Introduction
In recent years, a significant progression in the
field of cancer research has been the clarification of the
important role of tumor angiogenesis in the development of tumors
and, thus, the significance of antiangiogenic therapy for the
treatment of cancer (1,2). Tumor angiogenesis refers to the process
in which the growth of capillary vessels is induced by tumor cells
and blood circulation is established in the tumor microenvironment.
Angiogenesis is important in the growth, invasion and metastasis of
the tumor. The concept of the angiogenic switch, which was
initially proposed by Hanahan and Folkman (1) in 1996, further clarified that the
proliferation and metastasis of primary solid tumors is dependent
on angiogenesis, and is regulated by pro-angiogenesis factors and
angiogenic inhibitory factors. Consequently, the association
between capillary vessels, and tumor growth, invasion, metastasis
and prognosis has become a popular research topic (2).
Cluster of differentiation (CD) 34 is a specific
marker of vascular endothelial cells. In particular, CD34 is
particularly sensitive to tumor angiogenesis, as it can clearly
represent the state of neovascularization during the growth of a
tumor (3). Vascular endothelial
growth factor (VEGF), a factor involved in vascular endothelial
proliferation, can specifically promote cell proliferation and
angiogenesis, and is closely associated with the growth, invasion
and metastasis of tumors. Therefore, CD34 and VEGF are two
important indicators of tumor angiogenesis. Recently, the
associations between CD34 and VEGF expression, and the
clinicopathological characteristics of patients with cancer have
been reported; however, the association between these associations
and the survival of patients with cancer have rarely been reported.
It has previously been demonstrated that the expression of CD34 and
VEGF is of great importance for determining the prognosis of cancer
patients (4–7), however, certain studies have argued that
there is no significant association between the expression of CD34
and VEGF, and the survival of patients with cancer (8,9).
Therefore, the aim of the present study was to analyze the
association between the expression of CD34 and VEGF, and the
survival of patients with breast cancer, in order to identify the
clinical significance of these two indicators in determining the
prognosis of patients with breast cancer.
Subjects and methods
Subjects
Paraffin-embedded, formalin-fixed tissue blocks of
resected breast cancer from 44 female patients (mean age, 57.14
years; range, 32–82 years) were obtained from the histopathology
archives of Zhejiang Cancer Hospital (Hangzhou, China). All
subjects were hospitalized and received treatment in Zhejiang
Cancer Hospital between February 2006 and December 2006. Follow-up,
which terminated in April 2013, was conducted for 41–88 months,
with an average follow-up time of 62 months. Furthermore, no
patients had received pre-operative radiotherapy or chemotherapy. A
review of the clinicopathological data identified 1 case of
medullary carcinoma, 1 case of metaplastic carcinoma (squamous cell
carcinoma) and 42 cases of invasive ductal carcinoma. According to
the 2010 edition of the Union for International Cancer Control
(UICC) clinical staging system (10),
there were 8 cases of stage I disease, 27 cases of stage II and 9
cases of stage III. If typed according to estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor-2
(HER-2) immunohistochemical data, the tumors could be classified as
luminal A-type (ER+ or PR+, plus
HER-2−), luminal B-type (ER+ or
PR+, plus HER-2+), HER-2 overexpression-type
(ER−/PR−/HER-2+) and
triple-negative-type
(ER−/PR−/HER-2−) breast cancer.
Additionally, the p53 expression status was obtained from the
clinicopathological data of the 44 specimens, among which there
were 23 positive cases. The present study was conducted in
accordance with the declaration of Helsinki and with the approval
of the Ethics Committee of Zhejiang Cancer Hospital. Written
informed consent was obtained from all participants.
Immunohistochemical analysis
The expression of CD34 and VEGF was detected using
the EnVision immunohistochemical method (Dako, Glostrup, Denmark),
according to the manufacturer's instructions. Briefly, the tissue
sections were deparaffinized and rehydrated conventionally, and
then incubated with 1 mM EDTA for 10–15 min at 100°C in a pressure
cooker for antigen retrieval. Following incubation with 3%
H2O2 for 5 min to remove endogenous
peroxides, the sections were washed with phosphate-buffered saline
and then incubated with rabbit monoclonal CD34 (clone EP373Y;
dilution, 1:100; cat. no. ab81289; Abcam, Cambridge, MA, USA) or
polyclonal VEGF (clone A-20; dilution, 1:300; cat. no. sc-152G;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) antibodies for 2 h
at room temperature, followed by incubation with horseradish
peroxidase-conjugated secondary antibody (dilution, 1:100; cat. no.
SA00001-17; Proteintech Group, Inc., Chicago, IL, USA). The signal
was detected using 3,3′-diaminobenzidine substrate and then
sections were counterstained with hematoxylin to visualize the
nuclei.
Interpretation of immunohistochemical
results
CD34 expression in vascular endothelial
cells
According to the method described by Weidner
(11), endothelial cell clusters
expressing CD34 and forming lumen or vessels were counted as
individual microvessels. However, a luminal area larger than the
sum of the diameters of eight erythrocytes, or a blood vessel with
a thick muscular layer or a single positive cell were not counted
as microvessels. Tumor sections were scanned under light microscopy
(TH4-200; Olympus Corporation, Tokyo, Japan) at low power
(magnification, x40) to identify vascular intensive areas (hot
spots). Individual tumor microvessels were then counted at high
power (magnification, x100) in five fields and the mean vessel
count in three hot spots was used as the microvessel density (MVD).
