Introduction
Pancreatic cancer is characterized by a poor
prognosis and frequent early distant metastasis (1). Early detection of pancreatic cancer
remains difficult, despite advances in diagnostic imaging (2). Therefore, curative surgery is rarely
possible for patients with this disease. Chemotherapy is
administered to patients with advanced pancreatic cancer, and has
been shown to significantly improve prognosis (3). Multiple signaling pathways are involved
in pancreatic cancer (1) and thus,
inhibitors of these pathways may represent potential novel
therapeutic targets.
Mesenchymal-epithelial transition factor (c-Met) is
a receptor for hepatocyte growth factor (HGF) (4). When HGF binds to c-Met, the signal is
transmitted downstream via the phosphatidylinositol 3-kinase
pathway and MAP kinase. HGF/c-Met is involved with the
proliferation and motility of cancer cells (5). Pancreatic cancer cells express c-Met,
and overexpression of the gene encoding c-Met is associated with a
poor prognosis in this disease (6).
Therefore, c-Met is hypothesized to be involved in the development
of pancreatic cancer. Notably, HGF is a potent mitogen in normal
human pancreatic exocrine cells via its action on c-Met (7). c-Met is involved in the self-renewal of
pancreatic cancer stem cells in combination with CD44 (8). This indicates that c-Met is involved in
cell proliferation and that inhibitors of c-Met may present a novel
molecular target for the treatment of pancreatic cancer. A previous
study demonstrated that treatment with monoclonal antibodies
against c-Met, suppressed tumor growth in a mouse xenograft model
of pancreatic cancer and improved the survival of the mice
(9). SU11274 is a specific inhibitor
of c-Met, which competes with adenosine triphosphate to bind with
the activation loop of c-Met (10).
Therefore, in the present study, the suppression of proliferation
and motility of pancreatic cancer cells following treatment with
SU11274 was investigated.
Materials and methods
Cell culture
The PANC-1, MIA-Paca2, NOR-P1, PK-45H, PK-1 and
PK-59 pancreatic cancer cell lines were obtained from RIKEN Cell
Bank (Tsukuba, Japan). The MIA-Paca2 cell line was cultured in
Dulbecco's modified Eagles medium (DMEM; Sigma-Aldrich, St. Louis,
MO, USA) and the NOR-P1, PK-45H, PK-1 and PK-59 cell lines were
cultured in RPMI-1640 medium (Sigma-Aldrich), supplemented with 10%
fetal bovine serum (FBS; Life Technologies, Grand Island, NY, USA).
Cell lines were cultured in a humidified atmosphere of 5%
CO2 at 37°C. The cultured cells were then observed under
a microscope (CKX41N-31PHP; Olympus Corporation, Tokyo, Japan).
Scratch assay
Cells were grown on 4-well chamber slides
(Becton-Dickinson, Franklin Lakes, NJ, USA). When the cells had
reached 80% confluence, cell sheets were scratched with a sterile
razor and 0 or 30 µM SU11274 (Wako Pure Chemical Industries, Ltd.,
Tokyo, Japan) was added to the media. Two days later, cells were
stained with hematoxylin and eosin (Muto Pure Chemicals Co., Ltd.,
Tokyo, Japan). The cells were then observed and images were
captured using an AX80 microscope (Olympus Corporation). The number
of cells that had migrated >150 µm from the cut surface were
counted in 5 different fields from each slide.
Immunostaining
Serial sections of human pancreatic tissue from a
62-year-old male with adenocarcinoma and normal human pancreatic
tissue from a 74-year-old male were purchased from BioChain
(Hayward, CA, USA). The sections were deparaffinized, autoclaved
and incubated with hydrogen peroxide, followed by 2% FBS in
phosphate-buffered saline (PBS) for 30 min. After incubation
overnight at 4°C with a monoclonal rabbit anti-human c-Met antibody
(cat. no. 8198; 1:300; Cell Signaling Technology, Danvers, MA,
USA), specimens were washed with PBS and subsequently incubated
with a polyclonal horseradish-peroxidase-labeled donkey anti-rabbit
secondary antibody (cat. no. NA934-100UL; 1:2,000; GE Healthcare,
Pittsburgh, PA, USA) for 2 h at 4°C. Subsequently, diaminobenzidine
(Dako, Glostrup, Denmark) was applied as the chromogen to tissue
sections and nuclei were stained with hematoxylin for 15 sec.
