Introduction
Prostate cancer has become one of the most common
types of cancer, and is currently the second most lethal disease
affecting the elderly male population in developed countries. In
2010 and 2013, prostrate cancer led to 80,900 and 33,720
mortalities, respectively (1–3). Although progress has been made in the
treatment of prostate cancer, the majority of patients succumb to
tumor metastasis. Furthermore, tumors recur in ~30% of patients
within 12–18 months of undergoing a prostatectomy (4,5).
Therefore, an improved understanding of the molecular mechanisms
involved in the pathogenesis of prostate cancer, including those
underlying metastasis and invasion, is urgently required.
The Piwi genes, which were identified a number of
decades ago, were the first class of genes known to be required for
stem cell self-renewal in a diverse range of organisms (6,7). Piwi-like
RNA-mediated gene silencing 2 (Piwil2) belongs to the Ago/Piwi
family, which is comprised of Piwil1/Hiwi, Piwil2/Hili, Piwil3 and
Piwil4/Hiwi2. The human-derived Piwil2 gene regulates RNA silencing
and transcription, functions in spermatogenesis, and is involved in
the self-renewal and differentiation of normal testis and fetal
tissues (8,9). Piwil2, as a candidate oncogene in
previous studies, is highly expressed in breast, gastric,
colorectal and papillary thyroid cancer, and is closely associated
with the occurrence and progression of tumors (10–14).
The association between Piwil2 and prostate cancer
is rarely reported. The present study therefore aimed to
investigate this association and discern the potential underlying
molecular mechanisms.
Methods
Cell lines and primary tumor
specimens
The PC-3, 22RV1, DU-145 and LNCaP cell lines
(American Type Culture Collection, Manassas, VA, USA) were cultured
in RPMI-1640 complete medium (GE Healthcare Life Sciences, Logan,
UT, USA) containing 10% fetal calf serum, 100 units/ml penicillin
and 100 µg/ml streptomycin. The immortalized normal prostate
epithelial cell line RWPE-1 was cultured in keratinocyte serum-free
medium containing 0.05 mg/ml bovine pituitary extract and 5 ng/ml
recombinant epidermal growth factor (Gibco Life Technologies,
Carlsbad, CA, USA). The cell lines were cultivated in a 37°C
incubator with 5% CO2. Next, the cells were digested
with 0.25% trypsin every three to four days for passage. All cells
used in the experiments were in the logarithmic growth phase.
In total, 30 tumor specimens were obtained from
patients with prostate cancer at The First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China). The patients, who were
aged between 45 and 89 years old, underwent a radical
prostatectomy, without receiving any other treatments, between July
and November 2012. The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Zhengzhou University.
Informed consent was obtained from each patient, conforming to the
tenets of the Declaration of Helsinki (15).
Total RNA extract and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Human tumor tissues and pericarcinomatous tissues
obtained from patients with prostate cancer were immediately frozen
in liquid nitrogen and stored at −80°C. The total RNA from the
tumor tissues, pericarcinomatous tissues and all aforementioned
cell lines were extracted using a total RNA extraction kit,
according to the manufacturer's instructions (Invitrogen Life
Technologies, Carlsbad, CA, USA). Next, 100 ng total RNA was used
for qPCR, according to the instructions of the SYBR Green PCR
master mix kit (Invitrogen Life Technologies). Each amplification
was performed using one of the following primers: β-actin forward,
5′-CACCCAGCACAATGAAGAT-3′ and reverse, 5′-CAAATAAAGCCTGCCAAT-3′;
Piwil2 forward, 5′-TCATGGGGCCATCAGAAG-3′ and reverse,
5′-CCATCCCGATCACCATTAAC-3′; matrix metalloproteinase (MMP)-2
forward, 5′-GATACCCCTTGACGGTAAGG-3′ and reverse,
5′-CCTTCTCCCAAGGTCCATAGC-3′; MMP-9 forward,
5′-GGGACGCAGACATCGTCATC-3′ and reverse, 5′-TCGTCATCGTCGAAATGGGC-3′;
E-cadherin forward, 5′-TGCTCTTCCAGGAACCTCTGTG-3′ and reverse,
5′-GGTGACCACACTGATGACTCCTG-3′; N-cadherin forward,
5′-GGTGGAGGAGAAGAAGACCAG-3′ and reverse, 5′-GGCATCAGGCTCCACAGT-3′;
vimentin forward, 5′-GGGACCTCTACGAGGAGGAG-3′ and reverse,
5′-CGCATTGTCAACATCCTGTC-3′; and Twist forward,
5′-GCTGTGCTTACTCTAGCCATC-3′ and reverse,
5′-TGAGGCATTTGCTCACATCAC-3′. All PCR experiments were performed in
triplicate using the Bio-Rad C1000 Touch™ Thermal Cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). PCR was performed under the
following conditions: Denaturation at 93°C for 2 min, followed by
40 cycles of 93°C for 1 min, 55°C for 1 min and 72°C for 1 min, and
extension at 72°C for 7 min.
