Introduction
Brain glioma, which originates from neuroglia cells,
is the most common type of intracranial tumor, accounting for ~45%
of all cases. Worldwide, 20–50 million individuals succumb due to
malignant glioma each year (1).
While, in recent years, with the development of diagnostic
instruments and numerous novel antitumor agents, the efficacy of
glioma treatment has greatly improved, it remains unsatisfactory
(2). A previous study reported that,
even when patients with low-grade glioma are considered cured by
surgery, 45% later succumbed, due to recurrence or metastasis of
glioma within 2 years (3). This
indicates that certain patients with low-grade gliomas that are
considered to be completely resected, may have developed
micrometastases prior to surgery (4).
Often, the micrometastases cannot be located using conventional
methods, such as imaging and histopathology, particularly in
low-grade tumors. Therefore, investigation into the micrometastasis
of low-grade glioma is important for improving the treatment and
prognosis of patients with glioma (5).
The thymidylate synthase (TYMS) gene is a malignant
tumor marker that was demonstrated to have high sensitivity and
specificity for malignant metastasis in low-grade glioma (6). The present study detected the expression
levels of the TYMS gene in low-grade malignant glioma tissues and
adjacent lymph nodes, using immunohistochemical analysis. The
current study aimed to provide novel recommendations for diagnosis,
disease evaluation, treatment strategies and prognosis of low-grade
malignant glioma, by exploring the association between TYMS gene
expression in the primary foci and metastatic lymph nodes of
low-grade malignant glioma, and to analyze the function of TYMS in
the lymph node metastases of low-grade glioma.
Patients and methods
Patients
The present study included 93 patients with
surgically resected and pathologically confirmed low-grade
malignant glioma, who were treated at Huaihe Hospital of Henan
University (Kaifeng, China) between July 2009 and January 2014. The
following clinical data was obtained from each patient: Gender,
age, subjective symptoms (dizziness, headache, a feeling of
pressure in the head, etc.), disease site, tumor type, pathological
stage, degree of differentiation and lymph node involvement.
According to the 2007 World Health Organization
(WHO) classification (7), glioma is
classified into four grades with respect to the malignancy of the
glioma. Grade I glioma is pilocytic astrocytoma; grade II is
low-grade malignant glioma; grade III is anaplastic astrocytoma;
and grade IV is glioblastoma multiforme. WHO has also classified
glioma as low-grade glioma, including general pathological
classification of glioma grades I–II, and high-grade glioma,
including grades III and IV glioma. Following treatment, the
survival rate for patients with grade I or II glioma is good, with
patients typically surviving a number of years.
The current cohort of 93 cases included 49 males and
44 females, with an age range of 23–72 years (mean age, 46.14±8.52
years). According to the 2007 WHO classification criteria, the
cohort included 28 cases of astrocytoma, 12 cases of
oligodendroglioma, 10 cases of ependymoma, 11 cases of mixed
glioma, 7 cases of choroid plexus tumor, 7 cases of mixed neuronal
glial tumor, 12 cases of pineal parenchymal tumor and 6 cases of
embryonal tumor. 15 patients with benign brain tumors [7 cases of
meningioma, 6 cases of pituitary adenoma and 2 cases of acoustic
neuroma] were also included in the study, comprising 9 males and 6
females, with an age range of 20–71 years (mean, 45.37±7.61 years).
The present study was conducted in accordance with the declaration
of Helsinki and approval was obtained from the Ethics Committee of
Huaihe Hospital of Henan University. Written informed consent was
obtained from all participants.
Immunohistochemistry
The surgically resected glioma specimens (n=93) and
dissected cervical lymph nodes (n=157) were resected from the
middle of the tissue in the longitudinal direction. These 250
samples were randomly marked by Arabic numerals from 1–250. The
marked sample was resected from the middle of the tissue in the
longitudinal direction and was further marked by A or B (e.g., 1A,
1B; 250A, 250B). Specimen A or B from the 250 samples was then
randomly selected for pathological analysis.