Specimens were observed and images were captured under the AX80
microscope.
Cell proliferation assay
MIA-Paca2 and PK-45H cells were trypsinized,
harvested and seeded in 96-well flat-bottom plates (Asahi Glass
Co., Ltd., Tokyo, Japan) at a density of 1,000 cells/well.
MIA-Paca2 and PK-45H cells were incubated for 24 h in DMEM and
RPMI-1640 medium, respectively, supplemented with 10% FBS.
Following cell culture, the c-Met inhibitor, SU11274, was added at
the desired concentrations (0 or 30 µM) and cells were incubated
for 72 h. Cell proliferation was then assessed by a
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS), inner salt assay, according to the manufacturer's
instructions (Promega Corporation, Madison, WI, USA). MTS is
reduced by cells to a colored formazan product, with a maximum
absorbance at 490 nm. Absorbance at 490 nm was measured with an
iMark Microplate Absorbance Reader (Bio-Rad, Hercules, CA,
USA).
Quantitative polymerase chain reaction
(qPCR)
Cells were cultured in 6-well plates (Asahi Glass
Co., Ltd.). Forty-eight hours after the addition of SU11274 to the
media, total RNA was isolated with Isogen reagent (Nippon Gene,
Tokyo, Japan). Complementary DNA was synthesized with SuperScript
III and oligo(dT) primers according to the manufacturer's
instructions (Life Technologies). Normal pancreatic tissue total
RNA was purchased from Clontech Laboratories, Inc., (Mountain View,
CA, USA). The following PCR primers were used: Forward, 5-CAT TGG
GGA GCA CTA TGT C-3′ and reverse, 5-TGT CCA CCT CAT CAT CAG CG-3′
for c-Met (accession no. NM_000245), 110 bp; forward, 5-AGA GGC GGA
GGA GAA CAA ACA G-3′ and reverse, 5-AGG CGG TAG TAG GAC AGG AAG
TTG-3′ for cyclin D1 (accession no. NM_053056), 180 bp; and
forward, 5-CGA ATG CCA GAG AAG GTC AC-3′ and reverse, 5-CCA TGA GAA
TCC GCT TGT TT-3′ for RPL19 (accession no. BC095445), 157 bp. RPL19
was used as an internal control to monitor the amount of mRNA.
Quantitative PCR was performed using Fast SYBR-Green Master Mix
(Life Technologies) in a Mini Opticon system (Bio-Rad); 40 cycles
consisting of 5 sec of denaturation at 95°C and 5 sec of
annealing-extension at 60°C were performed. The expression levels
of genes were analyzed automatically by this system. The relative
c-MET expression levels were calculated as follows: Relative c-Met
expression = c-Met expression/RPL19 expression.