Western blot analysis
The cells were lysed at 2–8°C in RIPA solution
containing 1% proteasome inhibitor (Beyotime Institute of
Biotechnology, Jiangsu, China), for 30 min. The total protein
concentration was then determined using the Bradford method. In
total, 30 µg of denaturized total protein was loaded into each lane
for SDS-PAGE. The bands were then transferred to polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were incubated with blocking buffer (Sigma-Aldrich, St.
Louis, MO, USA) containing 5% bovine serum albumin in
phosphate-buffered saline for 2 h at room temperature. Incubation
was then performed using the primary monoclonal mouse anti-human
Piwil2 (dilution, 1:800; cat. no. LS-C62097-100; LifeSpan
BioSciences, Inc., Seattle, WA, USA), monoclonal mouse anti-human
E-cadherin (dilution, 1:800; cat. no. 5296S; Cell Signaling
Technology, Inc., Danvers, MA, USA), monoclonal mouse anti-human
N-cadherin (dilution, 1:500; cat. no. 14215S; Cell Signaling
Technology, Inc.), polyclonal rabbit anti-human Twist (dilution,
1:800; cat. no. ab50581; Abcam, Cambridge, UK), monoclonal mouse
anti-rabbit vimentin (dilution, 1:800; cat. no. 9775S; Cell
Signaling Technology, Inc.) and polyclonal mouse anti-human β-actin
(dilution, 1:1,500; cat. no. sc-7210; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) antibodies overnight at 4°C. The membranes
were subsequently incubated with horseradish peroxidase-conjugated
polyclonal rabbit anti-mouse (dilution, 1:4,000; cat. no. A0216;
Beyotime Institute of Biotechnology) or polyclonal goat anti-rabbit
(dilution, 1:4,000; cat. no. A0239; Beyotime Institute of
Biotechnology) secondary antibodies for 2 h at room temperature.
Detection was performed using the enhanced chemiluminescent
substrate (Bio-Rad Laboratories, Inc.).
Knockdown of the Piwil2 gene using
short hairpin RNA (shRNA)
A recombinant lentivirus containing a green
fluorescent protein (GFP) reporter and a sequence encoding a
Piwil2-specific shRNA (shRNA group) or a scramble shRNA (mock
group) was synthesized by Shanghai Rainbow Chemistry Co., Ltd.
(Shanghai, China). The sequences of the Piwil2 and scrambled shRNA
were 5′-AAACCTTTGGACCCAGCTCTG-3′ and 5′-GTACCGCACGTCATTCGTATC-3′,
respectively (14). The PC-3 cells
were transfected with viral supernatants according to the
manufacturer's instructions. In total, 5×105 cells were
washed three times prior to the cell transfection, and 20 pmol
shRNA, with 3 µg/ml polybrene in serum-free medium, was added to
the cells and incubated at 37°C. Fresh culture medium was added
after 6 h. The cells expressing GFP were sorted using a flow
cytometer (FACS AriaTM II; BD Biosciences, Franklin Lakes, NJ, USA)
when the cells had reached 70–80% confluence. The silencing effect
on the Piwil2 gene was assessed by qPCR and western blot analysis,
according to the aforementioned protocols.
Tumor invasion and migration
A Transwell unit was used to investigate cellular
invasion. The units were placed in 24-well plates and the upper
chamber was coated with Matrigel (BD Biosciences). In total, 200 ml
of cell solution, at a density of 5×104 cells/ml,
resuspended in serum-free medium, was added to the upper chamber,
and medium containing 10% fetal bovine serum was added to the lower
chamber. Subsequent to a 24-h incubation, the cells that had passed
through the membrane were fixed with 4% paraformaldehyde for 15
min, and then stained with crystal violet. The cells in five random
fields were counted under an Olympus CX41 light microscope
(magnification, x200; Olympus Corporation, Shanghai, China). In
addition, a wound-healing assay was performed in order to measure
the extent of cell migration. In total, 1×104 PC-3 cells
were transplanted into a 24-well plate. When the cells had grown to
cover the entire bottom of the plate well, a straight-line scratch
was made using a 20-µl pipette tip. Subsequent to a 48-h incubation
period in the serum-free medium, images of the cells were captured
using an Olympus CKX31 reverse-phase microscope (Olympus
Corporation).