The resected glioma specimens were formalin-fixed,
paraffin-embedded and sliced into 4-µm serial sections. The
sections were dewaxed, hydrated and flushed with phosphate-buffered
saline (PBS). Subsequently, polymer enhancer (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) and mouse anti-human
TYMS monoclonal antibody (catalog no. CAB-4990MH; Creative BioMart,
Shirley, NY, USA; dilution, 1:200) were applied. The sections were
then incubated at room temperature for 1 h and washed with PBS. The
secondary antibody was HRP-conjugated goat anti-mouse polyclonal
antibody (catalog no. ab97023; Abcam, Cambridge, UK; dilution,
1:500). The sections were then incubated again at room temperature
for 1 h. Finally, diaminobenzidine chromogenic liquid (Daan Gene
Co., Ltd.) was added. Following hematoxylin staining, the slices
were sealed with neutral resin and the coloring process was
performed using the immunohistochemical surfactant protein A
method, according to the manufacturer's instructions (Beijing
Dingguo Biotechnology Co. Ltd., Beijing, China). Positive and
negative immunohistochemical controls were used for comparison,
with PBS buffer solution used in place of the TYMS antibody as the
negative control. Subsequent to specimen processing, the expression
of TYMS in the glioma tissue and adjacent cervical lymph nodes was
observed under a BX51 microscope (Olympus Corporation, Tokyo,
Japan).
Polymerase chain reaction (PCR) and
reverse transcription-quantitative PCR (RT-qPCR)
A MagNA Lyser Instrument (Roche Diagnostics, Basel,
Switzerland) was used to homogenate the other half of the 157 lymph
nodes and allow DNA extraction. A DNA sample (1 µg) was selected
for quality evaluation and concentration detection using a NanoDrop
2000 concentration detector (Thermo Fisher Scientific, Inc.,
Rockford, IL, USA). The samples were diluted to a working
concentration of 20–40 ng/µl and the TYMS gene was amplified by PCR
with the following primers: TYMS forward,
5′-GGTATTGAGACAGACAGTGc-3′; TYMS reverse,
5′-CATGGGATCTGTTTCTTTGC-3′; β-actin (control) forward,
5′-GCGGGAAATCGTGCGTGAC-3′; and β-actin reverse,
5′-CGTCATACTCCTGCTTGCTG-3′. The primers were synthezised by Sangon
Biotechnology Co. (Shanghai, China). Real-time quantitative PCR was
performed using a SYBR Premix EX TaqTM kit (TaKaRa, Dalian, China),
according to manufacturer's instructions. The PCR cycling
conditions consisted of 95°C for 30 s followed by 35 cycles of
denaturation at 95°C for 3 s and annealing at 60°C for 30 s,
extension at 72°C for 1 min, and final extension at 72°C for 5 min.
The standard curve was then constructed, and the results were
quantified using the 2−ΔΔCt method. The PCR products
were purified and sequenced using an ABI 3130XL sequencer (Applied
Biosystems, Foster City, CA, USA). In addition, the expression of
TYMS mRNA in the lymph nodes was determined by performing RT-qPCR.
The expression of the TYMS gene, as assessed by RT-qPCR, was used
as marker with which to identify lymph node metastases. The
associations between TYMS gene expression, and lymph node
metastasis, clinical and pathological factors, postoperative
survival and disease-free survival were also analyzed.
Evaluation criteria
Positive expression of the TYMS gene was defined as
the observation of brown granules in the cytomembrane and/or
cytoplasm. The intensity of the expression was determined by
performing 10 high-magnification observations for every slice, and
applying a semi-quantitative integral analysis method, according to
the color intensity and percentage of positively stained cells.
Staining intensity was quantified according to the number of the
positive cells, as follows: <5% positive cells, 0 points; 6–25%
positive cells, 1 point; 25–50% positive cells, 2 points; 51–75%
positive cells, 3 points; and >75% positive cells, 4 points. The
color intensity criterion was as follows: Dark brown, 3 points;
light brown, 2 points; and light yellow, 1 point. Points from the
two subcriteria were combined and the following overall scores were
determined: 6–7 points, strong positive (+++); 4–5 points, moderate
positive (++); 2–3 points, weak positive (+); and 0–1 points,
negative (−) (8).
