Introduction
Transcription factor hypoxia-inducible factor-1
(HIF-1) is composed of α and β subunits (1). When mammalian cells contain sufficient
oxygen, HIF-1α is degraded by proteasomes. Thus, under normal
conditions, mammalian cells contain HIF-1β, but not HIF-1α
(2). However, in hypoxic conditions,
HIF-1α stabilizes and associates with HIF-1β. The resulting HIF-1
complex regulates target genes that encode growth and angiogenic
factors, such as vascular endothelial growth factor (VEGF),
glycolysis enzymes [for example, hexokinase (HK)] and glucose
transporters (3). Therefore, HIF-1
expression increases the viability of hypoxic cells and the rate of
glycolysis.
Intratumoral hypoxia develops in malignant tumors,
including pancreatic cancer (4). A
hypoxic environment increases HIF-1α stability and induces its
expression in cancer cells (5,6).
Subsequently, HIF-1α expression in cancer cells leads to an
increase in cell growth and migration, glycolytic activity, and
angiogenesis (3,7,8).
Healthy cells produce energy by oxidative
phosphorylation (OXPHOS) in the mitochondria. However, OXPHOS is
inhibited in the majority of cancer cells, which instead derive the
preponderance of their energy from an increase in the rate of
glycolysis (9). Glycolysis is an
inefficient method of cellular energy production; therefore, cancer
cells increase the expression levels of glucose transporters and
glycolytic enzymes to maintain a high rate of glycolysis (10). This is known as the Warburg effect.
HIF-1α makes a significant contribution to the Warburg effect by
enhancing the expression of glucose transporters and glycolytic
enzymes. Furthermore, HIF-1α expression increases the growth,
migration and angiogenesis of cancer cells (3,7,8).
Endocrine and exocrine pancreatic tissues are
separated by a permeable membrane, thus, insulin secreted from
endocrine β-cells diffuses into the pancreatic interstitium at high
concentrations (11). When pancreatic
cancer occurs, intrapancreatic insulin activates insulin receptors
(IRs) expressed on cancer cells, and induces cell growth, migration
and angiogenesis (12–15). Notably, IR activation and HIF-1α
expression have the same effect on pancreatic cancer cell growth,
migration and angiogenesis. In consideration of this, we
hypothesize that the IR and HIF-1 signaling pathways interact with
each other in pancreatic cancer cells. In agreement with this
hypothesis, intracellular kinases, such as phosphoinositide
3-kinase, Akt and extracellular signal-regulated kinases 1/2, are
involved in the signaling cascades that induce HIF-1α expression
and those that follow IR activation (6,12,13).
The present study investigated whether the insulin
and HIF-1 signaling pathways cooperate in pancreatic cancer cells.
Wild-type MiaPaCa2 cells (wt-MiaPaCa2) and MiaPaCa2 cells
transfected with small interfering RNA against HIF-1α (si-MiaPaCa2)
were orthotopically transplanted into separate nude mice. In our
previous studies, it was demonstrated that wt-MiaPaCa2 cells
expressed HIF-1α when cultured in vitro under hypoxic
conditions and when grown as tumor grafts in nude mice. By
contrast, si-MiaPaCa2 cells did not express HIF-1α in the
aforementioned conditions (7). The
present study varied intrapancreatic insulin levels in
tumor-carrying mice under the following conditions: i) Untreated
(miceun); ii) single injection of the β-cell toxin
streptozotosin (SZ) prior to cancer cell transplantation
(micesz) and iii) daily injection of insulin following
cancer cell transplantation (micein). By altering levels
of insulin in these mice, the current study aimed to investigate
possible cooperation between interstitial insulin and
cancer-induced HIF-1 expression.
Materials and methods
Animal experiments
The wt-MiaPaCa2 pancreatic cancer cells were
purchased from the American Type Culture Collection (Rockville, MD,
USA). The si-MiaPaCa2 cell line was created in our previous study
(7), and the growth, glycolysis and
migration of the two cell types were characterized in our previous
studies (7,16,17). Mice
were handled following the principles of laboratory animal care
described by the National Institutes of Health (grants1.nih.gov/grants/olaw/references/phspol.htm).
Following acclimation, 45 male nude mice (age, 5 weeks; weight,
23±5 g; Taconic, Ry, Denmark) were randomly designated to one
intact control group and six tumor carrier groups (Table I). The control mice
(micectr, n=5) were left untreated, the wt-MiaPaCa2
cells were orthotopically transplanted into three groups of nude
mice (miceun-wt, n=6; micein-wt, n=7; and
micesz-wt, n=7) and the si-MiaPaCa2 cells were
orthotopically transplanted into the remaining three groups of nude
mice (miceun-si, n=6; micein-si, n=7; and
micesz-si, n=7), as previously described (7). All tumor carriers were subjected to one
of the following three conditions: i) Miceun-wt and
miceun-si were untreated; ii) following cancer cell
transplantation, 50 mU insulin (Novo Nordisk, Bagsværd, Denmark)
was subcutaneously (s.c.) injected into micein-wt and
micein-si once a day; and iii) one day prior to
cancer-cell transplantation, SZ (cat no. S0130; Sigma Aldrich, St.