However, in contrast to the methods described by Keyhani et
al (12), the present study
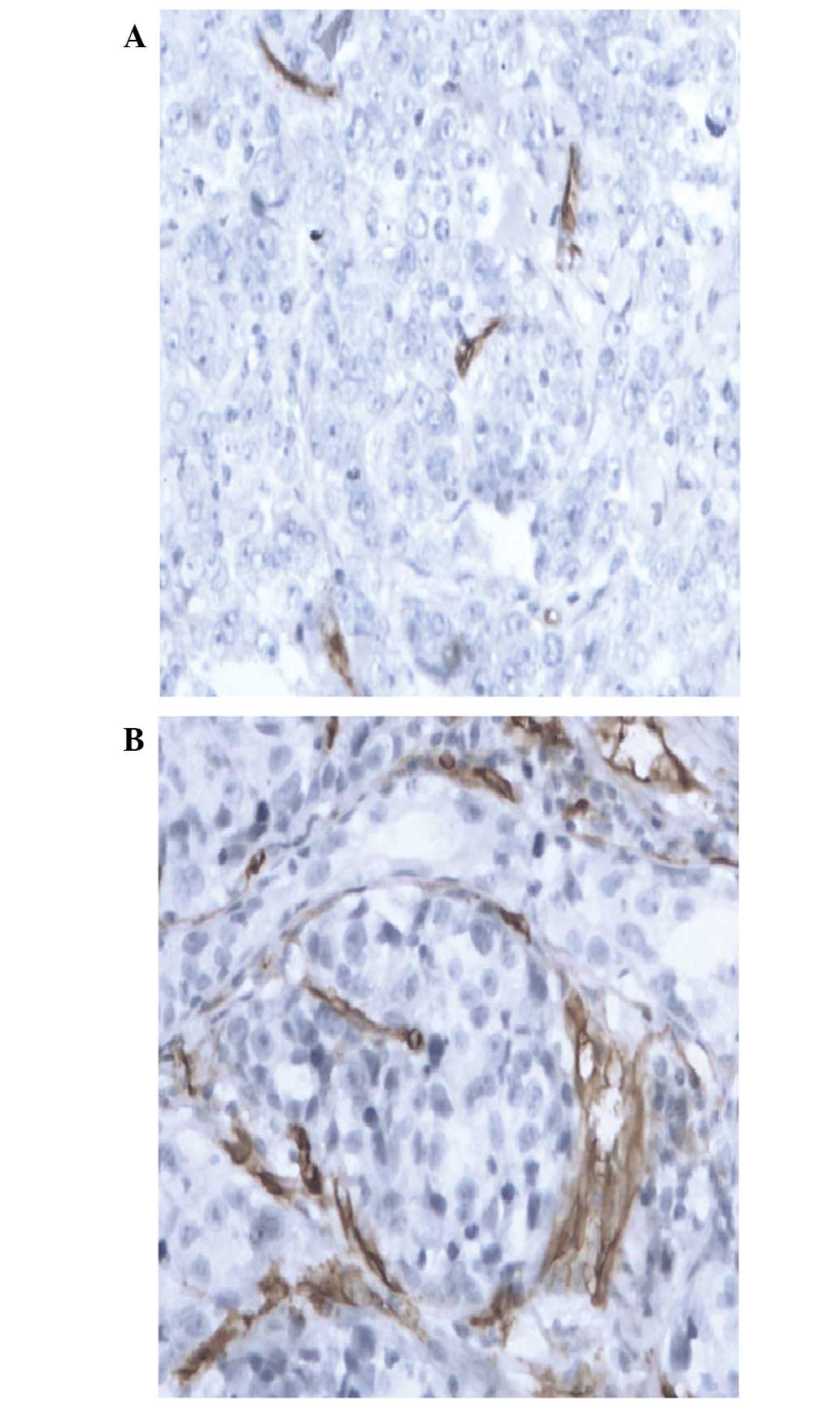
defined a low expression CD34 level as an MVD value of ≤15/HPF
(Fig. 1A) and a high CD34 expression
level as an MVD value of >15/HPF (Fig.
1B).
VEGF expression in vascular endothelial
cells
The expression intensity of VEGF and its
distribution in the tumor samples were observed at high power
(magnification, x100), and semi-quantitatively analyzed.
Cytoplastic brown granules in the cytoplasm or membrane of the
tumor cells were considered as positive for VEGF expression when
the proportion of immunoreactive cells was ≥5% [<5% staining,
negative (0); 5–25% staining, weakly positive (+); 26–50% staining,
positive (++); and >50% staining, strongly positive (+++)]. For
statistical analysis, samples with 0/+ staining were included in
the low VEGF expression group, while samples with ++/+++ expression
were included in the high VEGF expression group.