Association between the expression of
TYMS and relevant factors in patients with glioma
In accordance with a previous study (9), data on gender, age, subjective symptoms,
disease site, tumor type, pathological stage, degree of
differentiation and metastasis of the lymph nodes were collected
from all current patients exhibiting low-grade malignant brain
glioma. The present study used statistics to analyze the
association between lymph node metastasis and these factors.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 14.0; SPSS Inc., Chicago, IL, USA). Each
parameter was split into two categories, which were compared by
performing a t-test. The associations between lymph node metastasis
of low-grade primary malignant glioma tumors and other factors were
analyzed using logistic regression analysis. Furthermore, the
expression of the TYMS gene and the postoperative survival rate
were analyzed using Kaplan-Meier survival analysis, and independent
risk factors were analysis using Cox regression analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
TYMS protein expression and lymph node
metastasis
In the 15 patients with benign brain tumors, TYMS
protein expression was negative in all cervical lymph nodes
examined. However, for the 93 patients with low-grade malignant
glioma, TYMS protein expression was positive in all primary lesions
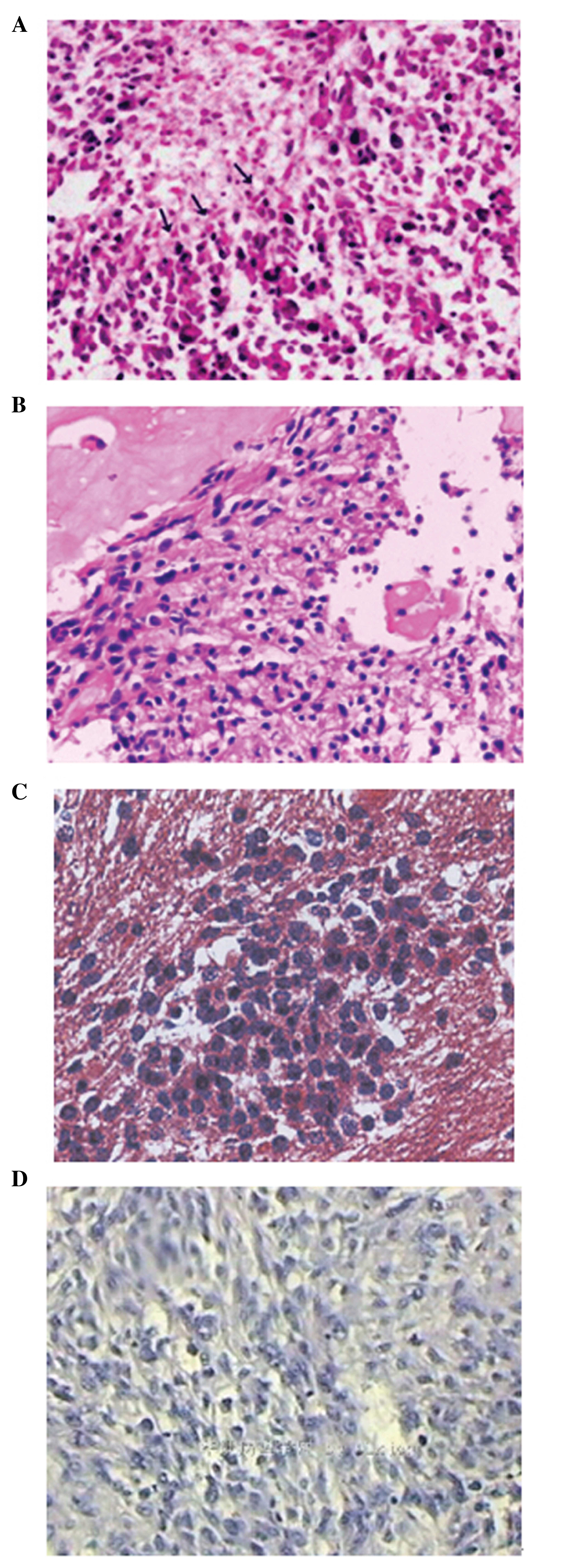
(100%) and in 43/157 (27.93%) lymph nodes, as indicated in Fig. 1.