Louis, MO, USA) was s.c. injected into micesz-wt and
micesz-si at a dose of 50 mg/kg. This dose was derived
from a pilot study wherein different doses of SZ (20–240 mg/kg)
were administered to nude mice carrying wt-MiaPaCa2 cells. The aim
of the pilot study was to determine the maximum possible dose of SZ
that allowed the tumor carriers to survive for 12 weeks. Notably,
the selected SZ dose (50 mg/kg) was considerably smaller than that
used to induce diabetes in normal nude mice (240 mg/kg) (18). In addition, all 45 mice survived
treatment. The mice were sacrificed under anesthesia (50 mg
pentobarbital/kg, intraperitoneally), and the chest and abdomen
were opened by incision. The heart was punctured using a needle
(gauge #18) connected to a syringe. Blood was collected in the
syringe, and the plasma was separated. Furthermore, healthy
pancreas and tumor biopsies were obtained, and stored at −80°C.
 | Table I.Groups of nude mice (n=7). |
Table I.
Groups of nude mice (n=7).
| Groups | n | Transplanted
cells |
Treatmenta |
|---|
|
micectr | 5 | None | NA |
|
miceun-wt | 6 | wt-MiaPaCa2 | None |
|
micein-wt | 7 | wt-MiaPaCa2 | Insulin injection
following cancer induction |
|
micesz-wt | 7 | wt-MiaPaCa2 | SZ injection prior to
cancer induction |
|
miceun-si | 6 | si-MiaPaCa2 | None |
|
micein-si | 7 | si-MiaPaCa2 | Insulin injection
following cancer induction |
|
micesz-si | 7 | si-MiaPaCa2 | SZ injection prior
to cancer induction |
Assays
Insulin and c-peptide were extracted from tumor-free
pancreatic tissue using acid ethanol, as previously described
(19). Pancreatic insulin and
c-peptide concentrations were determined using radioimmunoassay
kits (Linco Research Inc., St. Charles, MO, USA). Plasma insulin
and c-peptide concentrations were determined using the same kits,
however, plasma glucose and lactate were measured in a biochemical
analyzer (2700; YSI, Inc., Yellow Springs, OH, USA). Cryosections
were prepared from the tumor biopsies (8-µm thick) using a
microtome cryostat (CM1950; Leica Microsystems GmbH, Wetzlar,
Germany). Subsequently, apoptosis was stained by terminal
deoxynucleotidyl transferase dUTP nick end labeling using an
ApopTag® kit (EMD Millipore, Temecula, CA, USA) (20) and the sections were counterstained
with hematoxylin. Microscopic morphology was captured
region-by-region with a DM4000B microscope (Leica Microsystems
GmbH) using a charge-coupled device camera (DFC500; Leica Camera
AG). Images were merged using Leica Application Suite software
version 3.8 (Leica Microsystems GmbH) and a global view was
reconstituted for each section. Western blotting was used to
determine the protein expression levels of IR, HK-II, and VEGF in
the tumor grafts (7,16,17,20).
Briefly, whole-cell protein was extracted following homogenization
of the tumor grafts. Proteins were separated on an 8% SDS gel and
transferred to polyvinylidene difluoride membranes. The membranes
were then incubated overnight at 4°C with monoclonal mouse
anti-human IR-β (1:1,000; cat no. 69508; Abcam, Cambridge, UK),
monoclonal mouse anti-human VEGF (1:1,000; cat no. 555036; BD
Pharmingen, San Diego, CA, USA) and polyclonal goat anti-human
HK-II (1:1,000; cat no. 6521; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) primary antibodies. After rinsing, membranes were
incubated with the appropriate horseradish peroxidase-conjugated
secondary antibodies [sheep anti-mouse IgG (1:2,000; cat no. NA931;
GE Healthcare Life Sciences, Chalfont, UK) and polyclonal rabbit
anti-goat IgG (1:2,000; cat no. AP106P; EMD Millipore)] for 1 h at
room temperature and treated with chemiluminescence detection
reagents. Specific blotting was recorded using an image analysis
system (ChemiDoc™ XRS System, Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Statistical analysis
Analyses were performed using SPSS software version
17.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean
± standard error of the mean. When three or more groups were
involved, results were analyzed using analysis of variance.
However, when fewer than three groups were involved, Student's
t-test was performed. P<0.05 was considered to indicate a
statistically significant difference.
Results
Pancreatic insulin concentration significantly
decreased in five out of six groups of tumor carriers compared with
the micectr (P<0.05; Fig.
