discontinuation patients compared with 5 (18.5%) of
the other 27 patients. However, the early discontinuation was not
associated with other clinical characteristics, such as gender
(P=0.229), percutaneous transhepatic biliary drainage insertion
(0.404), stage (P=0.229), relapse (P=0.284), primary lesions
(P=0.700) and metastatic site (liver, P=0.700; peritoneum, P=0.214;
lung, P=0.303; and distant lymph node, P=0.128). Also, the
incidence of grade 3/4 neutropenia and thrombocytopenia was not
associated with early discontinuation (P=0.537 and P=0.201,
respectively). The non-hematological toxicities could not be
analyzed due to a low incidence in this study.
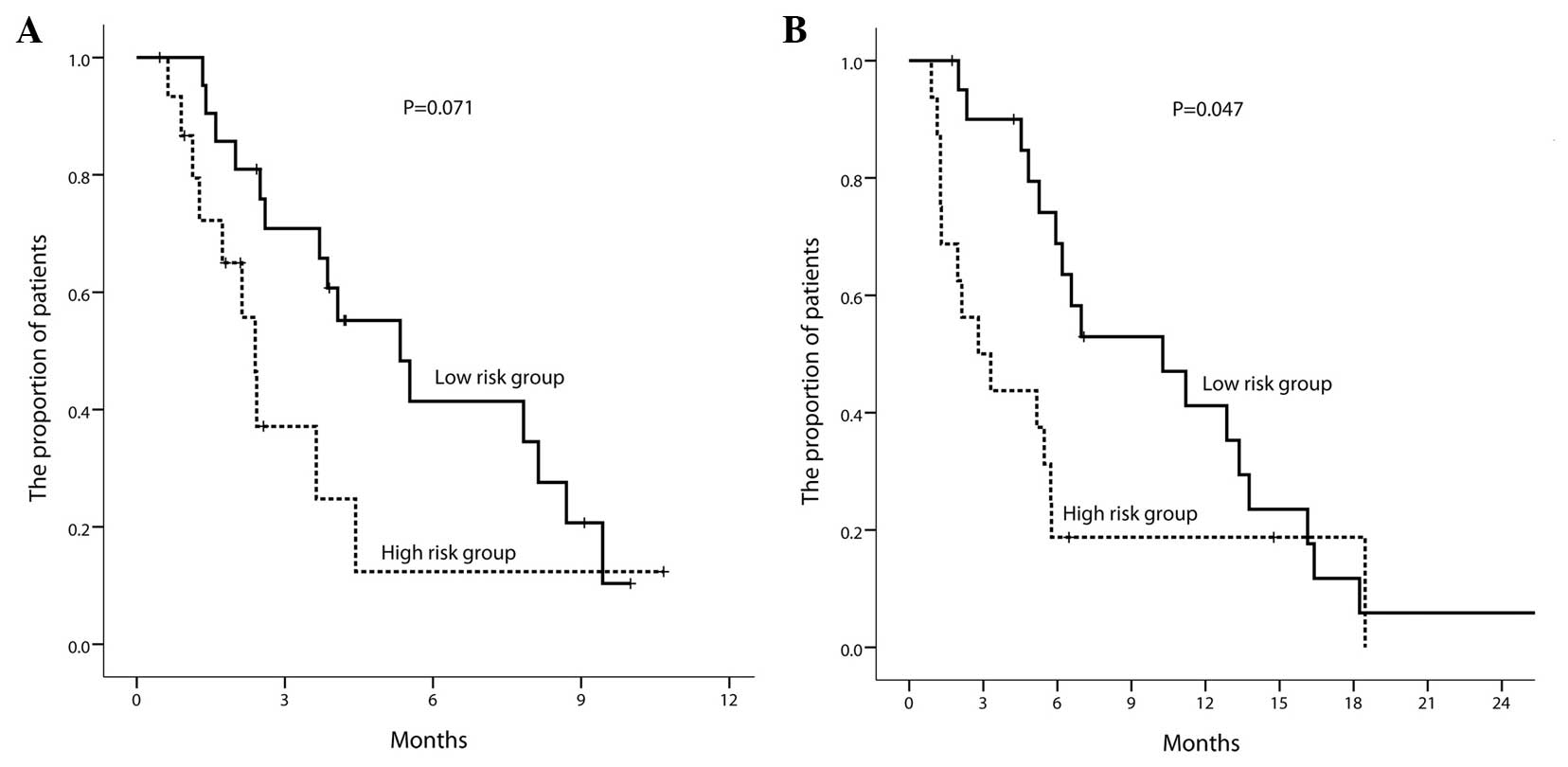
As old age and a poor PS were correlated with early
discontinuation, the patients were separated into two groups: A
high-risk group (n=16; age ≥65 or PS ≥2) and a low-risk group
(n=21; age <65 and PS <2). According to these criteria, the
median PFS time was 2.4 months (95% CI, 2.0–2.8) in the high-risk
group and 5.3 months (95% CI, 2.9–7.7) in the low-risk group
(P=0.071; P=0.021 in Breslow test) (Fig.