Statistical analysis
SPSS statistical software (version 17.0; SPSS, Inc.,
Chicago, IL, USA) was used to perform the statistical analyses in
the present study. Comparisons between groups were analyzed using a
χ2 test and univariate survival analysis was conducted
using the Kaplan-Meier method. In addition, a log-rank test was
performed to identify significant factors for Cox regression
multivariate analysis and ultimately to determine independent
factors affecting the survival of the patients. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of CD34 in breast
cancer
As determined by immunohistochemical analysis, 32
specimens of breast cancer expressed low levels of CD34, with an
MVD of ≤15/HPV, accounting for 72.7% of cases. By contrast, 12
specimens expressed high levels of CD34, with an MVD of >15/HPV,
accounting for 27.3% of cases. The expression of CD34 had no
significant correlation with the clinicopathological factors of the
patients enrolled in the present study (P>0.05; Table I). However, Kaplan-Meier analysis
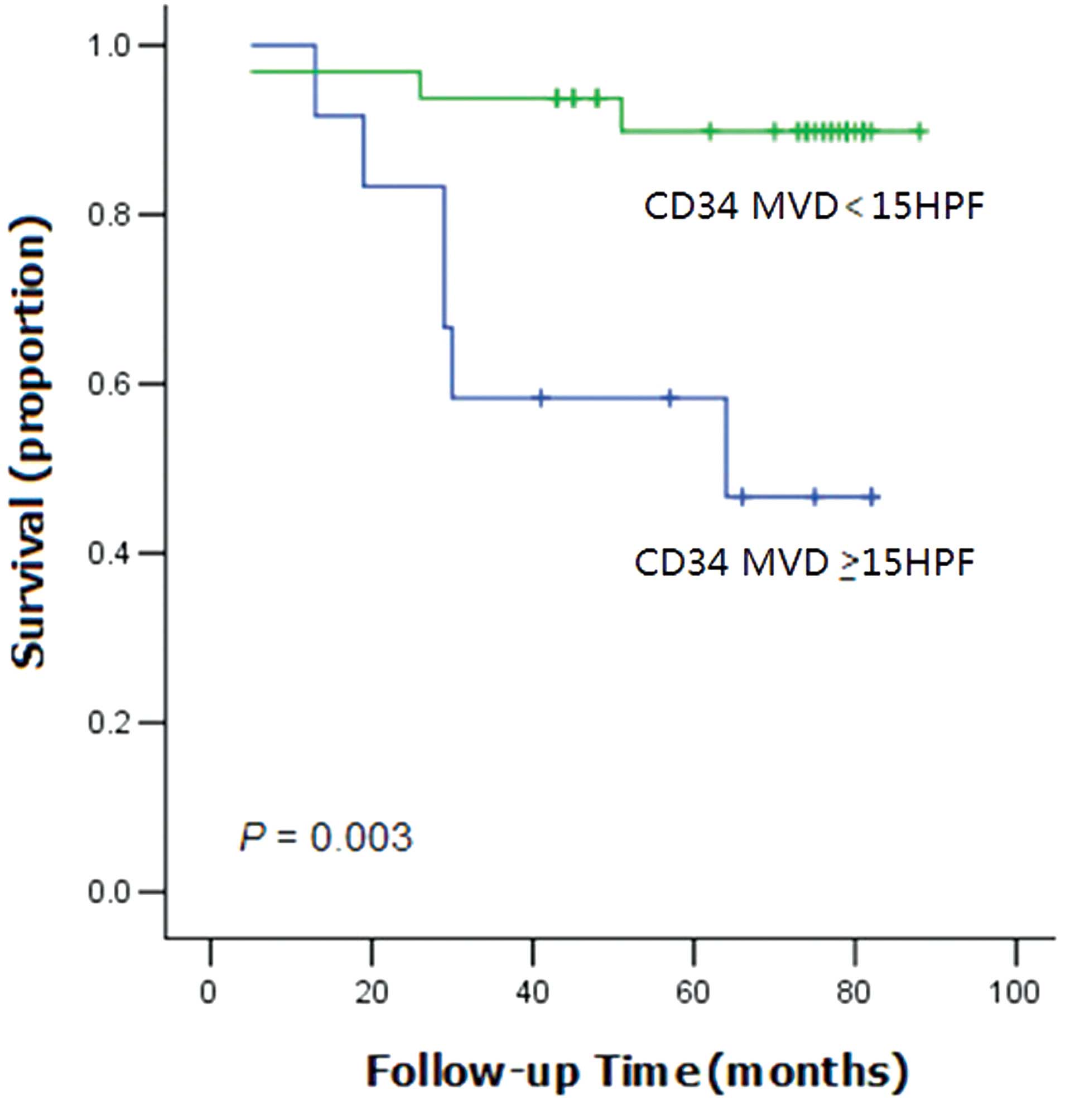
demonstrated that the overall survival (OS) time of the patients
with high CD34 expression levels was significantly shorter than the
OS time of patients with low CD34 expression (P=0.003; Fig. 2).
 | Table I.Association between CD34 and VEGF
expression and various clinicopathological factors. |
Table I.
Association between CD34 and VEGF
expression and various clinicopathological factors.