1A). Furthermore, pancreatic c-peptide concentration
significantly decreased in miceun-wt and
micesz-si (P<0.05; Fig.
1B), and plasma insulin concentration levels were significantly
decreased in micesz-wt and micesz-si
(P<0.05; Fig. 1C) compared with
micectr. No tumor carriers exhibited significant changes
in plasma c-peptide concentration (Fig.
1D). However, plasma glucose concentration significantly
decreased in micein-si (P<0.05; Fig. 1E), and plasma lactate concentration
significantly increased in micesz-wt and
miceun-si (P<0.05; Fig.
1F) compared with micectr.
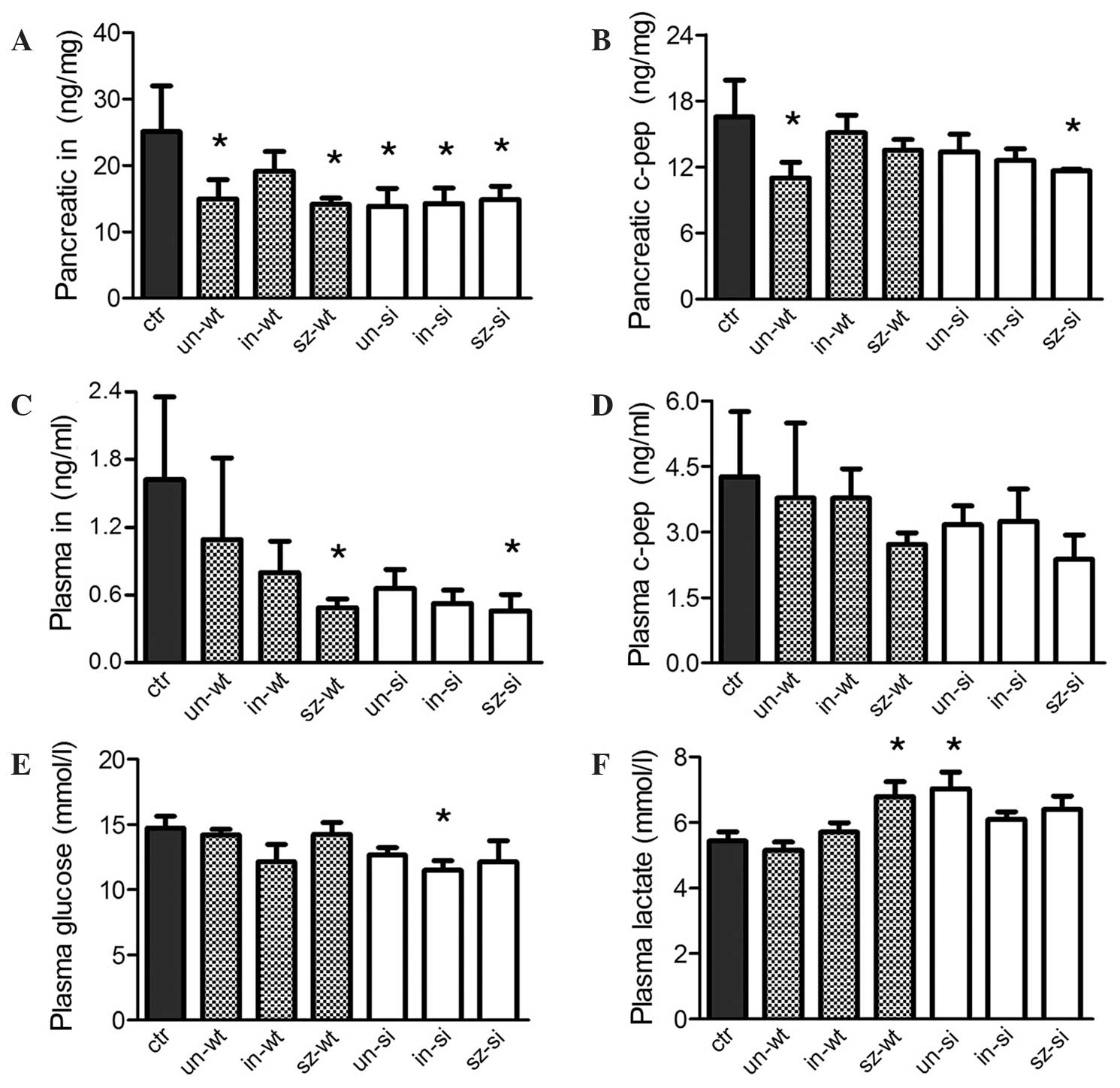 | Figure 1.Nude mice (n=45) were designated to
micectr and six pancreatic tumor carrier groups. In
three groups (miceun-wt, micein-wt and
micesz-wt), pancreatic tumors were composed of
wt-MiaPaCa2 pancreatic cancer cells. In the other carriers
(miceun-si, micein-si and
micesz-si), tumors were composed of hypoxia-inducible
factor-1-negative si-MiaPaCa2 cells. Miceun-wt and
miceun-si were otherwise untreated. For
micesz-wt and micesz-si, sz (50 mg/kg) was
injected prior to cancer induction. For micein-wt and
micein-si, in (50 mU) was injected daily following
cancer induction. Pancreatic concentrations of (A) in and (B) c-pep
were determined, and the results were normalized to the weight of
the samples (in mg). Plasma samples were analyzed for (C) in, (D)
c-pep, (E) glucose and (F) lactate concentrations levels. Error
bars indicate the standard error of the mean. *P<0.05 vs.
