Introduction
Gastric cancer is the second most common cause of
cancer-associated mortality in the world. In particular, it is one
of the predominant cancer types in Korean and East Asian
populations (1–4). Gastric cancer is a multifactorial
malignant disorder caused by a wide range of risk factors,
including genetic predisposition, the environment and
Helicobacter pylori infection (1). Persistent H. pylori infection in
the human stomach elicits a chronic inflammatory response, the
extent of which may vary between individuals depending on the
genetic makeup of the host. This phenomenon may aid in explaining
the diverse range of outcomes observed in individuals infected with
H. pylori. Therefore, polymorphisms in genes that are
important in the host inflammatory response to this infection may
alter an individual's susceptibility to gastric cancer (2). Notably, associations have been
identified between gastric cancer and the expression of various
genes involved in folate metabolism, such as methionine synthase
(MTR), methylenetetrahydrofolate reductase (MTHFR) and MTR
reductase (MTRR) (3).
MTHFR is an essential component of folate metabolism
that has been indicated to be involved in DNA methylation and
synthesis (4). The common MTHFR C677T
polymorphism results in the substitution of alanine by valine,
producing of a thermolabile variant that retains only ~30% of the
activity of the wild-type MTHFR enzyme (5). The association between this gene
polymorphism and the risk of gastric cancer has drawn increasing
attention in the scientific community and has been investigated
extensively, with 27 original studies (3,6–32) examining the role of the MTHFR C667T
polymorphism in the development of gastric cancer. However, these
studies have yielded conflicting results, partially due to the
small effect of the gene polymorphism on the risk of gastric cancer
and the relatively small sample sizes used. Therefore, the aim of
the current meta-analysis was to determine a more precise
estimation of the association between the MTHFR C677T polymorphism
and gastric cancer risk.
Materials and methods
Identification and eligibility of
relevant studies
The current meta-analysis was performed according to
the guidelines for systematic reviews of genetic association
studies (33). Two investigators
independently searched the MEDLINE, EMBASE and Wanfang electronic
databases for studies published from inception to May 2013.
Combining text words and Medical Subject Headings (MESH) terms, the
following keywords were used to perform the literature search:
‘MTHFR’ or ‘methylenetetrahydrofolate reductase’ to search for
MTHFR; ‘gastric cancer’ or ‘stomach cancer’ to search for gastric
cancer; and ‘gene’, or ‘polymorphism’ or ‘genetic variation’ to
search for genetic variations. The aforementioned search terms were
used in conjunction with the ‘explode’ feature where applicable.
Full studies published in the English and Chinese languages were
considered for inclusion in the present study. In addition, the
reference lists of all primary studies and reviews were manually
searched. All case-control studies that investigated the
association between the MTHFR 677C>T polymorphism and gastric
cancer were included. Furthermore, when the same series was used in
more than one case-control study, the study with the largest cohort
was selected.
Data extraction
The following data was extracted from each of the
selected studies: First author, year of publication, ethnicity of
study population, and the number of cases and controls for each
C677T genotype.
Statistical analysis
Crude odds ratios (ORs) with 95% confidence
intervals (CIs) were calculated to evaluate the association between
the MTHFR 677C>T polymorphism and the risk of gastric cancer.
The pooled ORs were obtained for homozygous (TT vs. CC),
heterozygous (CT vs. CC), dominant (TT+CT vs. CC) and recessive (TT
vs. CC +CT) models. Heterogeneity assumption was examined using the
χ2-based Q test (34),
with P≤0.01 considered to indicate heterogeneity among studies.
Subsequently, the pooled OR estimate was calculated for each study
using the fixed-effects model (Mantel-Haenszel method) (35). Otherwise, the random-effects model
(DerSimonian and Laird method) was used (36). To evaluate the source of between-study
heterogeneity, Galbraith plots was constructed to identify outliers
that may be acting as major sources of between-study heterogeneity.
In addition, subgroup analyses by ethnicity were performed. The
potential publication bias of the present study was estimated by
constructing a funnel plot in which the standard error of log(OR)
was plotted against log(OR), for each study. Funnel plot asymmetry,
which indicates a possible publication bias, was evaluated using
Egger's linear regression test. Furthermore, the significance of
the intercept was determined by performing a t-test, as proposed by
Egger (37). P<0.05 was considered
to indicate a statistically significant publication bias (38). All statistical analyses were performed
using Stata software (version 10.0; Stata Corporation, College
Station, TX, USA).