Statistical analysis
The data were analyzed using SPSS software version
15.0 (SPSS, Inc., Chicago, IL, USA). The data derived from the
tumor invasion assay of the shRNA and mock groups were assessed
using a t-test for two independent samples or the rank-sum
test for non-normal distribution, and the data are presented as the
mean ± standard deviation. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of Piwil2 in clinically
obtained specimens of prostate cancer
In order to investigate the expression of the Piwil2
gene and protein in patients with different histological grades of
prostate cancer, RT-qPCR and western blot analysis was performed.
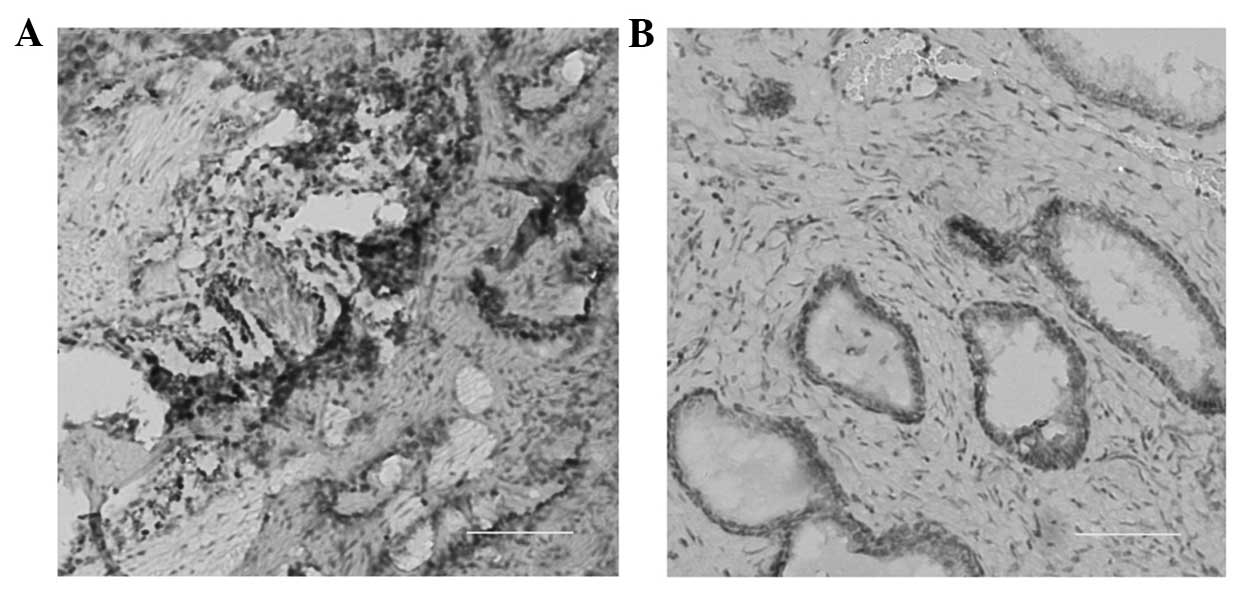
Immunohistochemical staining revealed a high expression of
Piwi-like protein 2 in prostate cancer cells (Fig. 1A) when compared with adjacent normal
tissue (Fig. 1B). The results of
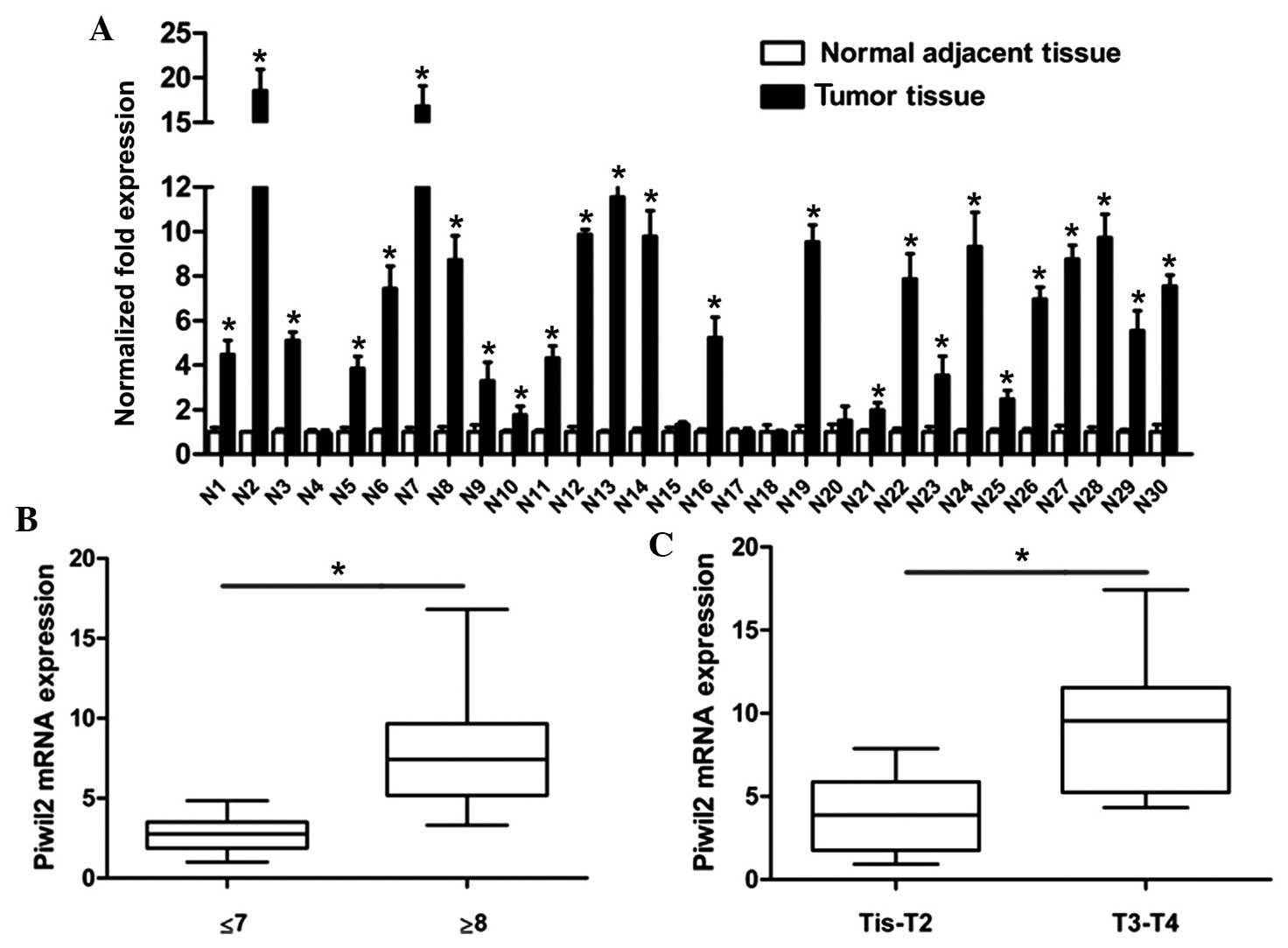