micectr. Ctr, control; un, untreated; wt, wild-type; in,
insulin; sz, streptozosin; si, small interfering RNA; c-pep,
c-peptide. |
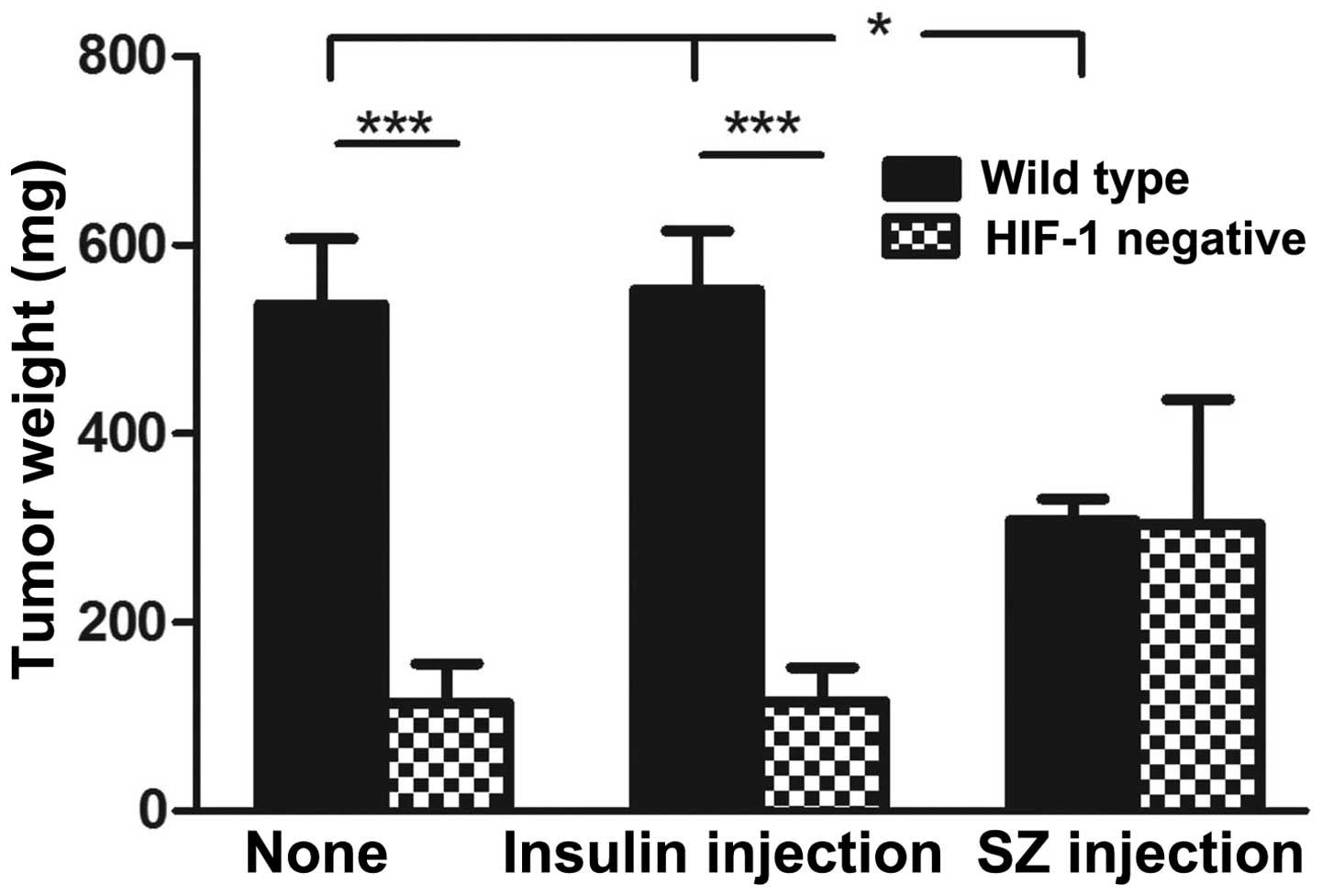
wt-MiaPaCa2 tumors from miceun-wt and
micein-wt were heavier than their si-MiaPaCa2
counterparts from miceun-si and micein-si
(P<0.001), as well as those from micesz-wt
(P<0.05; Fig. 2). However, no
significant differences in tumor weight were observed between the
three groups of si-MiaPaCa2 tumors (Fig.