Results
Study characteristics
A total of 27 publications met the inclusion
criteria of the current meta-analysis (3,6–32), thus, a total of 5,757 cases and 8,501
controls were used in the pooled analyses. Tables I and II list the included studies and their major
characteristics. In the 27 studies, the sample sizes ranged between
72 and 1,230 individuals. Furthermore, the studies included 12
European and 17 Asian populations, and the majority of controls
were matched for gender and age.
 | Table I.Major characteristics of all studies
included in the current meta-analysis. |
Table I.
Major characteristics of all studies
included in the current meta-analysis.
| First author
(ref.) | Year of
publication | Country | Ethnicity | Cases, n | Controls, n |
|---|
| Miao et al
(8) | 2002 | China | Asian | 217 | 468 |
| Gao et al
(7) | 2002 | China | Asian | 107 | 200 |
| Gao et al
(32) | 2001 | China | Asian | 107 | 200 |
| Stolzenberg-Solomon
et al (21) | 2003 | China | Asian | 90 | 398 |
| Bi et al
(9) | 2005 | China | Asian | 309 | 188 |
| Shen et al
(10) | 2005 | China | Asian | 320 | 313 |
| Sarbia et al
(14) | 2005 | Germany | Caucasian | 332 | 255 |
| Wang et al
(20) | 2005 | China | Asian | 129 | 315 |
| Si et al
(18) | 2005 | China | Asian | 122 | 101 |
| Kim et al
(19) | 2005 | Korea | Asian | 133 | 445 |
| Li et al
(30) | 2006 | China | Asian | 170 | 140 |
| Graziano et al
(13) | 2006 | Italy | Caucasian | 162 | 164 |
| Lacasaña-Navarro
et al (24) | 2006 | Mexico | Caucasian | 201 | 427 |
| Weng et al
(17) | 2006 | China | Asian | 38 | 34 |
| Zeybek et al
(26) | 2007 | Turky | Caucasian | 35 | 144 |
| Wang et al
(16) | 2007 | China | Asian | 467 | 540 |
| Götze et al
(12) | 2007 | Germany | Caucasian | 103 | 106 |
| Zhang et al
(3) | 2007 | USA | Caucasian | 295 | 399 |
| Mu et al
(6) | 2007 | China | Asian | 194 | 391 |