RT-qPCR revealed that 83.33% of tumor tissues (25/30) possessed a
higher level of Piwil2 than the associated adjacent tissues
(Fig. 2A). In addition, Piwil2
expression was positively associated with the Gleason score
(P=0.002) and the tumor-node-metastasis (TNM) stage (P=0.003) of
the tumor tissue. As shown in Fig. 2B and
C, Piwil2 was overexpressed in patients with a high Gleason
score (≥8) and an advanced TNM stage (T3-T4).
Expression of Piwil2 in prostate
cancer cell lines
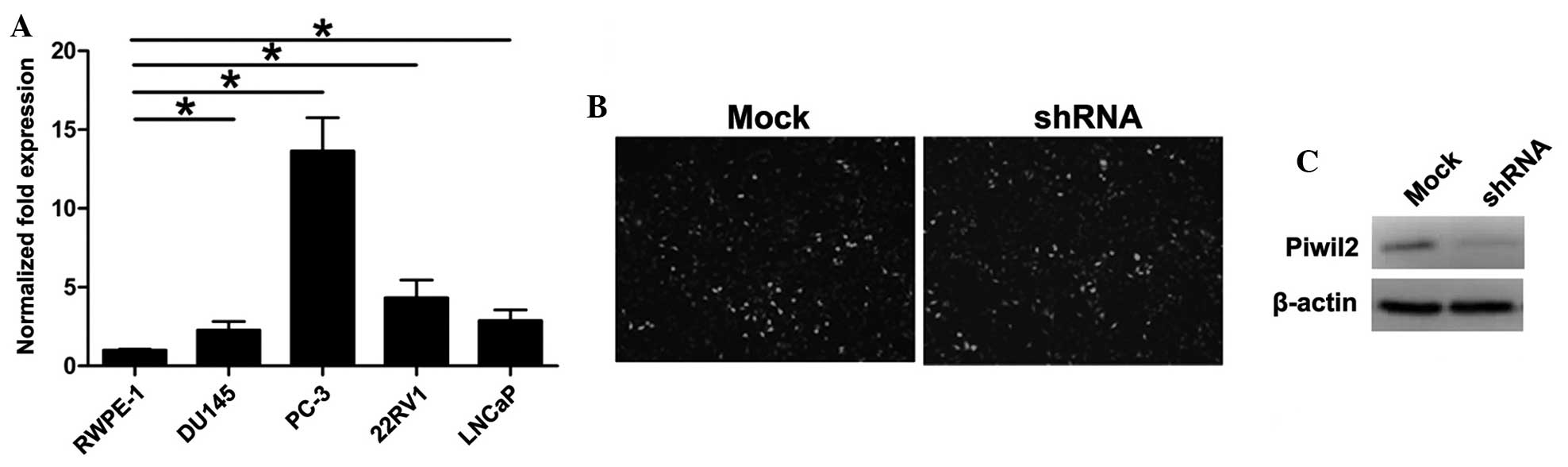
RT-qPCR was performed in order to detect the level
of Piwil2 in the normal prostate RWPE-1 cell line and in the PC-3,
22RV1, DU-145 and LNCaP prostate cancer cell lines. The level of
Piwil2 in the tumor cell lines was higher compared with the normal
prostate cell line (Fig. 3A). Of the
four prostate cancer cell lines analyzed, Piwil2 expression was
highest in the PC-3 cells.