|
| CD34 expression, n
(%) |
| VEGF expression, n
(%) |
|
|---|
|
|
|
|
|
|
|---|
| Parameter | ≤15/HPF | >15/HPF | P-value | 0/+ | ++/+++ | P-value |
|---|
| Age, years |
|
| 0.249 |
|
| 0.006 |
| ≥50 | 6
(21.4) | 22 (78.6) |
| 6
(21.4) | 22 (78.6) |
|
|
<50 | 6
(37.5) | 10 (62.5) |
| 10 (62.5) | 6
(37.5) |
|
| TNM stage |
|
| 0.647 |
|
| 0.798 |
| I–II | 9
(25.7) | 26 (74.3) |
| 13 (37.1) | 22 (62.9) |
|
| III | 3
(33.3) | 6
(66.7) |
| 3
(33.3) | 6
(66.7) |
|
| Vascular
invasion |
|
| 0.873 |
|
| 0.018 |
|
Negative | 10 (27.8) | 26 (72.2) |
| 16 (44.4) | 20 (55.6) |
|
|
Positive | 2
(25.0) | 6
(75.0) |
| 0
(0) | 8
(100) |
|
| Tumor size, cm |
|
| 1.000 |
|
| 1.000 |
| ≤2 | 3
(27.3) | 8
(72.7) |
| 4
(36.4) | 7
(63.6) |
|
|
>2 | 9
(27.3) | 24 (72.7) |
| 12 (36.4) | 21 (63.6) |
|
| Lymph node
metastasis |
|
| 0.255 |
|
| 0.907 |
|
Negative | 3
(17.6) | 14 (82.4) |
| 6
(35.3) | 11 (64.7) |
|
|
Positive | 9
(33.3) | 18 (66.7) |
| 10 (37.0) | 17 (63.0) |
|
| p53 |
|
| 0.622 |
|
| 0.305 |
|
Negative | 5
(23.8) | 16 (76.2) |
| 6
(28.6) | 15 (71.4) |
|
|
Positive | 7
(30.4) | 16 (69.6) |
| 10 (43.5) | 13 (56.5) |
|
| Molecular type |
|
| 0.214 |
|
| 0.186 |
|
Non-triple-negative | 5
(20.0) | 20 (80.0) |
| 7
(28.0) | 18 (72.0) |
|
|
Triple-negative | 7
(36.8) | 12 (63.2) |
| 9
(47.4) | 10 (52.6) |
|
| ER |
|
| 0.658 |
|
| 0.160 |
|
Negative | 8
(29.6) | 19 (70.4) |
| 12 (44.4) | 15 (55.5) |
|
|
Positive | 4
(23.5) | 13 (76.5) |
| 4
(23.5) | 13 (76.5) |
|
| PR |
|
| 0.736 |
|
| 0.851 |
|
Negative | 8
(25.8) | 23 (74.2) |
| 11 (35.5) | 20 (64.5) |
|
|
Positive | 4
(30.8) | 9
(69.2) |
| 5
(38.5) | 8
(61.5) |
|
| HER-2 |
|
| 0.222 |
|
| 0.323 |
|
Negative | 11 (31.4) | 24 (68.6) |
| 14 (40.0) | 21 (60.0) |
|
|
Positive | 1
(11.1) | 8
(88.9) |
| 2
(22.2) | 7
(77.8) |
|
Expression of VEGF in breast
cancer
Among the 44 cases of breast cancer tissue
investigated, 16 cases (36.4%) weakly expressed (0/+) VEGF and 28
cases (63.6%) strongly expressed (++/+++) VEGF. The OS of patients
exhibiting high VEGF expression demonstrated no significant
difference from that of the patients with low expression (P=0.366).