| Boccia et al
(11) | 2007 | Italy | Caucasian | 102 | 254 |
| Vollset et
al (25) | 2007 | Europe | Caucasian | 295 | 399 |
| Li et al
(15) | 2007 | China | Asian | 170 | 140 |
| Zúñiga-Noriega
et al (23) | 2008 | Mexico | Caucasian | 51 | 83 |
| Galván-Portillo
et al (22) | 2009 | Mexico | Caucasian | 248 | 478 |
| Yang et al
(31) | 2010 | China | Asian | 139 | 165 |
| De Re et al
(27) | 2010 | Italy | Caucasian | 57 | 454 |
| Saberi et al
(29) | 2012 | Iran | Caucasian | 450 | 780 |
| Gao et al
(28) | 2013 | China | Asian | 264 | 535 |
 | Table II.Genotypes of the
methylenetetrahydrofolate reductase C677T polymorphism included in
the meta-analysis. |
Table II.
Genotypes of the
methylenetetrahydrofolate reductase C677T polymorphism included in
the meta-analysis.
|
|
| Cases, n | Controls, n |
|---|
|
|
|
|
|
|---|
| First author
(ref.) | Year of
publication | CC | CT | TT | CC | CT | TT |
|---|
| Gao et al
(32) | 2001 | 22 | 61 | 24 | 63 | 99 | 38 |
| Miao et al
(8) | 2002 | 47 | 107 | 63 | 151 | 217 | 100 |
| Gao et al
(7) | 2002 | 22 | 61 | 24 | 63 | 99 | 38 |
| Stolzenberg-Solomon
et al (21) | 2003 | 17 | 36 | 37 | 65 | 209 | 124 |
| Bi et al
(9) | 2005 | 139 | 150 | 20 | 97 | 76 | 15 |
| Shen et al
(10) | 2005 | 105 | 171 | 44 | 113 | 172 | 28 |
| Sarbia et al
(14) | 2005 | 138 | 153 | 41 | 107 | 115 | 33 |
| Wang et al
(20) | 2005 | 25 | 45 | 59 | 74 | 143 | 98 |
| Si et al
(18) | 2005 | 58 | 48 | 16 | 49 | 43 | 9 |
| Kim et al
(19) | 2005 | 42 | 64 | 27 | 143 | 239 | 63 |
| Li et al
(30) | 2006 | 61 | 78 | 31 | 67 | 56 | 17 |
| Graziano et
al (13) | 2006 | 34 | 86 | 42 | 67 | 68 | 29 |
| Lacasaña-Navarro
et al (24) | 2006 | 56 | 85 | 60 | 144 | 179 | 104 |
| Weng et al
(17) | 2006 | 14 | 19 | 5 | 15 | 11 | 8 |
| Zeybek et al
(26) | 2007 | 18 | 12 | 5 | 64 | 65 | 15 |
| Wang et al
(16) | 2007 | 74 | 203 | 190 | 119 | 234 | 187 |