Analysis of the postoperative overall
survival rate
Relevant clinical factors and lymph node metastasis
were analyzed using Kaplan-Meier survival analysis. The results
indicated that cervical lymph node metastasis (P<0.01), the
number of dissected cervical lymph nodes (P<0.05) and the degree
of differentiation (P<0.05) were significant factors influencing
postoperative survival. However, gender, age, subjective symptoms,
disease site, tumor type and pathological stage exhibited a limited
association with the survival rate of the patients (Table I).
 | Table I.Association between postoperative
overall survival rate and other factors in patients with low-grade
malignant glioma. |
Table I.
Association between postoperative
overall survival rate and other factors in patients with low-grade
malignant glioma.
|
| Category |
|
|
|---|
|
|
|
|
|
|---|
| Factor | 1 | 2 | χ2 | P-value |
|---|
| Gender | Male | Female |
0.481 |
0.539 |
| Age, years | <60 | ≥60 |
0.164 |
0.738 |
| Subjective
symptoms | No | Yes |
2.922 |
0.079 |
| Disease site | Cerebral
hemisphere | Cerebellum |
0.897 |
0.348 |
| Tumor type | Astrocytoma |
Oligodendroglioma |
0.189 |
0.718 |
| Pathological
stage | Ia | Ib |
0.849 |
0.361 |
| Degree of
differentiation | Low | High |
4.875 |
0.041a |
| Metastasis of
cervical lymph nodesb | No | Yes | 13.133 |
<0.001a |
| Dissected cervical
lymph nodes, n | <5 | ≥5 |
6.693 |
0.022a |
Additional analysis of the association between
metastases to cervical lymph nodes and the postoperative survival
rate, identified that the survival rate of patients with cervical
lymph node metastasis was 6.3-fold higher than in those without
cervical lymph node metastasis (P<0.01; data not shown).
Furthermore, the greater the number of lymph nodes dissected, the
higher the patient mortality.
Analysis of the postoperative
disease-free survival rate
The same method was applied in order to analyze the
factors associated the postoperative disease-free survival rate.
The following factors were demonstrated to be significantly
correlated with disease-free survival, using Kaplan-Meier survival
analysis: Metastasis to the cervical lymph nodes (P<0.01), the
number of dissected cervical lymph nodes (P<0.05), the degree of
differentiation (P<0.05) and the presence of subjective symptoms
(P<0.05; Table II). Additional
analysis of the association between metastases to the cervical
lymph nodes and postoperative disease-free survival, demonstrated
that the mortality of patients with metastasis to the cervical
lymph nodes was 8.4-fold higher than that in patients without
metastasis (P<0.01; data not shown).
 | Table II.Association between postoperative
disease-free survival rate and other factors in patients with
low-grade malignant glioma. |
Table II.
Association between postoperative
disease-free survival rate and other factors in patients with
low-grade malignant glioma.
|
| Category |
|
|
|---|
|
|
|
|
|
|---|
| Factor | 1 | 2 | χ2 | P-value |
|---|
| Gender | Male | Female |
0.514 |
0.492 |
| Age, years | <60 | ≥60 |
0.128 |
0.780 |
| Subjective
symptoms | No | Yes |
4.975 |
0.040a |
| Disease site | Cerebral
hemisphere | Cerebellum |
0.833 |
0.371 |
| Tumor type | Astrocytoma |
Oligodendroglioma |
0.328 |
0.609 |
| Pathological
stage | Ia | Ib |
2.691 |
0.102 |
| Degree of
differentiation | Low | High |
5.693 |
0.032a |
| Metastasis of
cervical lymph nodesb | No | Yes | 18.268 |
<0.001a |
| Dissected cervical
lymph nodes, n | <5 | ≥5 | 11.482 |
0.002a |
Independent risk factors affecting the
postoperative disease-free survival rate
The following factors were substituted into the Cox
regression analysis: Gender, age, disease site, tumor type,
pathological stage, lymph node metastasis, cervical lymph node
metastasis and number of dissected lymph nodes. The results
indicated that metastasis to the cervical lymph nodes was the only
independent risk factor affecting postoperative disease-free
survival. The risk of recurrence in patients with metastasis to the
cervical lymph nodes was 7.3-fold higher than that in those without
metastasis (P<0.01; data not shown).