Statistical analysis
One-way analysis of variance was performed using JMP
10.0.2 statistical software (SAS Institute, Cary, NC, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
c-Met expression is higher in
pancreatic cancer cells than in normal pancreatic cells
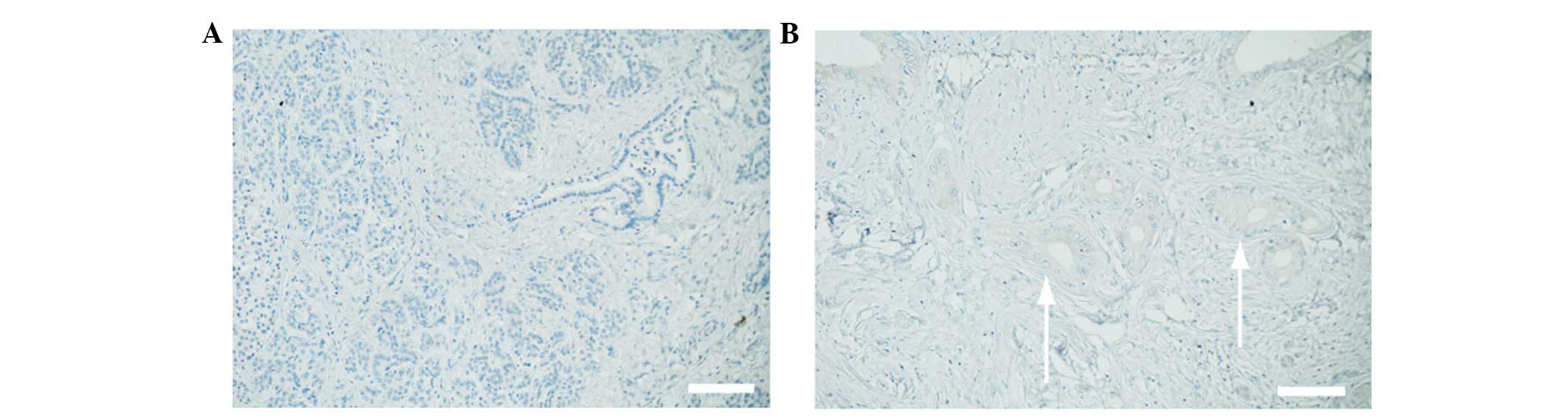
A commercially available surgical specimen was
immunostained in order to determine whether pancreatic cancer cells
express c-Met. Normal pancreatic tissue was negative for c-Met
(Fig. 1A), while the cell surface and
cytoplasm of pancreatic cancer cells were positive for c-Met
(Fig. 1B). Therefore, the results
demonstrated that c-Met is only expressed in pancreatic cancer
cells.
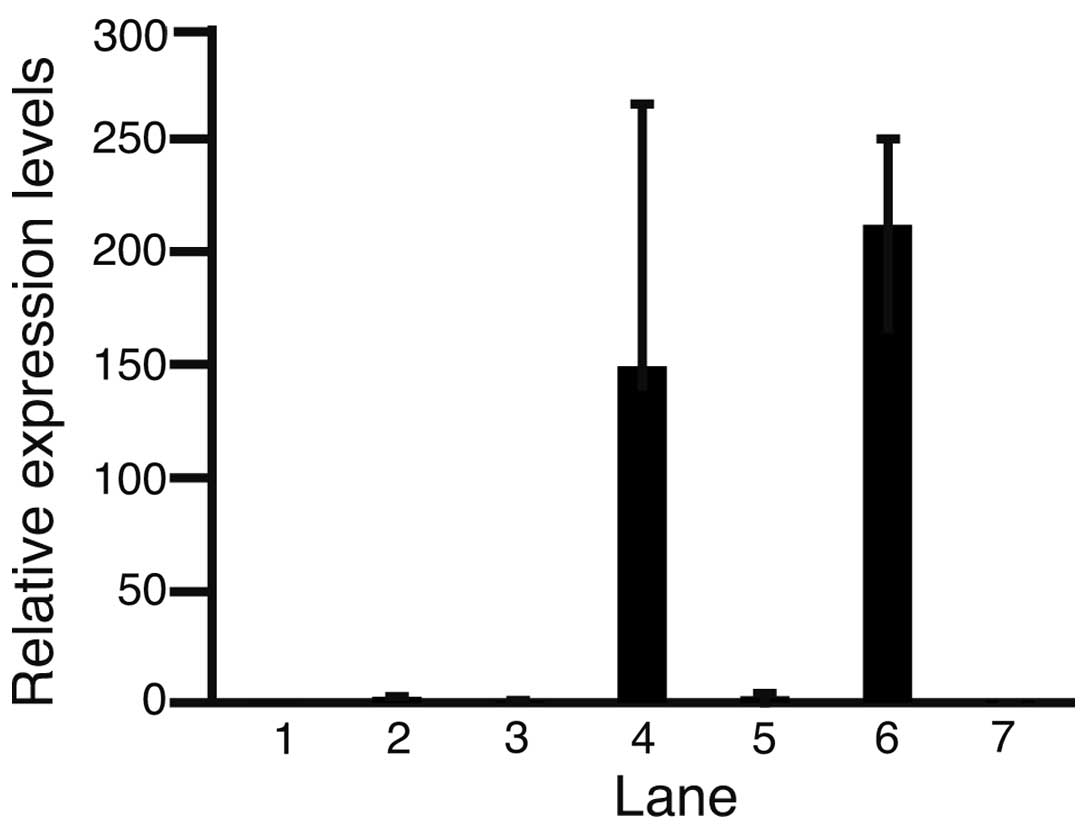
Expression levels of c-Met in normal pancreatic
cells and pancreatic cancer cell lines were compared using
quantitative PCR, and normalized against that of the normal
pancreas (Fig. 2). The relative
expression levels of c-Met in the MIA-Paca2, PANC-1, NOR-P1,