| Götze et al
(12) | 2007 | 46 | 45 | 12 | 41 | 49 | 16 |
| Zhang et al
(3) | 2007 | 146 | 116 | 33 | 185 | 178 | 36 |
| Mu et al
(6) | 2007 | 50 | 106 | 38 | 135 | 199 | 57 |
| Boccia et al
(11) | 2007 | 29 | 51 | 22 | 98 | 115 | 41 |
| Vollset et
al (25) | 2007 | 109 | 104 | 32 | 248 | 277 | 94 |
| Li et al
(15) | 2007 | 61 | 78 | 31 | 67 | 56 | 17 |
| Zúñiga-Noriega
et al (23) | 2008 | 16 | 23 | 12 | 17 | 49 | 17 |
| Galván-Portillo
et al (22) | 2009 | 37 | 132 | 79 | 89 | 217 | 172 |
| Yang et al
(31) | 2010 | 44 | 80 | 15 | 62 | 75 | 28 |
| De Re et al
(27) | 2010 | 18 | 25 | 14 | 152 | 238 | 64 |
| Saberi et al
(29) | 2012 | 422 | 308 | 50 | 198 | 172 | 35 |
| Gao et al
(28) | 2013 | 115 | 105 | 44 | 277 | 207 | 51 |
Meta-analysis of the MTHFR C677T
polymorphism
Table III indicates
the major results of the current meta-analysis. When all the
studies were pooled into the meta-analysis, the MTHFR T allele was
determined to be associated with a significantly increased risk of
developing gastric cancer (homozygous model: OR, 1.39; 95% CI,
1.20–1.62; heterozygous model: OR, 1.18; 95% CI, 1.05–1.32;
dominant model: OR, 1.23; 95% CI, 1.10–1.38; recessive model: OR,
1.26; 95% CI, 1.12–1.42) (P<0.001). In the subgroup analysis by
ethnicity, no significantly increased risk of gastric cancer was
identified in Caucasians with the MTHFR C677T polymorphism
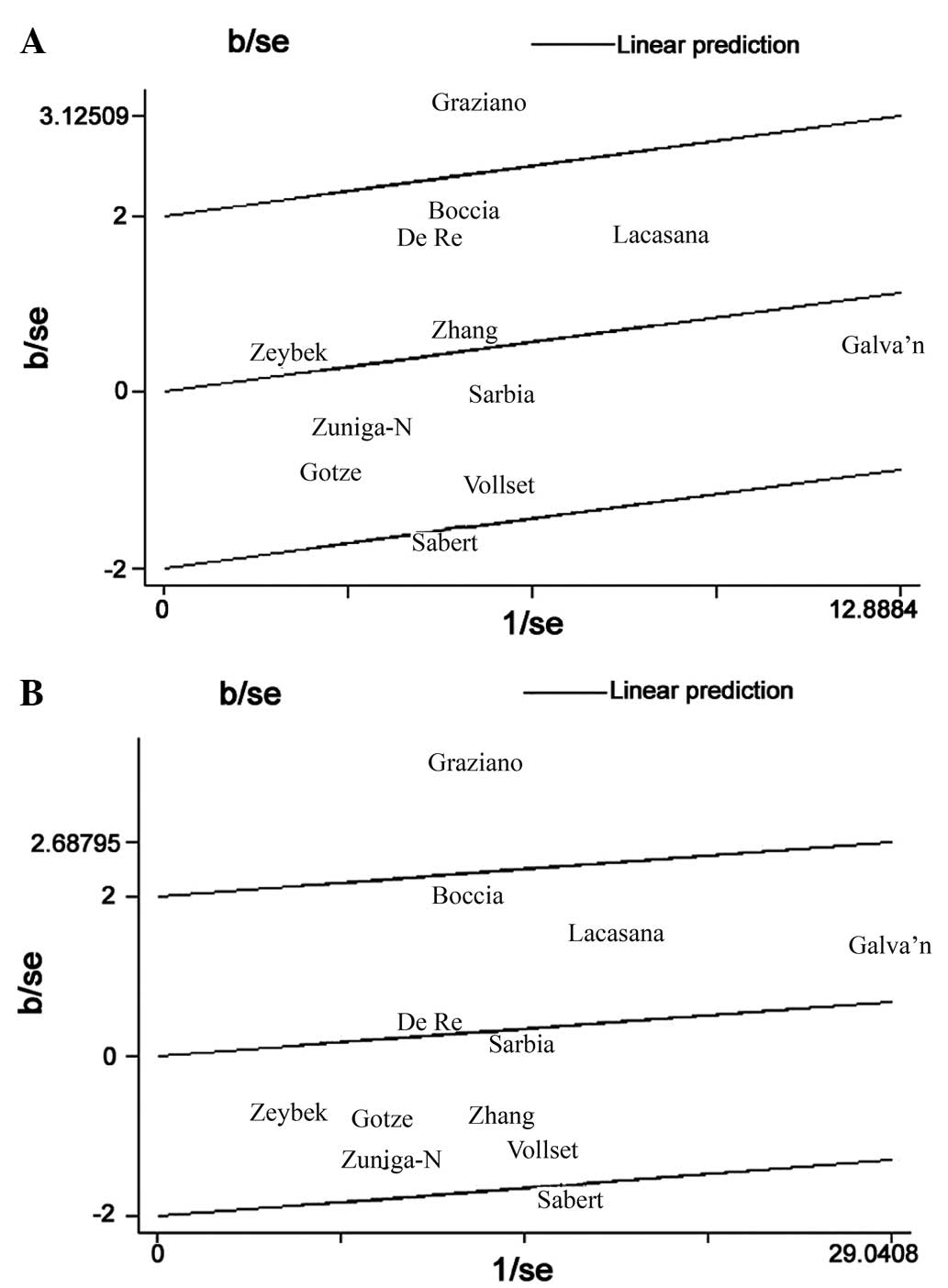
[homozygous model: OR, 1.15; 95% CI, 0.89–1.48 (Fig. 1A); heterozygous model: OR, 1.03; 95%
CI, 0.84–1.25; dominant model: OR, 1.05; 95% CI, 0.86–1.28
(Fig. 1B); recessive model: OR, 1.09;
95% CI, 0.91–1.31]; however, significantly increased risks were
identified in Asian populations (homozygous model: OR, 1.64; 95%
CI, 1.43–1.90; heterozygous model: OR, 1.30; 95% CI, 1.16–1.45;
dominant model: OR, 1.39; 95% CI, 1.25–1.54; recessive model: OR,