Follow-up
The patient was followed-up for 3–65 months until
December 2013, with a median follow-up time of 37 months. A total
of 93 patients dropped out of the follow-up process, resulting in a
dropout rate of 5.38%. It was noted that 28 patients (30.11%) did
not exhibit recurrence or metastasis until after the follow-up
process had ended; 9 patients (9.68%) experienced recurrence or
metastasis, but remained alive; and 51 patients (54.84%) succumbed
due to disease progression during the follow-up period.
Discussion
Brain glioma is a tumor type that continues to be
associated with a poor prognosis. It is well known that the
likelihood of curing glioma is closely associated with disease
stage, in addition to the methods of diagnosis and treatment. Early
detection is critical to improving the therapeutic effect and
increasing the survival of patients with glioma (10). In recent years, with the development
of novel medical technologies, the early diagnosis and rate of
successful treatment of low-grade malignant glioma have
significantly improved. However, the overall survival rate of
patients with glioma remains unsatisfactory. Therefore, there has
been increased interest in improving the therapeutic response of
glioma, particularly malignant glioma. In addition, the molecular
mechanism underlying the pathogenesis of glioma, as well as novel
therapeutic approaches for glioma, have been explored in recent
years (11). A previous clinical
study demonstrated that the same type of glioma exhibited
significant differences in treatment effect, prognosis, invasion,
recurrence and metastasis (12). In
the current study, molecular biology and immunohistochemistry
technologies were employed to explore the role of TYMS genes in
gliomas, which may provide valuable guidance for future treatment
and prognosis evaluation.
The 5-year survival rate of patients with low-grade
malignant glioma is not satisfactory, possibly due to
micrometastasis to lymph nodes, blood or bone marrow (13). These micrometastatic deposits may be a
major cause of recurrence in glioma. Micrometastases are difficult
to detect using conventional methods, such as imaging and clinical
pathology (14). The malignant tumor
cells of micrometastatic deposits may spread and survive in the
lymphatic circulatory system, blood, bone marrow, various tissues
and organs, resulting in numerous relevant clinical manifestations
(15).
A number of preliminary studies have demonstrated
that differences in treatment effect, prognosis, invasion,
recurrence and metastasis among the same pathological type of
glioma may be caused by differences in the expression levels of
specific genes. Therefore, it is important to detect any changes in
such genes and associated proteins, using molecular biology and
immunohistochemical techniques (16,17).
Clinically, optimal and individualized treatment regimes may be
designed for each individual patient with glioma, according to the
results of genetic tests, clinical data, surgery, pathological
analysis and diagnosis (18). Certain
studies have demonstrated that TYMS has high specificity and
sensitivity in detecting low-grade malignant metastasis of glioma.
TYMS may be specifically expressed in glioma tissues, while is not
expressed in normal lymph nodes, indicating that TYMS may be useful
as a marker with which to identify lymph node metastasis.
Therefore, detecting TYMS expression in low-grade malignant lymph
node metastases of glioma may enable a more accurate determination
of prognosis in patients with glioma (19).
The TYMS gene is located on the short arm of
chromosome 18 and encodes TYMS, a key enzyme involved in DNA
synthesis. TYMS is important for DNA synthesis and repair, and is
critical in folate metabolism circulation. TYMS is also currently
an important target enzyme in clinical chemotherapy based on
5-fluorouracil (5-FU) (20).
Metabolites of 5-fluorodeoxyuridine monophosphate bind to TYMS,
blocking the function of TYMS and thereby inhibiting DNA synthesis.