2). All tumor sections contained apoptotic regions (Fig. 3). Tumors from miceun-wt
exhibited the greatest cell viability, with only small and sporadic
necrotic regions (Fig. 3A). By
contrast, large, central necrosis was observed in wt-MiaPaCa2
tumors in carriers treated with SZ. In this group, the large,
central necrotic regions were surrounded by smaller necrotic
regions (Fig. 3B). The third group of
wt-MiaPaCa2 tumors from micein-wt exhibited a loose
organization that appeared to be the result of chronic cell death
(Fig. 3C). Thus, wt-MiaPaCa2 tumors
in carriers treated with SZ or insulin exhibited poorer cell
viability than the wt-MiaPaCa2 tumors in which the carriers were
untreated. This indicates that intact interstitial insulin
concentration is the most favorable condition for cancer cell
survival. However, examination of si-MiaPaCa2 tumors always
revealed poor cell viability. For example, tumors from
miceun-si were largely composed of hollow regions
(Fig. 3D). However, a high rate of
apoptosis was observed in tumors from micesz-si, even in
peripheral regions (Fig. 3E).
Additionally, si-MiaPaCa2 tumors from micein-si
contained numerous apoptotic regions despite their small size
(Fig. 3F).
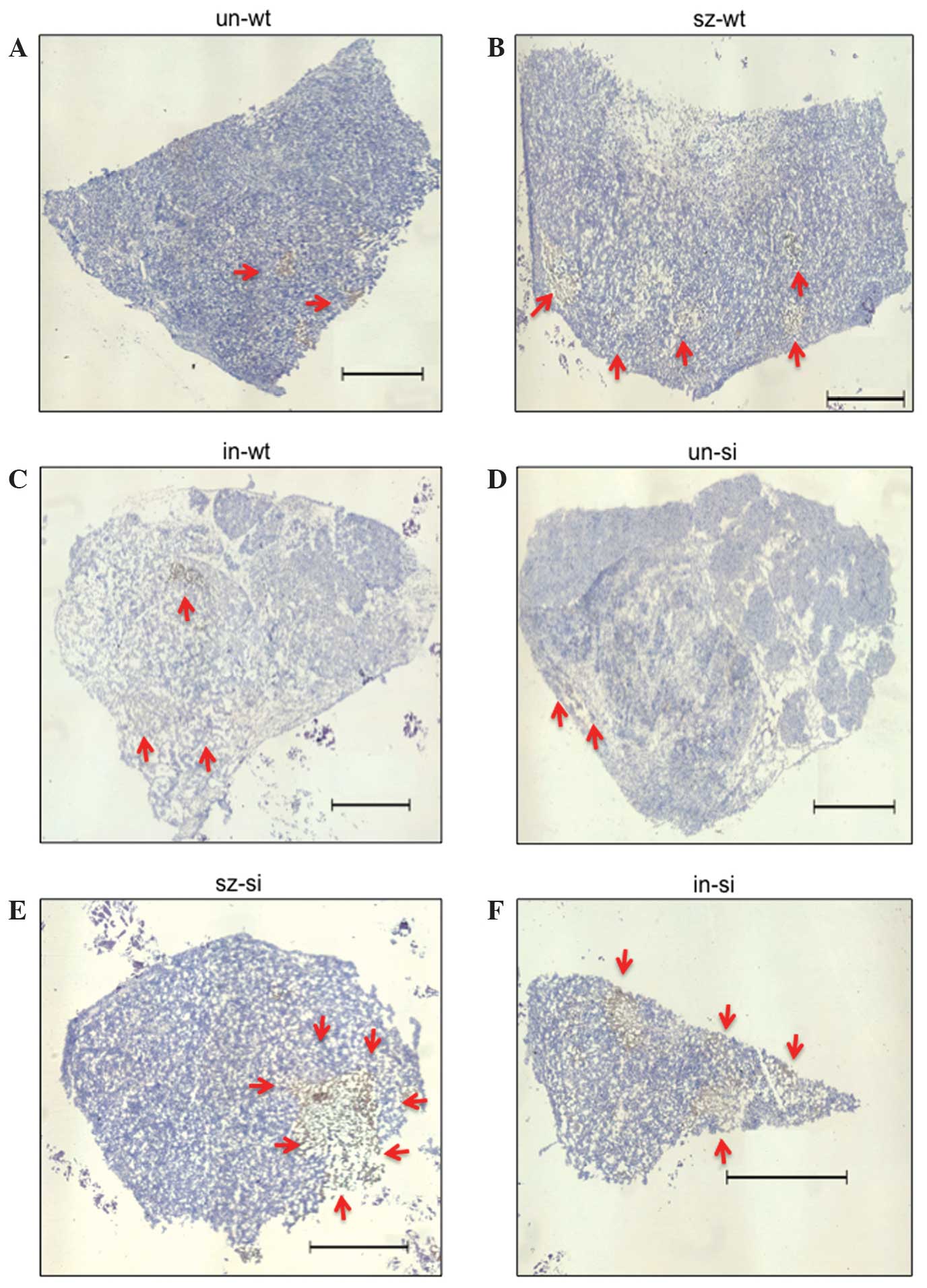 | Figure 3.Apoptosis in tumor grafts of (A)
miceun-wt, (B) micesz-wt, (C)