1.41; 95% CI, 1.25–1.51).
 | Table III.Pooled OR data obtained in the
current meta-analysis. |
Table III.
Pooled OR data obtained in the
current meta-analysis.
| Contrast model | Studies, n | OR (95% CI) | P-value | Model | I2,
% | P-value |
|---|
| Total studies |
|
|
|
|
|
|
|
Homozygous | 27 | 1.39
(1.20–1.62) | <0.001 | Random | 41.5 |
0.011 |
|
Heterozygous | 27 | 1.18
(1.05–1.32) |
0.006 | Random | 47.3 |
0.003 |
|
Recessive | 27 | 1.26
(1.12–1.42) | <0.001 | Random | 34.1 |
0.039 |
|
Dominant | 27 | 1.23
(1.10–1.38) | <0.001 | Random | 52.8 |
0.016 |
| Caucasian |
|
|
|
|
|
|
|
Homozygous | 12 | 1.15
(0.89–1.48) |
0.791 | Random | 58.2 |
0.006 |
|
Homozygous (adjusted for
heterogeneity) | 10 | 1.13
(0.93–1.36) |
0.215 | Fixed | 10.6 |
0.345 |
|
Heterozygous | 12 | 1.03
(0.84–1.25) | <0.001 | Fixed |
0.0 |
0.674 |
|
Recessive | 12 | 1.09
(0.91–1.31) |
0.367 | Fixed | 32.1 |
0.134 |
|
Dominant | 12 | 1.05
(0.86–1.28) |
0.609 | Random | 63.3 |
0.002 |
|
Dominant (adjusted for
heterogeneity) | 10 | 1.00
(0.88–1.14) |
0.968 | Fixed | 27.6 | 0.19 |
| Asian |
|
|
|
|
|
|
|
Homozygous | 17 | 1.64
(1.43–1.90) | <0.001 | Fixed |
0.0 |
0.674 |
|
Heterozygous | 17 | 1.30
(1.16–1.45) | <0.001 | Fixed |
2.6 |
0.423 |
|
Recessive | 17 | 1.41
(1.25–1.61) | <0.001 | Fixed |
8.1 |
0.361 |
|
Dominant | 17 | 1.39
(1.25–1.54) | <0.001 | Fixed |
0.0 |
0.729 |
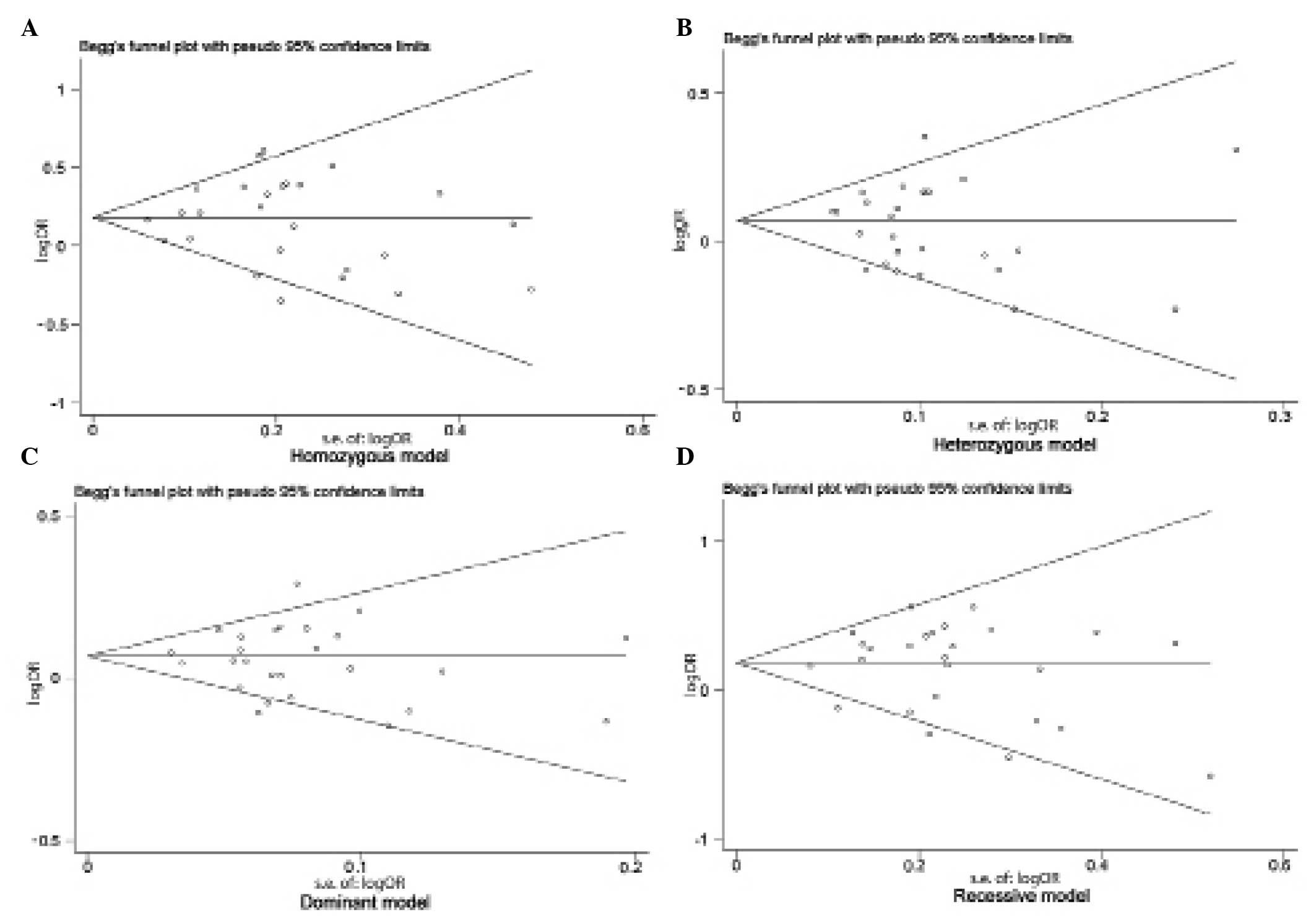
Publication bias
Begg's funnel plot and Egger's test were performed
to assess the publication bias of studies included in the current
meta-analysis. No evidence of marked asymmetry was observed in the
funnel plot (Fig. 2). Furthermore,
Egger's test did not indicate any statistical evidence of asymmetry
and therefore, publication bias (homozygous model, P=0.866;
heterozygous model, P=0.940; dominant model, P=0.851; recessive
model, P=0.358).