Numerous clinical studies in colorectal, lung, breast, head and
neck squamous cell carcinoma, as well as other types of tumor, have
identified that TYMS mRNA expression levels are closely associated
with the effects of 5-FU-based chemotherapy. In addition, TYMS has
an important role in the individualized treatment of glioma, as its
presence helps to predict patient sensitivity to radiotherapy and
chemotherapy. Previous clinical studies have demonstrated that
patients with low levels of TYMS mRNA expression exhibit good
responses to 5-FU chemotherapy (21,22). By
contrast, patients with high TYMS expression exhibit poor responses
to fluoride chemotherapy. Furthermore, a previous study identified
that the efficacy of pemetrexed, a novel antifolate agent, was
negatively correlated with the expression level of TYMS mRNA
(23).
In the present study, data regarding gender, age,
subjective symptoms, disease site, tumor type, degree of
differentiation, metastasis to cervical lymph nodes and the number
of dissected lymph nodes, were collected from patients with
low-grade malignant brain glioma. It was identified that lymph node
metastasis with TYMS mRNA expression had no significant correlation
with gender, age, smoking, pathological type and pathological
grade. However, lymph node metastasis was closely associated with
the number of dissected lymph nodes and the degree of
differentiation. Following metastasis to neck lymph nodes,
prognosis is poor and the postoperative survival time is short. The
greater the number of dissected lymph nodes, the lower the relative
probability of tumor metastasis and recurrence following surgery.
In addition, the lower the degree of tumor differentiation, the
higher the degree of malignancy, the poorer the prognosis, and the
shorter the postoperative survival time.
Following clarification of the associated risk
factors, the present study analyzed the association between the
aforementioned factors and disease-free survival of postoperative
patients. The results of the Kaplan-Meier survival analysis
indicated that the factors affecting postoperative survival
included cervical lymph node metastasis, the number of lymph nodes
dissected and the degree of differentiation (P<0.05). Additional
analysis of the association between disease-free survival and lymph
node metastasis of the neck, identified that the risk of mortality
was 28.4-fold higher in patients with cervical lymph node
metastasis compared with those without cervical lymph node
metastasis (P<0.01).
Although the sensitivity of RT-qPCR technology is
significantly greater than that of routine pathological
examination, the process of metastasis of low-grade malignant glial
neoplasm is complex and its underlying mechanisms remain unclear.
Furthermore, detection of abnormal expression of TYMS will not
identify every lymph node metastasis. Therefore, additional
investigation of the molecular mechanism underlying lymph node
metastasis is required, in order to provide a theoretical basis and
empirical evidence for the accurate staging of low-grade malignant
glioma, as well as informing multidisciplinary treatment strategies
for postoperative patients (24).
Due to the unique nature and diversity of gliomas,
the implementation of individualized therapy and targeted drug
treatment is the optimal clinical treatment strategy for clinicians
and patients (25). Adjuvant
therapies applied prior to surgery to patients with low-grade
malignant glioma, may reduce tumor size and decrease tumor stage,
allowing certain patients with locally advanced disease to undergo
surgical treatment. Detection of lymph node metastasis provides a
basis for more accurate staging of low-grade malignant glioma and
guides treatment selection (26). In
the present study, the association between TYMS gene expression and
the prognosis of patients with low-grade malignant glioma was
investigated by detecting the expression of the TYMS gene in
primary tumors and adjacent lymph nodes. The results of the present
study may facilitate the accurate prediction of the prognosis of
patients with low-grade malignant brain glioma, lowering invasive
intervention, reducing the conjecture involved in selecting
postoperative treatment strategies and improve therapeutic efficacy
(27).