3A). The median OS time was 2.8 months (95% CI, 0.5–5.1) in the
high-risk group and 10.3 months (95% CI, 4.1–16.4) in the low-risk
group (P=0.047; Fig. 3B).
Discussion
The triplet GFP chemotherapy showed an acceptable
disease control rate (24.1% for PR and 44.8% for SD) and a modest
efficacy for median PFS and OS times (3.9 and 5.8 months,
respectively). The 6-month PFS rate was 30.9% and the 1-year
survival rate was 30.6%.
In a recent phase III study, the folinic acid, 5-FU,
irinotecan and oxaliplatin (FOLFIRINOX) regimen resulted in a
6-month PFS rate of 52.8% and a 1-year OS rate of 48.4% in advanced
pancreatic cancer patients (26).
However, gemcitabine monotherapy remains the reference regimen for
advanced pancreatic cancer (2,27), as the
combination of gemcitabine with other cytotoxic drugs has not shown
significant benefit, except in three studies (28–30). In
phase II trials of triplet cytotoxic chemotherapy [i.e.,
gemcitabine/docetaxel/capecitabine, gemcitabine/oxaliplatin/5-FU,
gemcitabine/5-FU/cisplatin (GFP) or
mitomycin/docetaxel/irinotecan], the previously reported overall
response rate was between 0 and 33.3% and the median OS time was
between 6.1 and 14.5 months (31–35).
Although the response rate in the present study was acceptable, the
5.9-month median OS time may appear to be inferior to those of the
reported phase II triplet trials. However, if the time-point
survival rate is compared with those of previously reported triplet
trials, the 30.9% 1-year OS rate in the present study is comparable
with the results of the previous phase II GFP trials with 1-year OS
rates of 26–34% (34,35). As 10 patients in the present study
experienced early discontinuation, which is a major cause of
shortened median survival time, this discrepancy between time-point
survival rate and median survival time may indicate that the major
drawback of this triplet GFP regimen is the difficulty of long-term
maintenance rather than the efficacy of treatment. Also, this
discrepancy may explain why the median OS time of the GFP regimen
in the present study appears to be inferior to that of previous
duplet regimens, such as the combination of gemcitabine with
cisplatin (7.1–8.3 months) (12–14,29,36),
gemcitabine with oxaliplatin (9.2 months) (37), and gemcitabine with fluoropyrimidine
(6.7–10.3 months) (9,10,28,38).
To find the appropriate group for triplet GFP
chemotherapy, selection of the low-risk and high-risk group for
early discontinuation was performed according to age and PS. After
grouping all the enrolled patients into the two risk groups, the
low-risk group showed an acceptable 5.3-month median PFS time and a
10.3-month median OS time, which were comparable with previously
reported duplet or triplet regimens. Although the performance
scales are somewhat subjective, there is a possibility that the PS
at the initiation of chemotherapy may be a predictor of poor
tolerance in hematological toxicity to triplet GFP chemotherapy.