Discussion
It is well documented that individual susceptibility
to the development of cancer can vary, even when exposed to the
same environmental carcinogens (2,33). This
difference in susceptibility may be associated with genetic
variations, such as polymorphisms, in genes involved in
carcinogenesis. Therefore, genetic susceptibility to the
development of cancer has been the focus of considerable scientific
research. Recently, extensive investigation of genetic variants of
the MTHFR gene has taken place to determine its role in the
etiology of gastric cancer. Numerous studies have examined the role
of the MTHFR C677T polymorphism in gastric cancer risk, however,
the data is contradictory. Therefore, to improve understanding of
the association between the MTHFR C677T polymorphism and the risk
of gastric cancer, the present meta-analysis of pooled data from a
large sample was conducted. To the best of our knowledge, this is
the first meta-analysis regarding the association between the MTHFR
C677T polymorphism and the risk of gastric cancer to be conducted.
In addition, subgroup analysis and heterogeneity evaluations were
performed. The results indicated that the MTHFR 677 T allele is
associated with a significantly increased risk of developing
gastric cancer. Furthermore, significant associations were
identified in Asian individuals, but not in Caucasian individuals,
indicating a possible role of ethnicity in the risk of gastric
cancer, due to differences in genetic backgrounds, geography and
environment (37). However, it is
possible that the effect of the MTHFR 677 C allele is masked by the
expression of thus far unidentified causal genes involved in the
development of gastric cancer in Caucasian individuals. In
addition, the ethnic differences observed in the present study may
be due to chance, as studies with small sample sizes typically lack
the statistical power to detect marginal effects and may generate a
fluctuated risk estimate (39).
Considering the limited number of studies included in the present
meta-analysis and the small Caucasian populations, the current
results should be interpreted with caution.
Heterogeneity is a potential problem that may affect
the interpretation of the results of all meta-analyses. In the
present meta-analysis, significant between-study heterogeneity for
OR was identified in the overall comparisons (homozygous model,
P=0.011; heterozygous model, P=0.003; dominant model, P=0.016;
recessive model, P=0.039). However, subgroup analysis by ethnicity
demonstrated that heterogeneity was only evident between studies
involving Caucasian populations (homozygous model, P=0.006;
recessive model, P=0.002) but not for those involving Asian
populations (Table III).
Heterogeneity may also occur in poorly-designed studies that do not
exclude biases, as these biases may affect the estimation of the
real effects and cause incorrect conclusions to be drawn (40,41).
Therefore, Galbraith plots were used to identify the outlier
studies with poor quality designs. Following subgroup analysis of
Caucasian studies, the Galbraith plot identified two studies that
appeared to be major sources of heterogeneity (Fig. 1), with no between-study heterogeneity
observed among the remaining 10 studies (homozygous model, P=0.345;
recessive model, P=0.190). As a result, the fixed-effects model was
used to pool the ORs from the two outlier studies, effectively
removing heterogeneity from the current meta-analysis and thus
confirming that the two excluded studies contributed the
heterogeneity. Following adjustment for heterogeneity, the current
data demonstrated that the MTHFR MTHFR C677T polymorphism was
significantly associated with an increased risk of gastric cancer
in Asian individuals, but not in Caucasian individuals.
A number of limitations should be taken into
consideration when interpreting the findings of the current
meta-analysis. First, the controls were not uniformly defined.
Although the majority of the control subjects were recruited from
healthy populations, certain individuals exhibited benign medical
disorders. As a number of studies in the present meta-analysis
included control groups that may have different risks of developing
gastric cancer, non-differential misclassification bias may have
occurred. Second, the current results were based on unadjusted
estimates. If individual data is made available, future studies
should consider using it to perform more precise analyses, as
individual data would allow for the adjustment for additional
co-variates, such as age, smoking status, environmental factors and
lifestyle. Despite the aforementioned limitations, the current
meta-analysis exhibited high statistical power, as a large number
of cases and controls were pooled from different studies. In
addition, no publication bias was detected, indicating that the
overall pooled effects were unbiased.
In conclusion, the current meta-analysis indicated
that the MTHFR T allele is a low-penetrant genetic risk factor for
the development of gastric cancer. However, well-matched
case-control studies with homogeneous cancer patients of
multi-ethnic groups using standardized unbiased genotyping methods
are warranted in the future. Furthermore, it is recommended that
investigations should be conducted into the effects of gene-gene
and gene-environment interactions on the development of gastric
cancer.
References
|
1
|
Ahn DH, Rah H, Choi YK, et al: Association
of the miR-146aC>G, miR-149T>C, miR-196a2T>C, and
miR-499A>G polymorphisms with gastric cancer risk and survival
in the Korean population. Mol Carcinog (Suppl 1). 52:E39–E51. 2013.