micein-wt, (D) miceun-si, (E)
micesz-si and (F) micein-si. Tumor sections
were stained by terminal deoxynucleotidyl transferase dUTP nick end
labeling, and hematoxylin staining. Apoptotic cells are stained in
brown and indicated by arrows. The six panels are representative of
the six experimental groups. Scale bar, 1 mm. un, untreated; wt,
wild-type; sz, streptozotosin; in, insulin; si, small interfering
RNA. |
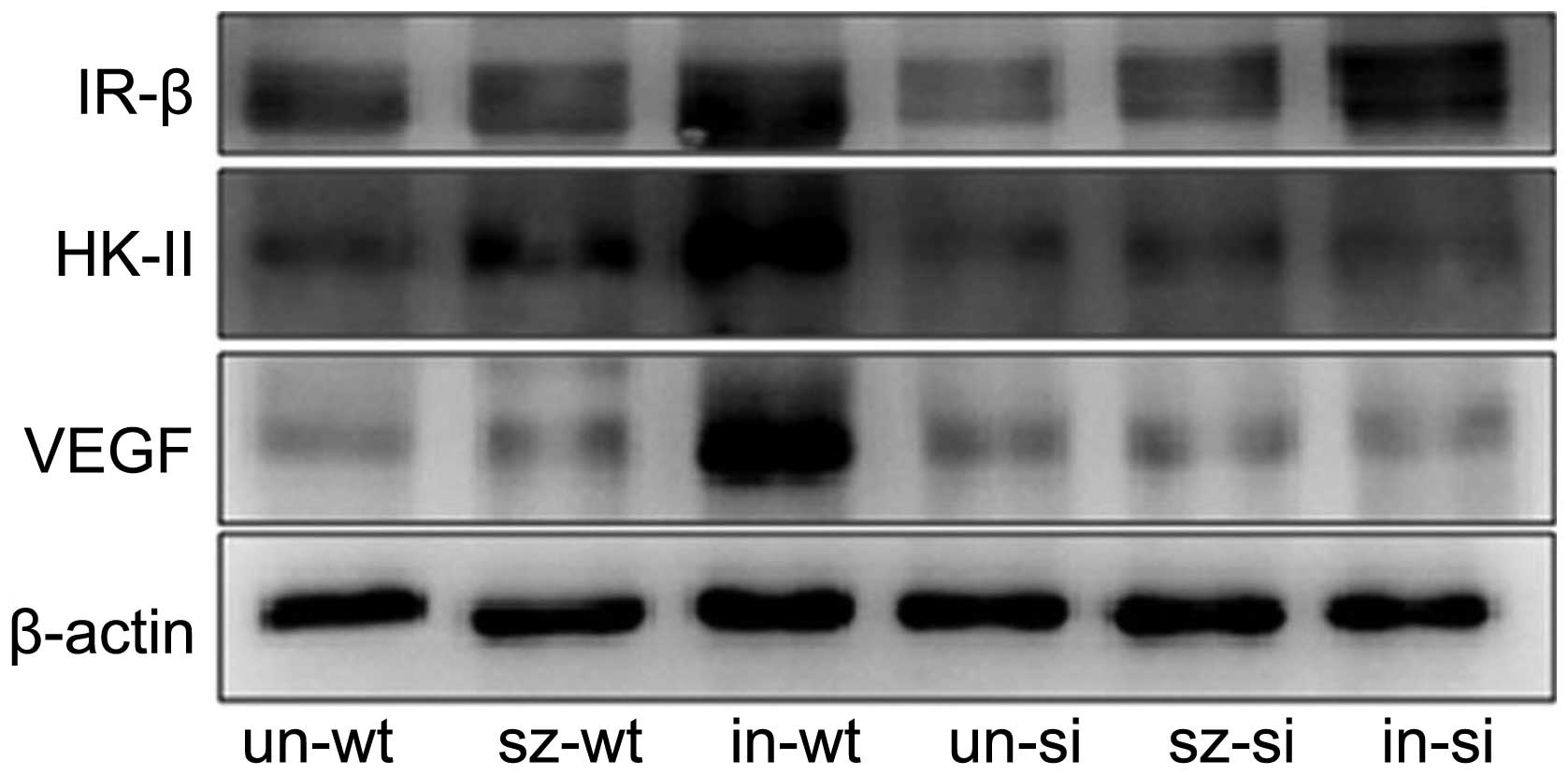
Protein expression analysis of IR and associated
proteins identified lower IR expression in si-MiaPaCa2 tumors from
miceun-si than their wt-MiaPaCa2 counterparts (Fig. 4). This indicates that HIF-1 is
required for basal IR expression. Insulin injections in
micein-wt and micein-si increased IR
expression levels in tumors from these carriers (Fig. 4). Thus, insulin treatment markedly
increased the expression of IR in cancer cells, independent of
HIF-1 expression. Although exogenous insulin induced similar
increases in IR expression in wt- and si-MiaPaCa2 cancer cells
in vivo, this effect was associated with increased HK-II and
VEGF protein expression in wt-MiaPaCa2 tumors but not si-MiaPaCa2
tumors (Fig. 4). Thus, cancer-induced
HIF-1 expression appears to be necessary for insulin to increase
HK-II and VEGF expression in the same cancer cells.