PK-45H, PK-1 and PK-59 cell lines and normal pancreatic cells were
0.05±0.01, 2.66±0.15, 0.98±0.60, 1.49±1.16, 2.92±2.00, 2.12±0.38
and 1.00±0.20 (mean ± standard deviation), respectively. c-Met
expression was higher in PANC-1, PK-45H, PK-1 and PK-59 cell lines
than that in normal pancreatic cells. These results indicate that
c-Met is involved in the development of pancreatic cancer. The
MIA-Paca2 and PK-45H cell lines were selected for further analysis;
PK-45H cells exhibited increased c-Met expression compared with
that in normal pancreatic cells, while MIA-Paca2 cells exhibited
reduced c-Met expression compared with that normal pancreatic
cells.
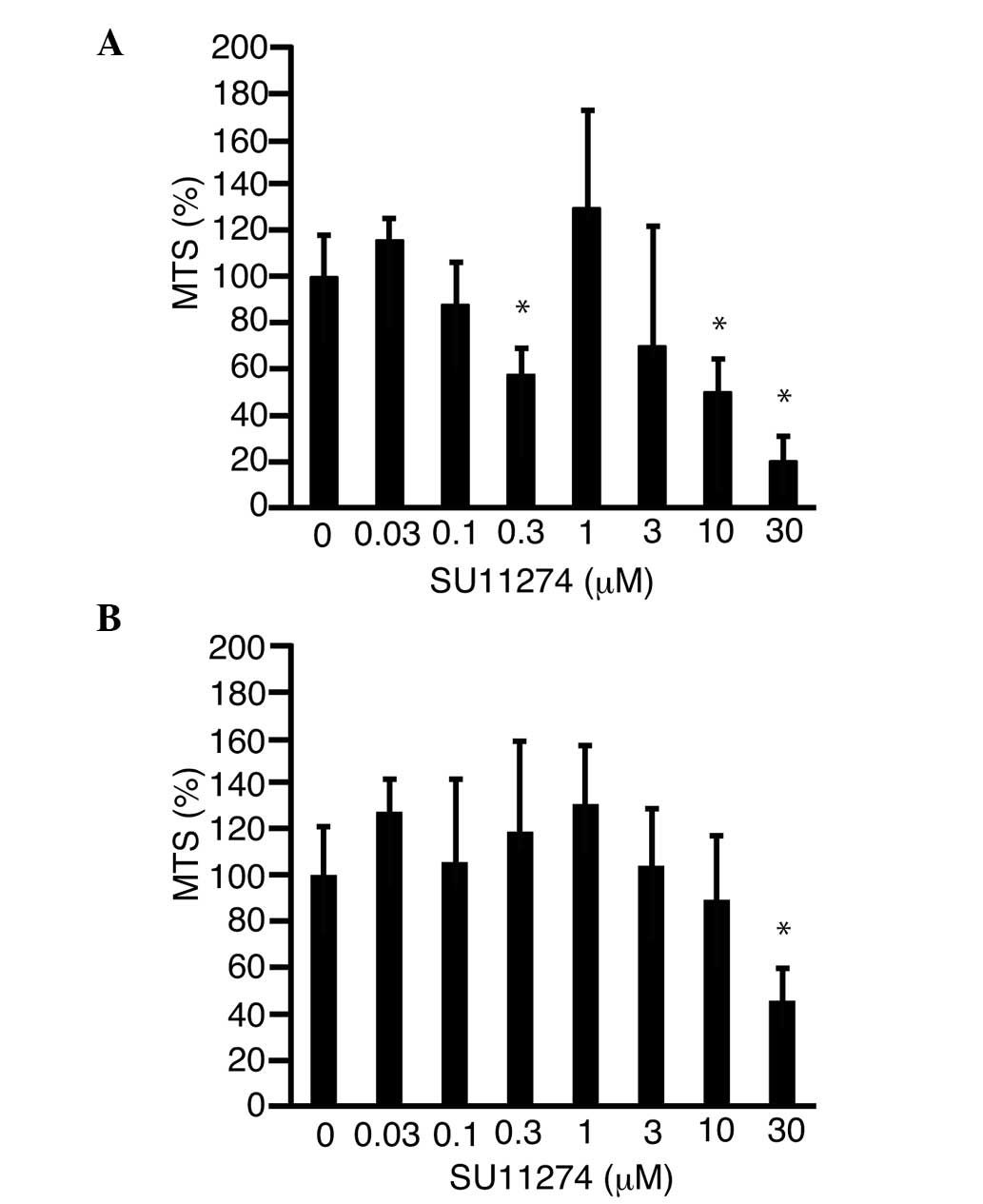
SU11274 suppresses pancreatic cancer
cell proliferation
SU11274 was added to the culture media and an MTS
assay was performed in order to determine whether this inhibitor of
c-Met suppressed cell proliferation. Proliferation of MIA-Paca2 and
PK-45H cells was suppressed to 19.8±10.7% (P<0.05; Fig. 3A) and 45.8±14.8% (P<0.05; Fig. 3B) of the control level, respectively,