When the tissues were divided into two groups by the patient's age
(≥50 and <50 years), the proportion of patients exhibiting high
VEGF expression in the ≥50 years group was significantly higher
than that in the <50 years group (78.6 vs. 37.5%; P=0.006). In
terms of vessel infiltration, VEGF was highly expressed in all 8
patients with vessel infiltration (100%), but was only expressed in
20/36 patients without vascular invasion (55.6%); this difference
was statistically significant (P=0.018) (Table I).
Cox regression multivariate
analysis
Cox multivariate analysis identified that clinical
stage, molecular type and age were independent prognostic factors
for breast cancer (P=0.005, P=0.006 and P=0.032, respectively),
while the expression of CD34 was identified as a potential
prognostic factor (P=0.055; Table
II).
 | Table II.Cox multivariate analysis of 44
patients with breast cancer. |
Table II.
Cox multivariate analysis of 44
patients with breast cancer.
| Parameter | Risk ratio (95%
CI) | P-value | Adjusted risk ratio
(95% CI) | P-value |
|---|
| CD34 (negative vs.
positive) | 0.155
(0.039–0.624) | 0.009a | 0.096
(0.009–1.050) | 0.055 |
| VEGF (negative vs.
positive) | 0.471
(0.098–2.270) | 0.348 | 0.497
(0.041–6.108) | 0.585 |
| TNM stage (I–II vs.
III) | 0.091
(0.022–0.372) | 0.001a | 0.023
(0.002–0.322) | 0.005a |
| Molecular type
(non-triple-negative vs. triple-negative) | 0.563
(0.151–2.098) | 0.392 | 0.021
(0.001–0.330) | 0.006a |
| Vascular invasion
(negative vs. positive) | 0.802
(0.166–3.869) | 0.784 | 0.476
(0.026–8.644) | 0.616 |
| Age (≤50 vs. >50
years) | 0.196
(0.025–1.570) | 0.125 | 0.007
(0.000–0.643) | 0.032a |
Association between the OS of patients
and lymph node metastasis
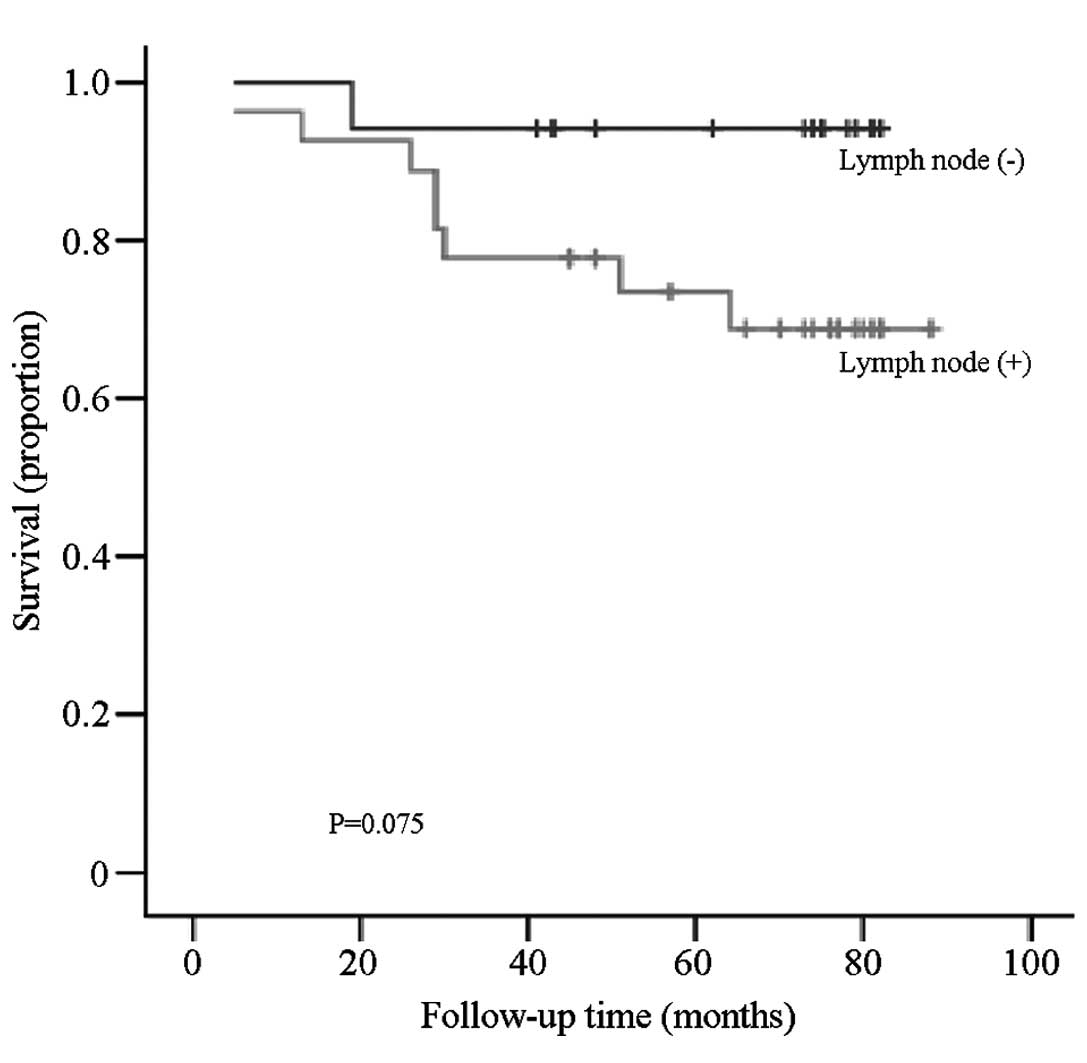
There was no significant difference between the OS
of breast cancer patients with and without lymph node metastasis
(P=0.075; Fig. 3). However, when the
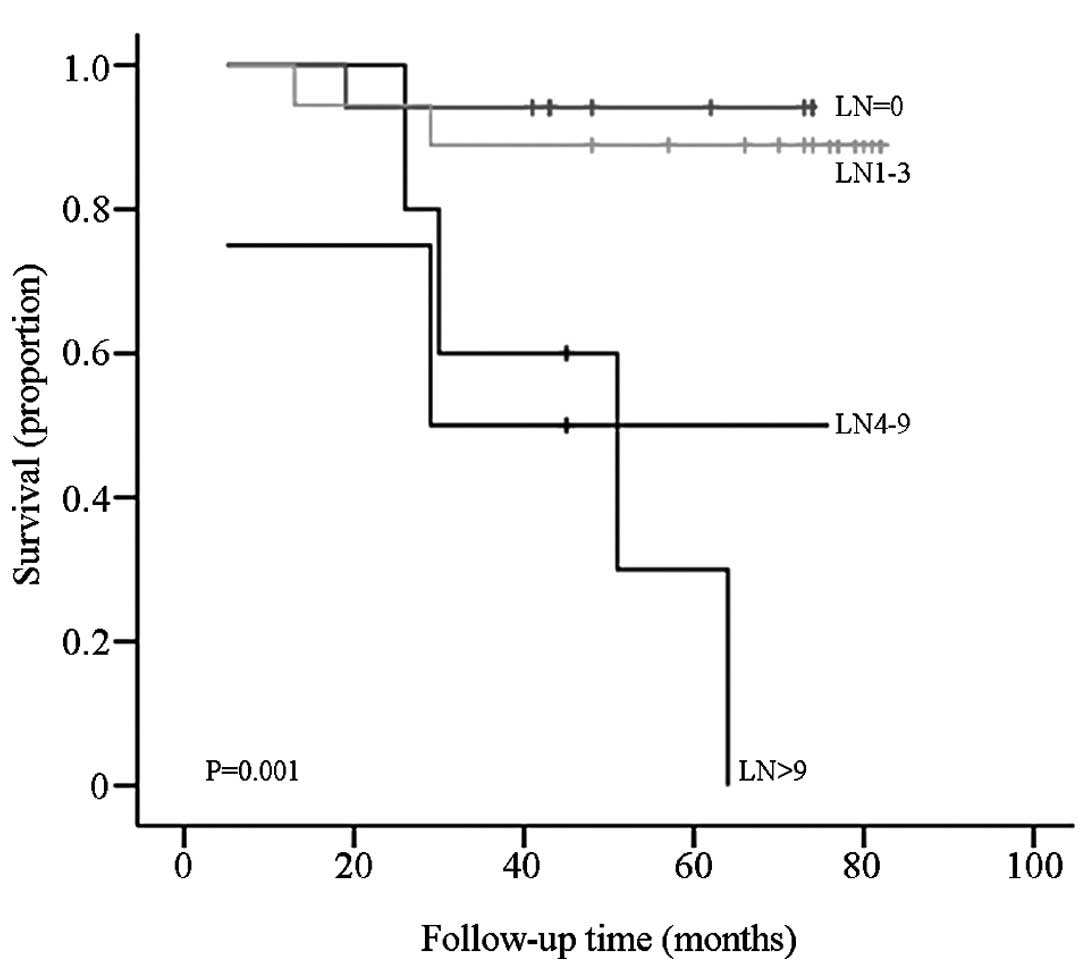
samples were divided into four groups according to the metastatic
lymph node number (N, 0; N1, 1–3; N2, 4–9; and N3, >10), the
difference in OS between each group was statistically significant
(P=0.001; Fig. 4).