Also, elderly patients (≥65) adhered poorly to the triplet GFP
chemotherapy. However, the exact reason is uncertain in the present
study, as non-hematological toxicities could not be analyzed due to
the low incidence in this study.
This triplet regimen showed substantial
hematological toxicities. Grade 3/4 neutropenia and
thrombocytopenia occurred in more than a half of the patients, who
required subsequent dose reduction. This frequent grade 3/4
hematological toxicity may inevitably deteriorate the general
medical condition of vulnerable patients with a poor PS, and this
probably caused early discontinuation prior to even attaining any
clinical benefits. As the study showed that the a poor PS was
associated with more frequent hematological toxicities, careful
monitoring of the CBC and prophylactic G-CSF use may be suggested
during chemotherapy.
In conclusion, the GFP regimen has comparable
activity to gemcitabine-based single or duplet chemotherapy in
disease control, and modest efficacy in survival. This triplet GFP
chemotherapy caused substantial hematological toxicity and a high
rate of early discontinuation in the patients with an old age and
poor PS. However, even when considering the result found in the
younger patients with a good PS, these results do not appear to
have marked superiority to gemcitabine-based single or duplet
chemotherapy.
References
|
1
|
Heinemann V: Gemcitabine: Progress in the
treatment of pancreatic cancer. Oncology. 60:8–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg Ml, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997.PubMed/NCBI
|
|
3
|
Carmichael J, Fink U, Russell RC, Spittle
MF, Harris AL, Spiessi G and Blatter J: Phase II study of
gemcitabine in patients with advanced pancreatic cancer. Br J
Cancer. 73:101–105. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crinò L, Mosconi AM, Calandri C, Corgna E,
Porrozzi S, Chiara S, Nobili MT and Tonato M: Gemcitabine in
advanced pancreatic cancer: A phase II trial. Am J Clin Oncol.
24:296–298. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rocha Lima CM, Savarese D, Bruckner H,
Dudek A, Eckardt J, Hainsworth J, Yunus F, Lester E, Miller W,
Saville W, et al: Irinotecan plus gemcitabine induces both
radiographic and CA 19-9 tumor marker responses in patients with
previously untreated advanced pancreatic cancer. J Clin Oncol.
20:1182–1191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stathopoulos GP, Mavroudis D, Tsavaris N,
Kouroussis C, Aravantinos G, Agelaki S, Kakolyris S, Rigatos SK,
Karabekios S and Georgoulias V: Treatment of pancreatic cancer with
a combination of docetaxel, gemcitabine and granulocyte
colony-stimulating factor: A phase II study of the Greek
Cooperative Group for Pancreatic Cancer. Ann Oncol. 12:101–103.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shepard RC, Levy DE, Berlin JD, Stuart K,
Harris JE, Aviles V, Thomas JP and Benson AB III: Phase II Study of
gemcitabine in combination with docetaxel in patients with advanced
pancreatic carcinoma (E1298). A trial of the eastern cooperative
oncology group. Oncology. 66:303–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oettle H, Arning M, Pelzer U, Arnold D,
Stroszczynski C, Langrehr J, Reitzig P, Kindler M, Herrenberger J,
Musch R, et al: A phase II trial of gemcitabine in combination with
5-fluorouracil (24-hour) and folinic acid in patients with
chemonaive advanced pancreatic cancer. Ann Oncol. 11:1267–1272.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hidalgo M, Castellano D, Paz-Ares L,
Gravalos C, Diaz-Puente M, Hitt R, Alonso S and Cortes-Funes H:
Phase I-II study of gemcitabine and fluorouracil as a continuous
infusion in patients with pancreatic cancer. J Clin Oncol.