Subsequent to transfection with shRNA or mock
recombinant lentiviruses, GFP-positive PC-3 cells were collected by
flow cytometry, sorted and then cultured (Fig. 3B). The silencing effect on the Piwil2
gene was confirmed by western blot analysis. Compared with the mock
group, the Piwil2 gene was significantly downregulated in cells of
the shRNA group (Fig. 3C). This
demonstrated that the lentivirus-encoding Piwil2-targeted shRNA
effectively decreased Piwil2 expression in the PC-3 cells.
Function of Piwil2 in invasion and
migration in vitro
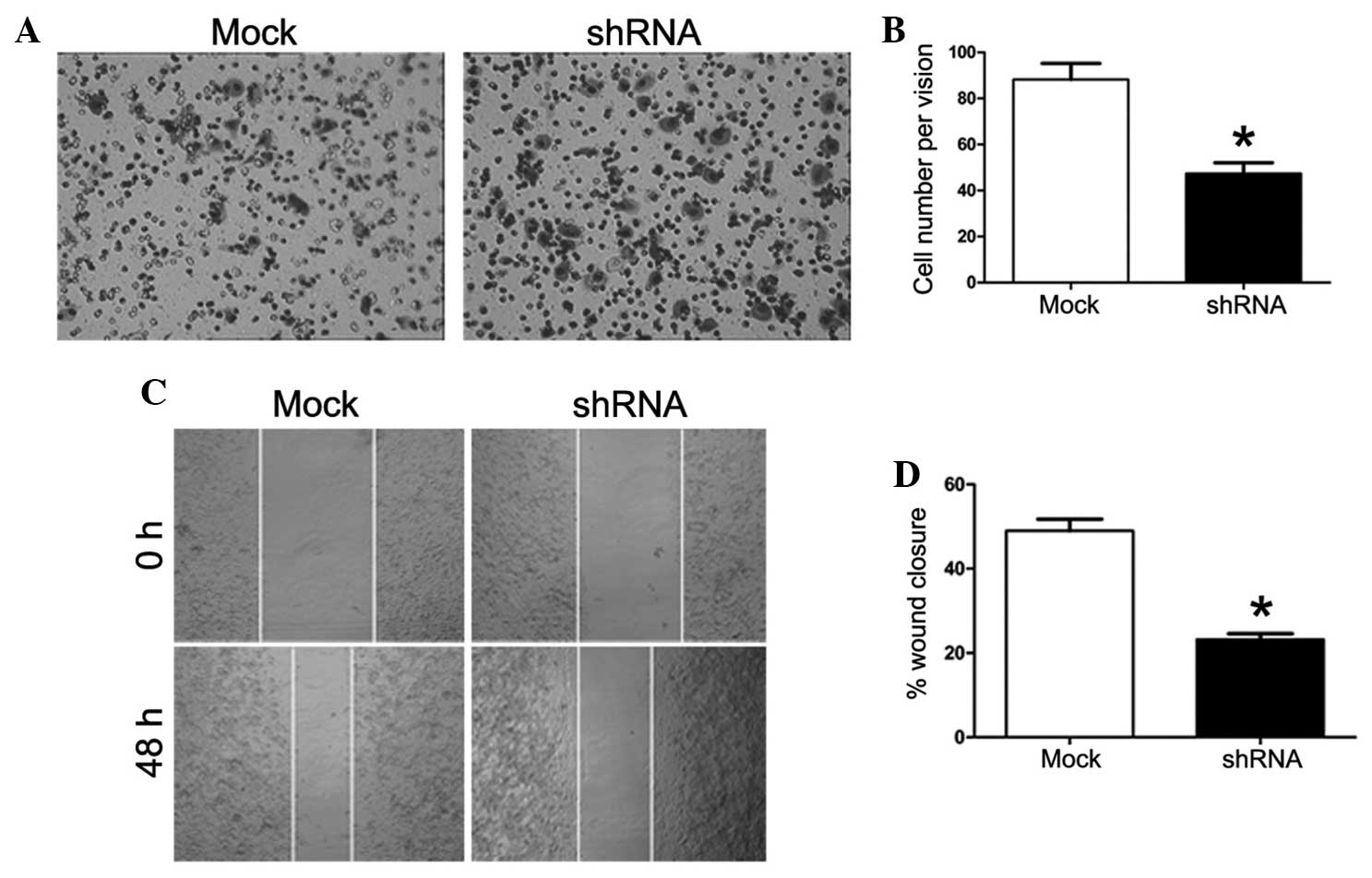
For the tumor invasion assay, the number of cells on
the lower chamber of the Transwell unit was counted. The number of
cells in the shRNA group (49.19±9.09) was significantly lower
compared with the mock group (90.43±14.54) (P=0.000; Fig. 4A and B). A wound-healing assay was
also performed in order to measure the distance of cell migration
in serum-free medium. The difference in the width of the scratch
between 0 and 48 h was processed using ImageJ 1.37 software
(National Institutes of Health, Bethesda, MA, USA). Knockdown of
the Piwil2 gene significantly decreased cell migration in the shRNA
group (Fig. 4C and D). The extent of
wound closure in the mock group (49.64±5.63) was significantly
higher compared with the shRNA group (23.53±2.66) (P=0.000). These
results indicate that knock-down of the Piwil2 gene in the PC-3
cells decreased invasion and migration in vitro.
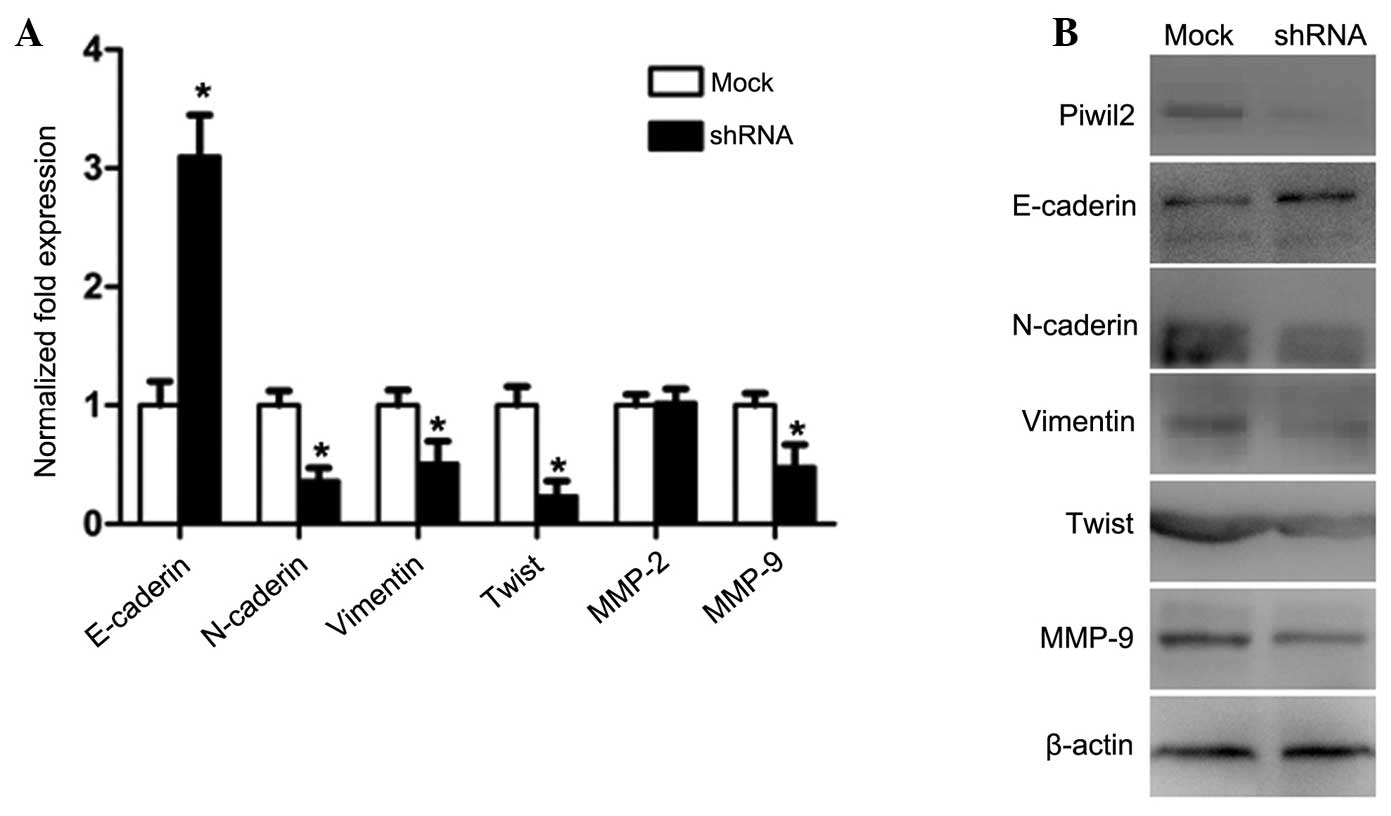
Knockdown of Piwil2 decreases the
expression of MMP-2/9 and inhibits epithelial-mesenchymal
transitions (EMT)
As MMPs and EMT have important roles in tumor