Discussion
Carcinogen-induced pancreatic cancer is associated
with a decrease in the population of pancreatic β-cells in hamsters
(21). This raises the possibility
that the presence of pancreatic cancer may cause a decrease in
pancreatic insulin production, thus resulting in decreased levels
of insulin in the pancreatic interstitial fluid. In the present
study, five out of six tumor carrier groups exhibited a decrease in
intrapancreatic insulin concentration. Furthermore, a significant
decrease in plasma insulin was observed in the mice that were
treated by the β-cell toxin SZ. Therefore, in addition to the
tumor-induced decrease in pancreatic insulin concentration, SZ
treatment may further decrease interstitial insulin levels in the
pancreas.
The Warburg effect increases glucose consumption and
lactate production in cancer cells. Following the transport of
cancer-induced lactate to the liver and its subsequent conversion
to glucose, it re-enters the bloodstream and can be returned back
to cancer cells. The glucose-lactate cycle increases energy
expenditure and causes cancer cachexia (22,23). In
the present study, certain tumor-carrying mice exhibited decreased
glucose levels and increased lactate levels in the plasma. These
results may be a consequence of increased glucose turnover in the
animals.
Tumors from miceun-si exhibited lower
levels of IR expression than their wild-type counterparts,
indicating that the HIF-1 signaling pathway is required for the
maintenance of basal IR levels in pancreatic cancer cells.
Significantly, IR expression was increased in tumors from
insulin-treated micein-wt and micein-si,
thus, the insulin-induced IR expression was independent of HIF-1
expression. However, an increase in VEGF and HK-II protein
expression levels, induced by exogenous insulin, was only observed
in tumors from micein-wt but not micein-si.
This indicates that although insulin-induced IR expression is
independent of HIF-1, HIF-1 is still required for insulin to
increase HK-II and VEGF expression levels in pancreatic cancer
cells.
In the present study, nude mice were treated with SZ
or insulin in an attempt to alter insulin concentrations in the
pancreatic interstitium. The results indicate that SZ treatment
decreased interstitial insulin in the pancreas. Exogenous insulin
increased IR, HK-II and VEGF expression levels in wt-MiaPaCa2
tumors carried by micein-wt, indicating that insulin
treatment may stimulate tumor cells in the pancreas. However,
wt-MiaPaCa2 tumors from micein-wt weighed as much as
those from miceun-wt, opposing the hypothesis that
exogenous insulin stimulated tumor cells in vivo. In
addition, histological examination indicated that tumors from
micein-wt had poor viability. Considering that exogenous
insulin may decrease insulin release by negative feedback, the
current data indicates that the ultimate effect of exogenous
insulin on pancreatic cancer cells may be of an inhibitory nature.
Notably, wt-MiaPaCa2 tumors from miceun-wt and
micein-wt were heavier than those from
micesz-wt. This is consistent with the hypothesis that
SZ reduces the stimulation of interstitial insulin in pancreatic
cancer cells. Finally, tumors from miceun-wt and
micein-wt were heavier than their si-MiaPaCa2
counterparts. This is in agreement with the results of a previous
study (7), which identified that the
HIF-1 signaling pathway is important for the growth of cancer
cells. To the best of our knowledge, the present study is the first
to investigate the effect of intra-pancreatic insulin on pancreatic
cancer cells in vivo. The results are consistent with those
of previous in vitro studies, which have demonstrated the
stimulatory effects of insulin on pancreatic cancer cells (14,15). Thus,
antagonizing the activity of intra-pancreatic insulin may
contribute to the inhibition of pancreatic cancer.
In conclusion, the present study demonstrated that
paracrine insulin and cancer-induced HIF-1 cooperate to provide
pancreatic cancer cells with a survival advantage. However,
additional studies are required to elucidate the mechanisms
underlying this cooperation.
Acknowledgements
The authors of the present study thank Dr Shasha Li
from Tianjin Medical University for her technical assistance and
the Qian-Ren Program of the Tianjin Municipal Government for their
financial support (grant no. 116001-20100004).