following treatment with 30 µM SU11274.
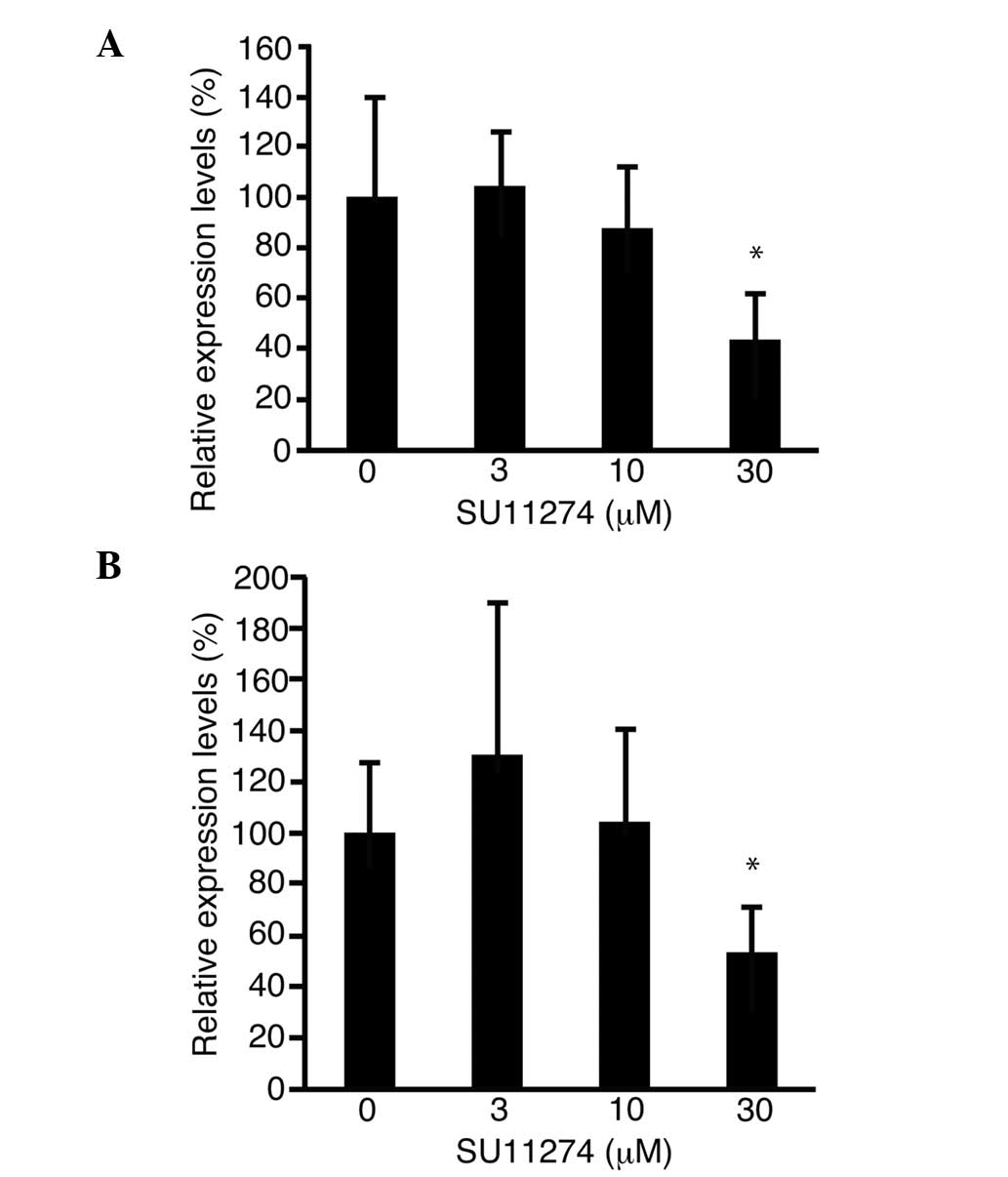
Cyclin D1 is involved in cell cycle progression.
Expression of the cyclin D1 gene was measured by quantitative PCR.
In the MIA-Paca2 and PK-45H cell lines, cyclin D1 expression was
downregulated to 43.7±17.9% (P<0.05; Fig. 4A) and 53.2±18.6% (P<0.05; Fig. 4B) of the control level, respectively,
following treatment with 30 µM SU11274.
SU11274 suppresses pancreatic cell
motility
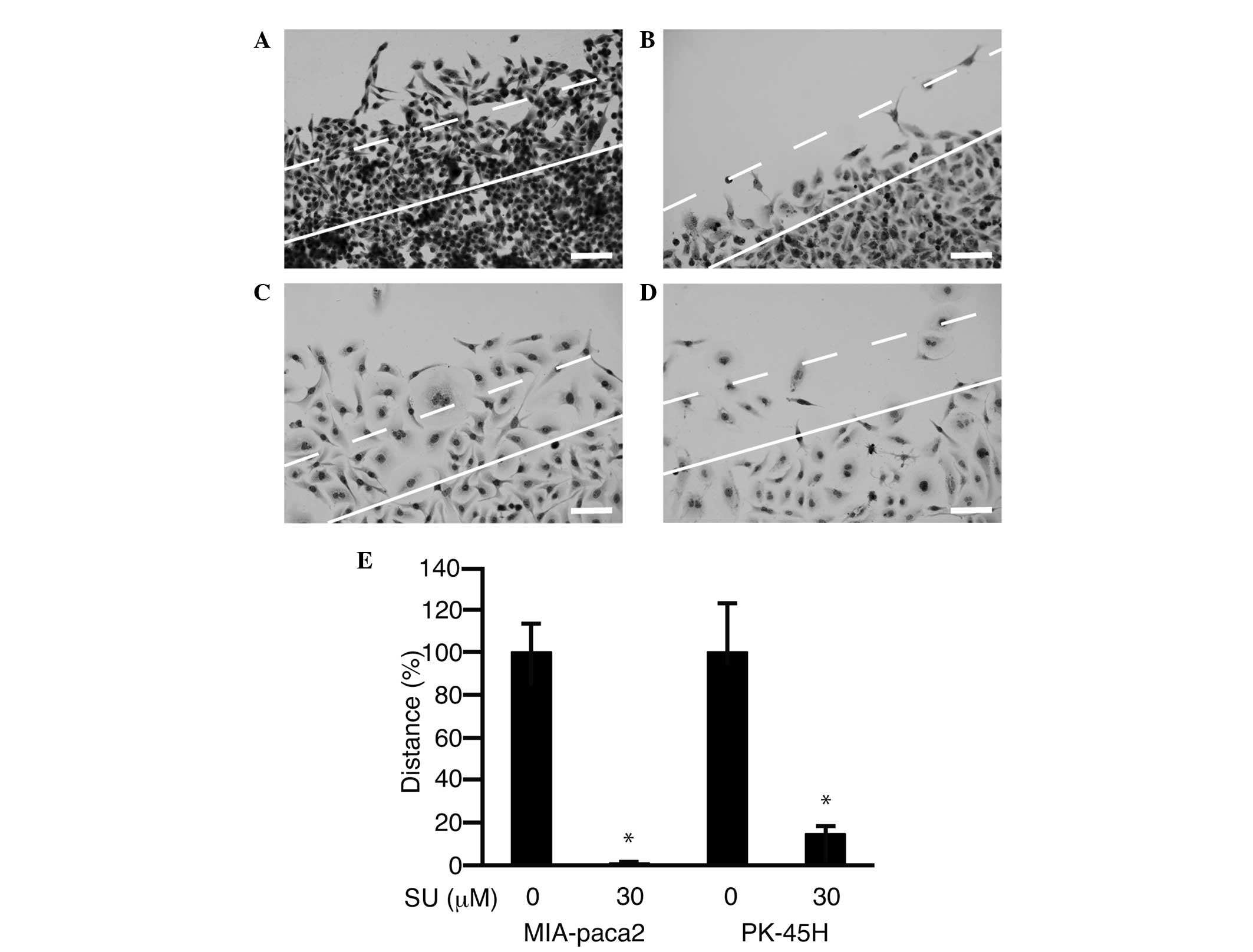
A scratch assay was performed in order to assess the
inhibition of cell motility following treatment with SU11274.
Forty-eight hours after scratching of the cell sheet, untreated
cells had migrated >150 µm from the cut surface (Fig. 5A and C). In cell cultures treated with
SU11274 (30 µM), a lower number of cells had migrated (Fig. 5B and D). Cells that had moved >150
µm were counted (Fig. 5E). Following
treatment with 30 µM SU11274, cell motility was suppressed to