The association between TYMS expression, and highly
malignant glioma and lymph nodes metastasis has been investigated
in previous studies. However, to the best of our knowledge, studies
analyzing the correlation between these factors are absent from the
literature. The present study observed the expression of TYMS in
low-grade malignant glioma tissue and adjacent lymph nodes, and
analyzed the correlation between TYMS expression, and low-grade
malignant glioma, lymph node metastasis, clinicopathological
factors and postoperative survival rate. These results may be
useful in guiding the clinical diagnosis, evaluations, treatment
and prognosis of patients with low-grade malignant glioma. Due to
the abnormal expression of TYMS in the lymph nodes adjacent to the
low-grade malignant glioma, TYMS expression is hypothesized to be
an indicator of lymph node metastasis. The present study
demonstrated that TYMS expression is significantly correlated with
overall and disease-free survival of patients with low-grade
malignant glioma. Therefore, certain high-risk patient subgroups
may be identifiable by detection of abnormal TYMS expression in
lymph nodes adjacent to the low-grade malignant brain glioma
lesion. In addition, this data may facilitate the accurate staging
of disease, allowing certain patients with low-grade malignant
brain glioma micrometastases to be more easily identified and,
thus, to undergo earlier intervention. Future studies should
determine the most comprehensive treatment strategy for low-grade
malignant brain glioma with lymph node metastasis in order to
improve the long-term survival rates of such patients.
References
|
1
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanai N, Chang S and Berger MS: Low-grade
gliomas in adults. J Neurosurg. 115:948–965. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stewart LA: Chemotherapy in adult
high-grade glioma: A systematic review and meta-analysis of
individual patient data from 12 randomised trials. Lancet.
359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Athanassiou H, Synodinou M, Maragoudakis
E, Paraskevaidis M, Verigos C, Misailidou D, Antonadou D, Saris G,
Beroukas K and Karageorgis P: Randomised phase II study of
temozolomide and radiotherapy compared with radiotherapy alone in
newly diagnosed glioblastoma multiforme. J Clin Oncol.
23:2372–2377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mason WP, Maestro RD, Eisenstat D, Forsyth
P, Fulton D, Laperrière N, Macdonald D, Perry J and Thiessen B:
Canadian GBM Recommendations Committee: Canadian recommendations
for the treatment of glioblastoma multiforme. Curr Oncol.
14:110–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masuda TA, Inoue H, Sonoda H, Mine S,
Yoshikawa Y, Nakayama K, Nakayama K and Mori M: Clinical and
biological significance of S-phase kinase-associated protein 2
(Skp2) gene expression in gastric carcinoma: Modulation of
malignant phenotype by Skp2 overexpression, possibly via p27
proteolysis. Cancer Res. 62:3819–3825. 2002.PubMed/NCBI
|
|
7
|
Rosenblum MK: The 2007 WHO Classification
of Nervous System Tumors: Newly recognized members of the mixed
glioneuronal group. Brain Pathol. 17:308–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weller M: Management of gliomas: Relevance
of molecular markers for clinical practice. Eur Assoc Neuro Oncol
Magazine. 2:6–10. 2012.
|
|
9
|
Shaw E, Arusell R, Scheithauer B, et al:
Prospective randomized trial of low-versus high-dose radiation
therapy in adults with supratentorial low-grade glioma: Initial
report of a North Central Cancer Treatment Group/Radiation Therapy
Oncology Group/Eastern Cooperative Oncology Group study. J Clin
Oncol. 20:2267–2276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takanami I: The prognostic value of
overexpression of Skp2 mRNA in non-small cell lung cancer. Oncol
Rep. 13:727–731. 2005.PubMed/NCBI
|
|
12
|
Kamata Y, Watanabe J, Nishimura Y, Arai T,
Kawaguchi M, Hattori M, Obokata A and Kuramoto H: High expression
of skp2 correlates with poor prognosis in endometrial endometrioid
adenocarcinoma. J Cancer Res Clin Oncol. 131:591–596. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pedeutour-Braccini Z, Burel-Vandenbos F,
Gozé C, Roger C, Bazin A, Costes-Martineau V, Duffau H and Rigau V:
Microfoci of malignant progression in diffuse low-grade gliomas:
Towards the creation of an intermediate grade in glioma
classification? Virchows Arch. 466:433–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Westhoff MA, Zhou S, Nonnenmacher L,
Karpel-Massler G, Jennewein C, Schneider M, Halatsch ME, Carragher
NO, Baumann B, Krause A, et al: Inhibition of NF-κ B signaling
ablates the invasive phenotype of glioblastoma. Mol Cancer Res.