Discussion
CD34, also known as human hematopoietic progenitor
cell antigen, is a single-chain transmembrane glycoprotein with a
molecular weight of 105–120 kDa, located on the long arm of
chromosome 1. CD34 is an endothelial cell-specific marker,
predominantly expressed on endothelial and hematopoietic progenitor
cells, and closely associated with the process of angiogenesis
(13). In addition, it is a more
objective indicator for evaluating the extent of tumor
angiogenesis. High expression of CD34 indicates a high MVD, i.e.,
more microvessels within the tumor tissue. Neovessels provide
nutrients and oxygen for tumor cells and remove metabolic waste,
promoting the growth rate of tumor (7). Furthermore, increased contact between
the tumor cells and the blood vessels, the weak and thin neovessel
wall, and the incomplete matrix membrane further promotes tumor
cell adhesion and entrance of tumor cells into the vessel. Tumor
cell metastasis can then occur via the blood circulation, allowing
spread to other areas of the body and form new metastatic lesions.
Therefore, an increase in the number of capillaries indicates an
increased probability of invasion and metastasis (2). Ch'ng et al (14) analyzed CD34 expression in 94 invasive
ductal breast cancer tissue samples and identified that CD34 was
highly expressed in the youth group (aged ≤55 years), but was not
associated with other clinicopathological factors. In addition, the
clinical stage of breast cancer was determined to be a major
contributor to a poor prognosis. Similarly, Murri et al
(15) reported the expression of CD34
in 168 patients with early invasive breast cancer. This study
identified that an increased tumor MVD was correlated with the OS
of the patients (P<0.05), and multivariate analysis identified
albumin concentration, topical therapy, systemic therapy and tumor
MVD as independent factors for predicting a poor prognosis
(P<0.05). By contrast, Chuangsuwanich et al (16) used immunomarkers on tissue microarrays
to classify the subtypes of breast cancer, and performed a
correlation analysis between breast cancer subtype, and various
clinicopathological features and prognostic markers, such as Ki-67
expression, p53 expression, MVD and VEGF expression. However, no
significant elevation in MVD or VEGF expression was apparent. In
the present study, the OS time of patients with high CD34
expression was significantly shorter compared with patients with
low CD34 expression (P=0.003). Additionally, Cox multivariate
analysis identified that CD34 expression at >15/HPV was a
potential indicator of a poor prognosis (P=0.055). Thus, detection
of CD34 expression may provide a novel potential tumor marker for
the clinical diagnosis and prognosis of patients with breast
cancer.
VEGF is a highly specific endogenous vascular
endothelial cell growth factor that activates tyrosine kinase
receptors, predominantly via binding to its specific surface
receptor, VEGF receptor-1 [VEGFR-1 (Flt-1)], and causing the
initiation of signal conduction. This signaling can promote the
mitosis of endothelial cells and eventually results in
neovascularization. Furthermore, VEGF signaling can increase
vascular permeability by increasing the production of enzymes
required for the degradation of extracellular matrix (4). Good vascularization allows tumor cells
to receive sufficient nutrients for rapid proliferation and to
enter blood vessels for distant metastasis. The majority of
previous studies have identified that VEGF expression is
significantly increased in metastatic breast ductal carcinoma and
is correlated with the poor clinical outcomes in patients with
early-stage breast cancer (12,17–19).
Furthermore, VEGF may be an independent prognostic factor for the
disease-free survival and OS of breast cancer patients (20). Thus, anti-VEGF therapy may have
potential in the treatment of patients with breast cancer.
Bevacizumab is a recombinant humanized monoclonal antibody for
human VEGF that can prevent the biological effects of VEGF by
neutralizing it. This neutralization of VEGF inhibits the formation
of new blood vessels and reduces the oxygen supply, blood supply
and nutrient supply to the tumor area, resulting in inhibited tumor
growth (2,21). An international multi-center open
randomized phase III clinical trial conducted by the Eastern
Cooperative Oncology Group (E2100) and reported by Miller et
al (21) appears to be the most
representative trial of bevacizumab conducted thus far (22). In March 2008, bevacizumab in
combination with paclitaxel was approved by the Food and Drug
Administration (FDA) for use in the first-line treatment of
metastatic breast cancer. However, a large number of clinical
studies of bevacizumab were subsequently conducted and identified
that, although improved progression-free survival was observed,
improved OS was not (23–25); therefore, the application of
bevacizumab for the treatment of metastatic breast cancer was
revoked by the FDA. However, the administration of bevacizumab
combined with paclitaxel for the treatment of metastatic breast
cancer is still retained in the American National Comprehensive
Cancer Network Guide (26). In
addition, it is still widely applied in the treatment of colon
cancer, lung cancer, glioblastoma, renal cell carcinoma and other
tumors (4,27).