invasion and metastasis, the levels of MMP-2 and -9, together with
the expression of the primary biomarkers of EMT, E-cadherin,
N-cadherin, vimentin and Twist, were evaluated by RT-qPCR and
western blotting. The results revealed that the expression of
vimentin, Twist and MMP-9 in the PC-3 cells of the shRNA group was
downregulated, but that E-cadherin was upregulated and MMP-2
exhibited no change in expression (Fig.
5A and B).
Discussion
The human-derived Piwil2 gene was first identified
as a stem and testis cell-specific gene (7). As tumor cells and stem cells have the
capacity of self-renewal, it is likely that their regulatory
mechanisms are similar. Piwil2 is also expressed in several
aforementioned tumors. The Piwil2 gene has been highly conserved
during evolution and serves as a key factor in maintaining the
self-renewal and differentiation of testis and embryonic cells in
normal tissue. In addition, Piwil2 has an important role in the
development, differentiation and regulation of precancerous stem
cells (9). An overexpression of
Piwil2 can disturb cell division, and therefore lead to malignant
transformation. Piwil proteins have been used as predictive markers
for a number of cancers (12,16), particularly for the early detection of
disease (6). Furthermore, an
overexpression of Piwil2 has been associated with a higher tumor
stage and a poorer prognosis (17,18).
Despite this, the role of Piwil2 in prostate cancer has been
investigated in relatively few studies (6,19).
The present study demonstrated that the Piwil2 gene
is highly expressed in surgical tissues and prostate cancer cell
lines. Furthermore, it revealed that the level of Piwil2 was