17:585–592. 1999.PubMed/NCBI
|
|
10
|
Berlin JD, Catalano P, Thomas JP, Kugler
JW, Haller DG and Benson AB III: Phase III Study of gemcitabine in
combination with fluorouracil versus gemcitabine alone in patients
with advanced pancreatic carcinoma: Eastern Cooperative Oncology
Group Trial E2297. J Clin Oncol. 20:3270–3275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wils JA, Kok T, Wagener DJT, Selleslags J
and Duez N: Activity of cisplatin in adenocarcinoma of the
pancreas. Eur J Cancer. 29A:203–204. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heinemann V, Wilke H, Mergenthaler HG,
Clemens M, König H, Illiger HJ, Arning M, Schalhorn A, Possinger K
and Fink U: Gemcitabine and cisplatin in the treatment of advanced
or metastatic pancreatic cancer. Ann Oncol. 11:1399–1403. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Colucci G, Giuliani F, Gebbia V, Biglietto
M, Rabitti P, Uomo G, Cigolari S, Testa A, Maiello E and Lopez M:
Gemcitabine alone or with cisplatin for the treatment of patients
with locally advanced and/or metastatic pancreatic carcinoma: A
prospective, randomized phase III study of the Gruppo Oncologia
dell'Italia Meridionale. Cancer. 94:902–910. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Philip PA, Zalupski MM, Vaitkevicius VK,
Arlauskas P, Chaplen R, Heilbrun LK, Adsay V, Weaver D and Shields
AF: Phase II study of gemcitabine and cisplatin in the treatment of
patients with advanced pancreatic carcinoma. Cancer. 92:569–577.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruckner HW, Zhou G, Haenel P, Szrajer L,
Greenspan E and Kurbacher C: Ex vivo ATP tumor testing of
gemcitabine for combination chemotherapy and biochemical
modulation. Proc Am Assoc Cancer Res. 39:310a(abstract 2116).
1998.
|
|
16
|
Bergman AM, Ruiz van Haperen VW, Veerman
G, Kuiper CM and Peters GJ: Synergistic interaction between
cisplatin and gemcitabine in vitro. Clin Cancer Res. 2:521–530.
1996.PubMed/NCBI
|
|
17
|
Peters GJ, Ruiz van Haperen VW, Bergman
AM, Veerman G, Smitskamp-Wilms E, van Moorsel CJ, Kuiper CM and
Braakhuis BJ: Preclinical combination therapy with gemcitabine and
mechanisms of resistance. Semin Oncol. 23:(Suppl 10). 16–24.
1996.PubMed/NCBI
|
|
18
|
Peters GJ, Bergman AM, Ruiz van Haperen
VW, Veerman G, Kuiper CM and Braakhuis BJ: Interaction between
cisplatin and gemcitabine in vitro and in vivo. Semin Oncol.
22:(Suppl 11). 72–79. 1995.PubMed/NCBI
|
|
19
|
Peters GJ, Bergman AM, Veerman G and Ruiz
van Haperen VWT: Synergism between cisplatin and gemcitabine (dFdC)
in resistant A2780 human ovarian cancer cell lines is schedule
dependent. Proc Am Assoc Cancer Res. 35:1950(abstract). 1994.
|
|
20
|
Braakhuis BJM, Ruiz van Haperen VWT,
Bergman AM, Welters MJP and Peters GJ: Preclinical in vivo
evaluation of the combination of 2,3 difluorodeoxycytidine (dFdC,
gemcitabine) and cisplatin (CDDP). Ann Oncol. 5:82(abstract 054).
1994.
|
|
21
|
Schabel FM Jr, Trader MW, Laster WR Jr,
Corbett TH and Griswold DP Jr: cis-Dichlorodiammineplatinum(II):
Combination chemotherapy and cross-resistance studies with tumors
of mice. Cancer Treat Rep. 63:1459–1473. 1979.PubMed/NCBI
|
|
22
|
Greene F, Page D, Fleming I, Fritz A,
Balch C, Haller D and Morrow M: AJCC Cancer Staging Manual. 6th.
Springer; New York, NY, USA: pp. 157–161. 2002
|
|
23
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fleming TR: One-sample multiple testing
procedure for phase II clinical trials. Biometrics. 38:143–151.