View Article : Google Scholar
|
|
2
|
Persson C, Canedo P, Machado JC, El-Omar
EM and Forman D: Polymorphisms in inflammatory response genes and
their association with gastric cancer: A HuGE systematic review and
meta-analyses. Am J Epidemiol. 173:259–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang FF, Terry MB, Hou L, et al: Genetic
polymorphisms in folate metabolism and the risk of stomach cancer.
Cancer Epidemiol Biomarkers Prev. 16:115–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Warshauer ME, Silverman DT, Schottenfeld D
and Pollack ES: Stomach and colorectal cancers in Puerto Rican-born
residents of New York City. J Natl Cancer Inst. 76:591–595.
1986.PubMed/NCBI
|
|
5
|
Jacques PF, Bostom AG, Williams RR, et al:
Relation between folate status, a common mutation in
methylenetetrahydrofolate reductase, and plasma homocysteine
concentrations. Circulation. 93:7–9. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mu LN, Cao W, Zhang ZF, et al:
Polymorphisms of 5, 10-methylenetetralydrofolate reductase (MTHFR),
fruit and vegetable intake, and the risk of stomach cancer.
Biomarkers. 12:61–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao C, Wu J, Ding J, et al: Polymorphisms
of methylenetetrahydrofolate reductase C677T and the risk of
stomach cancer. Zhonghua Liu Xing Bing Xue Za Zhi. 23:289–292.
2002.(In Chinese). PubMed/NCBI
|
|
8
|
Miao X, Xing D, Tan W, Qi J, Lu W and Lin
D: Susceptibility to gastric cardia adenocarcinoma and genetic
polymorphisms in methylenetetrahydrofolate reductase in an at-risk
Chinese population. Cancer Epidemiol Biomarkers Prev. 11:1454–1458.
2002.PubMed/NCBI
|
|
9
|
Bi J, Cai L and Zheng Z: Study on C667T
gene polymorphism and susceptibility to gastric cancer. Chin J
Public. 21:6612005.
|
|
10
|
Shen H, Newmann AS, Hu Z, et al:
Methylenetetrahydrofolate reductase polymorphisms/haplotypes and
risk of gastric cancer: A case-control analysis in China. Oncol
Rep. 13:355–360. 2005.PubMed/NCBI
|
|
11
|
Boccia S, Gianfagna F, Persiani R, et al:
Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms
and susceptibility to gastric adenocarcinoma in an Italian
population. Biomarkers. 12:635–644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Götze T, Röcken C, Röhl FW, et al: Gene
polymorphisms of folate metabolizing enzymes and the risk of
gastric cancer. Cancer Lett. 251:228–236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Graziano F, Kawakami K, Ruzzo A, et al:
Methylenetetrahydrofolate reductase 677C/T gene polymorphism,
gastric cancer susceptibility and genomic DNA hypomethylation in an
at-risk Italian population. Int J Cancer. 118:628–632. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sarbia M, Geddert H, Kiel S, et al:
Methylenetetrahydrofolate reductase C677T polymorphism and risk of
adenocarcinoma of the upper gastrointestinal tract. Scand J
Gastroenterol. 40:109–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Ji M, He N and Lu Z: Application of
microarray-based method for methylenetetrahydrofolate reductase
(MTHFR) polymorphisms in the risk of gastric carcinoma in east
China population. J Nanosci Nanotechnol. 7:3245–3249. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Guo W, He Y, et al: Association of
MTHFR C677T and SHMT1 C1420T with susceptibility to ESCC and GCA in
a high incident region of Northern China. Cancer Causes Control.
18:143–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weng YR, Sun DF, Fang JY, Gu WQ and Zhu
HY: Folate levels in mucosal tissue but not
methylenetetrahydrofolate reductase polymorphisms are associated
with gastric carcinogenesis. World J Gastroenterol. 12:7591–7597.
2006.PubMed/NCBI
|
|
18
|
Si PR, Fang DC, Zhang H, Yang LQ, Luo YH
and Liao HY: The relationship between methylenetetrahydrofolate
reductase gene polymorphism and microsatellite instability in
gastric cancer. Zhonghua Liu Xing Bing Xue Za Zhi. 26:794–799.
2005.(In Chinese). PubMed/NCBI
|
|
19
|
Kim JK, Kim S, Han JH, et al:
Polymorphisms of 5,10-methylenetetrahydrofolate reductase and risk
of stomach cancer in a Korean population. Anticancer Res.