positively associated with the Gleason score and TNM stage of the
tumor. These results indicate that Piwil2 has a positive role in
the development and progression of prostate cancer. Out of the four
prostate cancer cell lines that were analyzed, PC-3 cells possess
the capacity of high invasion and were derived from bone
metastasis, which accounts for up to 90% of all metastatic types of
prostate cancer (20). A recombinant
lentivirus encoding Piwil2-targeted-shRNA was therefore used to
knock down Piwil2 expression in PC-3 cells. As predicted, the
results revealed that the inhibition of Piwil2 significantly
reduced the invasion and metastasis of PC-3 cells.
A previous study identified that Piwil2 modulated
colon cancer metastasis via the regulation of MMP-9 transcriptional
activity (14). A further study
revealed that MMP-9 in PC-3 cells induced the activity of
osteoclasts, and enhanced the invasion of bone metastasis (21). In order to elucidate the potential
underlying mechanisms, the present study analyzed the expression of
MMPs and consequently identified a downregulation in the expression
of MMP-9 in the Piwil2-targeted shRNA PC-3 cells. EMT has gained
interest for its involvement in the early steps of invasion and
metastasis in malignant tumors of epithelial origin (22). Whether or not Piwil2 activates the
process of EMT has yet to be elucidated. Therefore, the present
study evaluated the expression of the primary biomarkers of EMT
using western blotting and RT-qPCR in the Piwil2-targeted shRNA
PC-3 cells. The epithelial marker, E-cadherin, was upregulated,
whereas the associated transcription factors involved in EMT,
namely vimentin, N-cadherin and Twist, were downregulated.