Tumor angiogenesis is an important part of the
process of breast cancer growth and metastasis; it is a complex
multi-factorial and multi-step process that can be used to
indicated if a tumor is malignant. In the process of tumor
development, various angiogenic factors are preferentially
expressed at different stages. However, as a key factor with the
strongest pro-angiogenic activity, VEGF is expressed throughout the
process of tumor development and is correlated with patient
survival (4,6). Ni et al (28) reported that VEGF expression in 75
patients with breast cancer was negatively correlated with survival
time, indicating that VEGF may be a malignant phenotype of breast
cancer, with increased expression predicting a poor prognosis. By
contrast, a study conducted by Dhakal et al (8) identified that VEGF expression is
associated with the differentiation of squamous cell carcinoma of
the vulva, but not with patient survival. Similarly, a previous
study determined that VEGF expression is not associated with
clinicopathological factors or survival in patients with colorectal
cancer (9). Furthermore, a
meta-analysis of 1,357 patients with breast cancer identified that
overexpression of VEGF-C was not correlated with the survival of
patients with breast cancer (29). In
the present study, high VEGF expression was identified in all 8
cases of breast cancer with tumor thrombi. All the breast cancer
tissues with vascular invasion highly expressed VEGF (100%); this
value was significantly higher than in the tissues not exhibiting
vascular invasion (55.6%; P=0.018). In addition, patients aged ≥50
years presented with a higher VEGF expression rate (78.6%) compared
with patients aged <50 years (37.5%) (P=0.006). However,
Kaplan-Meier analysis determined that differences in the OS of
patients were not significant between the high and low VEGF
expression groups (P=0.366). Thus, consistent with results reported
by Gao et al (29), VEGF
expression was not an independent prognostic factor for the breast
cancer patients. However, data obtained in the present study
identified that clinical stage, molecular typing and age were
independent prognostic factors for patients with breast cancer
(P=0.005, P=0.006 and P=0.032, respectively), while the expression
of CD34 was determined to be a potential independent prognostic
factor.
Srabovic et al (30) examined VEGFR-1 and VEGF expressions
levels in tumors and the adjacent tissue of 51 patients with breast
cancer, and in the healthy breast tissue of 30 patients with benign
breast diseases using immunohistochemical staining. The results
demonstrated that the expression of VEGFR-1 and VEGF were
significantly higher in the breast cancer tissues compared with the
healthy breast tissues (P<0.01). Furthermore, a significant
correlation was identified between VEGF and VEGFR-1 expression
levels (P<0.05); while no significant correlation was observed
between VEGF and VEGFR-1 expression, and tumor size, histological
grade or hormone receptor status. Increased expression of VEGFR-1
and VEGF in breast cancer tissues, and significant correlation
between the two proteins, indicates a possible role of the
VEGF/VEGFR-1 signaling pathway in the development of breast cancer,
although the potential prognostic value of VEGFR-1 has not yet been
confirmed. A study conducted by Keyhani et al (12) demonstrated that angiogenic markers in
breast cancer (for example, CD34) are potentially useful tools for
determining the most appropriate priority setting for delivering
antiangiogenic agents.
Although the sample size used in the present study
was small, the OS of patients according to the number of metastatic
lymph nodes (staged according to the 2010 edition of the UICC
staging system) demonstrated statistically significant differences.
Therefore, the current findings provide a greater scientific basis
for employing the UICC staging system in breast cancer.
Acknowledgements
The present study was supported by a grant from the
Medical Science and Technology Development Program of Zhejiang
Province (grant no. 2010KYB020).
References
|
1
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ziyad S and Iruela-Arispe ML: Molecular
mechanisms of tumor angiogenesis. Genes Cancer. 2:1085–1096. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao Y, Zhang ZL, Zhou M, et al: Pericyte
coverage of differentiated vessels inside tumor vasculature is an
independent unfavorable prognostic factor for patients with clear
cell renal cell carcinoma. Cancer. 119:313–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zorgetto VA, Silveira GG, Oliveira-Costa
JP, Soave DF, Soares FA and Ribeiro-Silva A: The relationship
between lymphatic vascular density and vascular endothelial growth
factor A (VEGF-A) expression with clinical-pathological features
and survival in pancreatic adenocarcinomas. Diagn Pathol.
8:1702013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horn LC, Schreiter C, Canzler A, Leonhardt
K, Einenkel J and Hentschel B: CD34(low) and SMA(high) represent
stromal signature in uterine cervical cancer and are markers for
peritumoral stromal remodeling. Ann Diagn Pathol. 17:531–535. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhan H, Liang H, Liu X, Deng J, Wang B and
Hao X: Expression of Rac1, HIF-1α and VEGF in gastric carcinoma:
correlation with angiogenesis and prognosis. Onkologie. 36:102–107.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sundov Z, Tomic S, Alfirevic S, et al:
Prognostic value of MVD, LVD and vascular invasion in lymph
node-negative colon cancer. Hepatogastroenterology. 60:432–438.
2013.PubMed/NCBI
|
|
8
|
Dhakal HP, Nesland JM, Førsund M, Trope CG
and Holm R: Primary tumor vascularity, HIF-1α and VEGF expression
in vulvar squamous cell carcinomas: their relationships with
clinicopathological characteristics and prognostic impact. BMC
Cancer. 13:5062013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anannamcharoen S and Nimmanon T: Study of
the vascular endothelial growth factor (VEGF) expression and
microvascular density (MVD) in primary colorectal cancer specimens.