References
|
1
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992.PubMed/NCBI
|
|
2
|
Salceda S and Caro J: Hypoxia-inducible
factor 1alpha (HIF-1alpha) protein is rapidly degraded by the
ubiquitin-proteasome system under normoxic conditions. Its
stabilization by hypoxia depends on redox-induced changes. J Biol
Chem. 272:22642–22647. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koong AC, Mehta VK, Le QT, et al:
Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol
Biol Phys. 48:919–922. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhong H, De Marzo AM, Laughner E, et al:
Overexpression of hypoxia-inducible factor 1alpha in common human
cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
6
|
Fukuda R, Hirota K, Fan F, Jung YD, Ellis
LM and Semenza GL: Insulin-like growth factor 1 induces
hypoxia-inducible factor 1-mediated vascular endothelial growth
factor expression, which is dependent on MAP kinase and
phosphatidylinositol 3-kinase signaling in colon cancer cells. J
Biol Chem. 277:38205–38211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang F, Li SS, Segersvärd R, Strömmer L,
Sundqvist KG, Holgersson J and Permert J: Hypoxia inducible
factor-1 mediates effects of insulin on pancreatic cancer cells and
disturbs host energy homeostasis. Am J Pathol. 170:469–477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Funasaka T, Yanagawa T, Hogan V and Raz A:
Regulation of phosphoglucose isomerase/autocrine motility factor
expression by hypoxia. FASEB J. 19:1422–1430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lyshchik A, Higashi T, Hara T, et al:
Expression of glucose transporter-1, hexokinase-II, proliferating
cell nuclear antigen and survival of patients with pancreatic
cancer. Cancer Invest. 25:154–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakagawa A, Samols E and Stagner JI:
Exocrine interstitial insulin and somatostatin in the perfused dog
pancreas. Am J Physiol. 264:G728–G734. 1993.PubMed/NCBI
|
|
12
|
Gallagher EJ and LeRoith D: The
proliferating role of insulin and insulin-like growth factors in
cancer. Trends Endocrinol Metab. 21:610–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trajkovic-Arsic M, Kalideris E and Siveke
JT: The role of insulin and IGF system in pancreatic cancer. J Mol
Endocrinol. 50:R67–R74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang F, Larsson J, Adrian TE, Gasslander T
and Permert J: In vitro influences between pancreatic
adenocarcinoma cells and pancreatic islets. J Surg Res. 79:13–19.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding XZ, Fehsenfeld DM, Murphy LO, Permert
J and Adrian TE: Physiological concentrations of insulin augment
pancreatic cancer cell proliferation and glucose utilization by
activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression.
Pancreas. 21:310–320. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Jia X, Duan Y, Xiao H, Sundqvist
KG, Permert J and Wang F: Excess glucose induces hypoxia-inducible
factor-1α in pancreatic cancer cells and stimulates glucose
metabolism and cell migration. Cancer Biol Ther. 14:428–435. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao H, Li S, Zhang D, Liu T, Yu M and
Wang F: Separate and concurrent use of 2-deoxy-D-glucose and
3-bromopyruvate in pancreatic cancer cells. Oncol Rep. 29:329–334.
2013.PubMed/NCBI
|
|
18
|
Graham ML, Janecek JL, Kittredge JA,
Hering BJ and Schuurman HJ: The streptozotocin-induced diabetic
nude mouse model: Differences between animals from different
sources. Comp Med. 61:356–360. 2011.PubMed/NCBI
|
|
19
|
Wang F, Larsson J, Abdiu A, Gasslander T,
Westermark P, Adrian TE and Permert J: Dissociated secretion of
islet amyloid polypeptide and insulin in serum-free culture media
conditioned by human pancreatic adenocarcinoma cell lines. Int J
Pancreatol. 21:157–164. 1997.PubMed/NCBI
|
|
20
|
Wang F, Kumagai-Braesch M, Herrington MK,
Larsson J and Permert J: Increased lipid metabolism and cell
turnover of MiaPaCa2 cells induced by high fat diet in an
orthotopic system. Metabolism. 58:1131–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asano N, Manabe T, Imanishi K and Tobe T:
Changes of A, B and D cells in Langerhans islets in pancreatic
cancers of hamsters. Nihon Geka Hokan. 60:233–242. 1991.PubMed/NCBI
|
|
22
|
Torosian MH, Bartlett DL, Chatzidakis C
and Stein TP: Effect of tumor burden on futile glucose and lipid
cycling in tumor-bearing animals. J Surg Res. 55:68–73. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lundholm K, Edström S, Karlberg I, Ekman L
and Scherstén T: Glucose turnover, gluconeogenesis from glycerol,
and estimation of net glucose cycling in cancer patients. Cancer.
50:1142–1150. 1982. View Article : Google Scholar : PubMed/NCBI
|