In summary, the present study revealed that the
expression of Piwil2 is positively correlated with the Gleason
score and TNM stage of patients with prostate cancer. The Piwil2
gene is involved in the invasion and metastasis of tumor cells by
regulating the levels of MMP-9 and modulating EMT. This suggests
that Piwil2 participates in tumor aggressiveness and progression.
Therefore, Piwil2 may be a novel therapeutic target in the
treatment of prostate cancer.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81272823), and the Henan
Science and Technology Research Foundation (no. 122102310047).
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beltran H, Beer TM, Carducci MA, et al:
New therapies for castration-resistant prostate cancer: efficacy
and safety. Eur Urol. 60:279–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang SS and Kibel AS: The role of
systemic cytotoxic therapy for prostate cancer. BJU Int. 103:8–17.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ni J, Cozzi P, Hao J, et al: Epithelial
cell adhesion molecule (EpCAM) is associated with prostate cancer
metastasis and chemo/radioresistance via the PI3K/Akt/mTOR
signaling pathway. Int J Biochem Cell Biol. 45:2736–2748. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JH, Schütte D, Wulf G, et al:
Stem-cell protein Piwil2 is widely expressed in tumors and inhibits
apoptosis through activation of Stat3/Bcl-XL pathway. Hum Mol
Genet. 15:201–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cox DN, Chao A, Baker J, et al: A novel
class of evolutionarily conserved genes defined by piwi are
essential for stem cell self-renewal. Genes Dev. 12:3715–3727.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sasaki T, Shiohama A, Minoshima S and
Shimizu N: Identification of eight members of the Argonaute family
in the human genome. Genomics. 82:323–330. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuramochi-Miyagawa S, Kimura T, Ijiri TW,
et al: Mili, a mammalian member of piwi family gene, is essential
for spermatogenesis. Development. 131:839–849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Sun Y, Guo J, et al: Expression of
hiwi gene in human gastric cancer was associated with proliferation
of cancer cells. Int J Cancer. 118:1922–1929. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He G, Chen L, Ye Y, et al: Piwil2
expressed in various stages of cervical neoplasia is a potential
complementary marker for p16. Am J Transl Res. 2:156–169.
2010.PubMed/NCBI
|
|
12
|
Liu JJ, Shen R, Chen L, et al: Piwil2 is
expressed in various stages of breast cancers and has the potential
to be used as a novel biomarker. Int J Clin Exp Pathol. 3:328–337.
2010.PubMed/NCBI
|
|
13
|
Yin DT, Li HQ, Wang YF, et al: Expression
of Piwil2 and its relationship with tumor invasion and metastasis
in papillary thyroid carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai
Ke Za Zhi. 46:237–239. 2011.(In Chinese). PubMed/NCBI
|
|
14
|
Li D, Sun X, Yan D, et al: Piwil2
modulates the proliferation and metastasis of colon cancer via
regulation of matrix metallopeptidase 9 transcriptional activity.
Exp Biol Med (Maywood). 237:1231–1240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
General Assembly of the World Medical
Association, . World Medical Association Declaration of Helsinki:
Ethical principles for medical research involving human subjects. J
Am Coll Dent. 81:14–18. 2014.PubMed/NCBI
|
|
16
|
Wang Y, Liu Y, Shen X, et al: The PIWI
protein acts as a predictive marker for human gastric cancer. Int J
Clin Exp Pathol. 5:315–325. 2012.PubMed/NCBI
|
|
17
|
Oh SJ, Kim SM, Kim YO and Chang HK:
Clinicopathologic implications of PIWIL2 expression in colorectal
cancer. Korean J Pathol. 46:318–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Greither T, Koser F, Kappler M, et al:
Expression of human Piwi-like genes is associated with prognosis
for soft tissue sarcoma patients. BMC Cancer. 12:2722012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shaikhibrahim Z, Lindstrot A, Ochsenfahrt
J, Fuchs K and Wernert N: Epigenetics-related genes in prostate
cancer: Expression profile in prostate cancer tissues,
androgen-sensitive and -insensitive cell lines. Int J Mol Med.
31:21–25. 2013.PubMed/NCBI
|
|
20
|
Bubendorf L, Schöpfer A, Wagner U, et al:
Metastatic patterns of prostate cancer: an autopsy study of 1,589
patients. Hum Pathol. 31:578–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong Z, Bonfil RD, Chinni S, et al: Matrix
metalloproteinase activity and osteoclasts in experimental prostate
cancer bone metastasis tissue. Am J Pathol. 166:1173–1186. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boyer B, Vallés AM and Edme N: Induction
and regulation of epithelial-mesenchymal transitions. Biochem
Pharmacol. 60:1091–1099. 2000. View Article : Google Scholar : PubMed/NCBI
|