J Med Assoc Thai. 95:1041–1047. 2012.PubMed/NCBI
|
|
10
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: Breast CancerAJCC Cancer Staging Manual.
7th. Springer-Verlag; New York, NY: pp. 223–240. 2009
|
|
11
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keyhani E, Muhammadnejad A, Behjati F, et
al: Angiogenesis markers in breast cancer - potentially useful
tools for priority setting of anti-angiogenic agents. Asian Pac J
Cancer Prev. 14:7651–7656. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Satterthwaite AB, Burn TC, Le Beau MM and
Tenen DG: Structure of the gene encoding CD34, a human
hematopoietic stem cell antigen. Genomics. 12:788–794. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ch'ng ES, Tuan Sharif SE and Jaafar H:
Characteristics of invasive breast ductal carcinoma, NOS, diagnosed
in a tertiary institution in the East Coast of Malaysia with a
focus on tumor angiogenesis. Asian Pac J Cancer Prev. 13:4445–4452.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murri AM, Hilmy M, Bell J, et al: The
relationship between the systemic inflammatory response, tumour
proliferative activity, T-lymphocytic and macrophage infiltration,
microvessel density and survival in patients with primary operable
breast cancer. Br J Cancer. 99:1013–1019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chuangsuwanich T, Pongpruttipan T,
O-Charoenrat P, Komoltri C, Watcharahirun S and Sa-Nguanraksa D:
Clinicopathologic features of breast carcinomas classified by
biomarkers and correlation with microvessel density and VEGF
expression: a study from Thailand. Asian Pac J Cancer Prev.
15:1187–1192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Staton CA, Hoh L, Baldwin A, Shaw L, Globe
J, Cross SS, Reed MW and Brown NJ: Angiopoietins 1 and 2 and Tie-2
receptor expression in human ductal breast disease. Histopathology.
59:256–263. 2011.PubMed/NCBI
|
|
18
|
Davidson B, Stavnes HT, Førsund M, Berner
A and Staff AC: CD105 (Endoglin) expression in breast carcinoma
effusions is a marker of poor survival. Breast. 19:493–498. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao YC, Ni XJ, Li Y, Dai M, Yuan ZX, Zhu
YY and Luo CY: Peritumoral lymphangiogenesis induced by vascular
endothelial growth factor C and D promotes lymph node metastasis in
breast cancer patients. World J Surg Oncol. 10:1652012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Acs G, Paragh G, Rakosy Z, et al: The
extent of retraction clefts correlates with lymphatic vessel
density and VEGF-C expression and predicts nodal metastasis and
poor prognosis in early-stage breast carcinoma. Mod Pathol.
25:163–177. 2012.PubMed/NCBI
|
|
21
|
Miller K, Wang M, Gralow J, et al:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. N Engl J Med. 357:2666–2676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miles DW, Chan A, Dirix LY, et al: Phase
III study of bevacizumab plus docetaxel compared with placebo plus
docetaxel for the first-line treatment of human epidermal growth
factor receptor 2-negative metastatic breast cancer. J Clin Oncol.
28:3239–3247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robert NJ, Diéras V, Glaspy J, et al:
RIBBON-1: randomized, double-blind, placebo-controlled, phase III
trial of chemotherapy with or without bevacizumab for first-line
treatment of human epidermal growth factor receptor 2-negative,
locally recurrent or metastatic breast cancer. J Clin Oncol.
29:1252–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gonçalves A, Deblock M, Esterni B, et al:
Docetaxel first-line therapy in HER2-negative advanced breast
cancer: a cohort study in patients with prospectively determined
HER2 status. Anticancer Drugs. 20:946–952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brufsky AM, Hurvitz S, Perez E, Swamy R,
Valero V, O'Neill V and Rugo HS: RIBBON-2: a randomized,
double-blind, placebo- controlled, phase III trial evaluating the
efficacy and safety of bevacizumab in combination with chemotherapy
for second-line treatment of human epidermal growth factor receptor
2-negative metastatic breast cancer. J Clin Oncol. 29:4286–4293.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Theriault RL, Carlson RW, Allred C, et al:
National Comprehensive Cancer Network: Breast cancer, version
3.2013: Featured updates to the NCCN guidelines. J Natl Compr Canc
Netw. 11:753–760. 2013.PubMed/NCBI
|
|
27
|
Huang H, Zheng Y, Zhu J, Zhang J, Chen H
and Chen X: An updated meta-analysis of fatal adverse events caused
by bevacizumab therapy in cancer patients. PLoS One. 9:e899602014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ni X, Zhao Y, Ma J, Xia T, Liu X, Ding Q,
Zha X and Wang S: Hypoxia-induced factor-1 alpha upregulates
vascular endothelial growth factor C to promote lymphangiogenesis
and angiogenesis in breast cancer patients. J Biomed Res.
27:478–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao S, Ma JJ and Lu C: Prognostic
significance of VEGF-C immunohistochemical expression in breast
cancer: a meta-analysis. Tumour Biol. 35:1523–1529. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Srabovic N, Mujagic Z,
Mujanovic-Mustedanagic J, Softic A, Muminovic Z, Rifatbegovic A and
Begic L: Vascular endothelial growth factor receptor-1 expression
in breast cancer and its correlation to vascular endothelial growth
factor a. Int J Breast Cancer. 2013:7467492013. View Article : Google Scholar : PubMed/NCBI
|