1982. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Di Marco M, Di Cicilia R, Macchini M,
Nobili E, Vecchiarelli S, Brandi G and Biasco G: Metastatic
pancreatic cancer: Is gemcitabine still the best standard
treatment? (Review). Oncol Rep. 23:1183–1192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Herrmann R, Bodoky G, Ruhstaller T,
Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A,
Pestalozzi B, et al: Gemcitabine plus capecitabine compared with
gemcitabine alone in advanced pancreatic cancer: A randomized,
multicenter, phase III trial of the Swiss Group for Clinical Cancer
Research and the Central European Cooperative Oncology Group. J
Clin Oncol. 25:2212–2217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heinemann V, Quietzsch D, Gieseler F,
Gonnermann M, Schönekäs H, Rost A, Neuhaus H, Haag C, Clemens M,
Heinrich B, et al: Randomized phase III trial of gemcitabine plus
cisplatin compared with gemcitabine alone in advanced pancreatic
cancer. J Clin Oncol. 24:3946–3952. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heinemann V, Boeck S, Hinke A, Labianca R
and Louvet C: Meta-analysis of randomized trials: Evaluation of
benefit from gemcitabine-based combination chemotherapy applied in
advanced pancreatic cancer. BMC Cancer. 8:822008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reni M, Panucci MG, Passoni P, Bonetto E,
Nicoletti R, Ronzoni M, Zerbi A, Staudacher C, Di Carlo V and Villa
E: Salvage chemotherapy with mitomycin, docetaxel, and irinotecan
(MDI Regimen) in metastatic pancreatic adenocarcinoma: A Phase I
and II Trial. Cancer Invest. 22:688–696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fine R, Moorer G, Sherman W, et al: Phase
II trial of GTX chemotherapy in metastatic pancreatic cancer. J
Clin Oncol. 27:(Suppl 15): abstract. 46232009.
|
|
33
|
Correale P, Montagnani F, Miano S,
Sciandivasci A, Pascucci A, Petrioli R, Testi W, Tanzini G and
Francini G: Biweekly triple combination chemotherapy with
gemcitabine, oxaliplatin, levofolinic acid and 5-fluorouracil
(GOLF) is a safe and active treatment for patients with inoperable
pancreatic cancer. J Chemother. 20:119–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
El-Rayes BF, Zalupski MM, Shields AF,
Vaishampayan U, Heilbrun LK, Jain V, Adsay V, Day J and Philip PA:
Phase II study of gemcitabine, cisplatin, and infusional
fluorouracil in advanced pancreatic cancer. J Clin Oncol.
21:2920–2925. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Novarino A, Chiappino I, Bertelli GF,
Heouaine A, Ritorto G, Addeo A, Bellone G, Merlano M and Bertetto
O: Phase II study of cisplatin, gemcitabine and 5-fluorouracil in
advanced pancreatic cancer. Ann Oncol. 15:474–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Colucci G, Labianca R, Di Costanzo F,
Gebbia V, Cartenì G, Massidda B, Dapretto E, Manzione L, Piazza E,
Sannicolò M, et al: Randomized phase III trial of gemcitabine plus
cisplatin compared with single-agent gemcitabine as first-line
treatment of patients with advanced pancreatic cancer: The GIP-1
study. J Clin Oncol. 28:1645–1651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Louvet C, André T, Lledo G, Hammel P,
Bleiberg H, Bouleuc C, Gamelin E, Flesch M, Cvitkovic E and de
Gramont A: Gemcitabine combined with oxaliplatin in advanced
pancreatic adenocarcinoma: Final results of a GERCOR multicenter
phase II study. J Clin Oncol. 20:1512–1518. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Di Costanzo F, Carlini P, Doni L, Massidda
B, Mattioli R, Iop A, Barletta E, Moscetti L, Recchia F, Tralongo
P, et al: Gemcitabine with or without continuous infusion 5-FU in
advanced pancreatic cancer: A randomised phase II trial of the
Italian oncology group for clinical research (GOIRC). Br J Cancer.
93:185–189. 2005. View Article : Google Scholar : PubMed/NCBI
|