25:2249–2252. 2005.PubMed/NCBI
|
|
20
|
Wang LD, Guo RF, Fan ZM, et al:
Association of methylenetetrahydrofolate reductase and thymidylate
synthase promoter polymorphisms with genetic susceptibility to
esophageal and cardia cancer in a Chinese high-risk population. Dis
Esophagus. 18:177–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stolzenberg-Solomon RZ, Qiao YL, Abnet CC,
et al: Esophageal and gastric cardia cancer risk and folate- and
vitamin B(12)-related polymorphisms in Linxian, China. Cancer
Epidemiol Biomarkers Prev. 12:1222–1226. 2003.PubMed/NCBI
|
|
22
|
Galván-Portillo MV, Cantoral A,
Oñate-Ocaña LF, et al: Gastric cancer in relation to the intake of
nutrients involved in one-carbon metabolism among MTHFR 677 TT
carriers. Eur J Nutr. 48:269–276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zúñiga-Noriega JR, Velazco-Campos Mdel R,
Aguirre-Rodríguez A, et al: C677T polymorphism of the MTHFR gene
and the risk of developing distal gastric cancer in a Mexican
population. Rev Gastroenterol Mex. 72:355–358. 2007.(In Spanish).
PubMed/NCBI
|
|
24
|
Lacasaña-Navarro M, Galván-Portillo M,
Chen J, López-Cervantes M and López-Carrillo L:
Methylenetetrahydrofolate reductase 677C>T polymorphism and
gastric cancer susceptibility in Mexico. Eur J Cancer. 42:528–533.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vollset SE, Igland J, Jenab M, et al: The
association of gastric cancer risk with plasma folate, cobalamin,
and methylenetetrahydrofolate reductase polymorphisms in the
European Prospective Investigation into Cancer and Nutrition.
Cancer Epidemiol Biomarkers Prev. 16:2416–2424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeybek U, Yaylim I, Yilmaz H, et al:
Methylenetetrahydrofolate reductase C677T polymorphism in patients
with gastric and colorectal cancer. Cell Biochem Funct. 25:419–422.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Re V, Cannizzaro R, Canzonieri V, et
al: MTHFR polymorphisms in gastric cancer and in first-degree
relatives of patients with gastric cancer. Tumour Biol. 31:23–32.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao S, Ding LH, Wang JW, Li CB and Wang
ZY: Diet folate, DNA methylation and polymorphisms in
methylenetetrahydrofolate reductase in association with the
susceptibility to gastric cancer. Asian Pac J Cancer Prev.
14:299–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saberi S, Zendehdel K, Jahangiri S, et al:
Impact of methylenetetrahydrofolate reductase C677T polymorphism on
the risk of gastric cancer and its interaction with Helicobacter
pylori infection. Iran Biomed J. 16:179–184. 2012.PubMed/NCBI
|
|
30
|
Li S, Cai MJ, Hou P and He NY: Single
nucleotide polymorphisms(C677T and A1298C) in
methylenetetrahy-drofolate reductase gene and susceptibility to
gastric carcinoma. J Southeast Univ. 25:321–324. 2006.
|
|
31
|
Yang XX, Li FX, Yi JP, Li X, Sun JZ and Hu
NY: Impact of methylenetetrahydrofolate reductase C677T
polymorphism on the risk of gastric cancer, colorectal cancer and
lung cancer. Guangdong Med J. 31:2375–2378. 2010.
|
|
32
|
Gao CM, Wu JZ, Ding JH, et al: MTHFR C677T
genotypes, lifestyle and the risk of stomach cancer. China J Cancer
Prev Treat. 8:187–190. 2001.
|
|
33
|
Sagoo GS, Little J and Higgins JP: Human
Genome Epidemiology Network: Systematic reviews of genetic
association studies. PLoS Med. 6:e282009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cochran WG: The combination of estimates
from different experiments. Biometrics. 10:101–129. 1954.
View Article : Google Scholar
|
|
35
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
36
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Galbraith RF: A note on graphical
presentation of estimated odds ratios from several clinical trials.
Stat Med. 7:889–894. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hirschhorn JN, Lohmueller K, Byrne E and
Hirschhorn K: A comprehensive review of genetic association
studies. Genet Med. 4:45–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wacholder S, Chanock S, Garcia-Closas M,
El Ghormli L and Rothman N: Assessing the probability that a
positive report is false: An approach for molecular epidemiology
studies. J Natl Cancer Inst. 96:434–442. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thorlund K, Imberger G, Johnston BC, et
al: Evolution of heterogeneity (I2) estimates and their
95% confidence intervals in large meta-analyses. PLoS One.
7:e394712012. View Article : Google Scholar : PubMed/NCBI
|