11:1611–1623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang G, Ayala G, De Marzo A, Tian W,
Frolov A, Wheeler TM, Thompson TC and Harper JW: Elevated Skp2
protein expression in human prostate cancer: Association with loss
of the cyclin-dependent kinase inhibitor p27 and PTEN and with
reduced recurrence-free survival. Clin Cancer Res. 8:3419–3426.
2002.PubMed/NCBI
|
|
16
|
Ezoe S, Matsumura I, Nakata S, Gale K,
Ishihara K, Minegishi N, Machii T, Kitamura T, Yamamoto M, Enver T,
et al: GATA-2/estrogen receptor chimera regulates
cytokine-dependent growth of hematopoietic cells through
accumulation of p21 (WAF1) and p27 (Kip1) proteins. Blood.
100:3512–3520. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kudo Y, Kitajima S, Sato S, Miyauchi M,
Ogawa I and Takata T: High expression of S-phase kinase-interacting
protein 2, human F-box protein, correlates with poor prognosis in
oral squamous cell carcinomas. Cancer Res. 61:7044–7047.
2001.PubMed/NCBI
|
|
18
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson J: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mamillapalli R, Gavrilova N, Mihaylova VT,
Tsvetkov LM, Wu H, Zhang H and Sun H: PTEN regulates the
ubiquitin-dependent degradation of the CDK inhibitor p27 (KIP1)
through the ubiquitin E3 ligase SCF (SKP2). Curr Biol. 11:263–267.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong Y, Sui L, Watanabe Y, Sugimoto K and
Tokuda M: S-phase kinase-associated protein 2 expression in
laryngeal squamous cell carcinomas and its prognostic implications.
Oncol Rep. 10:321–325. 2003.PubMed/NCBI
|
|
21
|
Koumarianou A, Tzeveleki I, Mekras D,
Eleftheraki AG, Bobos M, Wirtz R, Fountzilas E, Valavanis C,
Xanthakis I, Kalogeras KT, et al: Prognostic markers in early-stage
colorectal cancer: Significance of TYMS mRNA expression. Anticancer
Res. 34:4949–4962. 2014.PubMed/NCBI
|
|
22
|
Gotanda K, Hirota T, Matsumoto N and Ieiri
I: MicroRNA-433 negatively regulates the expression of thymidylate
synthase (TYMS) responsible for 5-fluorouracil sensitivity in HeLa
cells. BMC Cancer. 13:3692013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
von der Lehr N, Johansson S, Wu S, Bahram
F, Castell A, Cetinkaya C, Hydbring P, Weidung I, Nakayama K,
Nakayama KI, et al: The F-box protein Skp2 participates in c-Myc
proteosomal degradation and acts as a cofactor for c-Myc-regulated
transcription. Mol Cell. 11:1189–1200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Latres E, Chiarle R, Schulman BA,
Pavletich NP, Pellicer A, Inghirami G and Pagano M: Role of the
F-box protein Skp2 in lymphomagenesis. Proc Natl Acad Sci USA.
98:2515–2520. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qin L, Wang CZ, Fan HJ, Zhang CJ, Zhang
HW, Lv MH and Cui SD: A dual-targeting liposome conjugated with
transferrin and arginine-glycine-aspartic acid peptide for
glioma-targeting therapy. Oncol Lett. 8:2000–2006. 2014.PubMed/NCBI
|
|
26
|
Hua W, Yao Y, Chu YW, Zhong P, Zhang R,
Zhu W, Wu JS, Ma DX, Xu M, Mao Y and Zhou LF: Phase I study of
dendritic cells pulsed with tumor stem-like cells associated
antigens against malignant glioma in recurrent patients. Chin J
Neurosurg. 27:90–93. 2011.
|
|
27
|
Hart MG, Grant R, Garside R, Rogers G,
Somerville M and Stein K: Temozolomide for high grade glioma.
Cochrane Database Syst Rev. 8:CD0074152008.
|