1.0±0.3% in MIA-Paca2 cells (P<0.05) and 14.7±3.5% in PK-45H
cells (P<0.05) of the level of cells treated with 0 µM SU11274,
respectively.
Discussion
c-Met expression has been identified in pancreatic
cancer specimens (11). In the
present study, immunostaining detected no c-Met expression at the
protein level in normal pancreatic tissues. These results indicated
that c-Met is involved in the development of pancreatic cancer. HGF
is a potent mitogen in normal human pancreatic exocrine tissue and
functions in an autocrine manner (7,8). Thus, it
was hypothesized that an inhibitor of c-Met may suppress the
proliferation of pancreatic cancer cells. SU11274 is a specific
inhibitor of c-Met, which has been shown to suppress the
proliferation of hepatocellular carcinoma cells (12). In the present study, SU11274
effectively suppressed the proliferation of pancreatic cancer cells
via downregulation of cyclin D1.
c-Met is involved in cell motility and tumor-stromal
interactions (13) and thus,
inhibition of c-Met suppresses cell motility (14). In the present study, SU11274
significantly suppressed the motility of pancreatic cancer cells.
Cancer cells migrate and invade the surrounding tissues in clusters
(15). Pancreatic tumors
characteristically exhibit abundant stroma, and this
microenvironment represents a novel therapeutic target (16). c-Met inhibitors, such as SU11274, may
therefore be developed for use in the treatment of pancreatic
cancer.
Quantitative PCR demonstrated that c-Met was not
expressed in all pancreatic cancer cell lines. The expression of
the c-Met gene was lower in the MIA-Paca2 cell line compared with
that in normal pancreatic cells. However, SU11274 nevertheless
suppressed proliferation in this cell line. Although the mechanism
remains unclear, SU11274 may exert antitumor effects in all
pancreatic cancer cell lines, regardless of the level of c-Met
expression.
At present, c-Met inhibitors, such as tivantinib,
are undergoing clinical trials in patients with pancreatic cancer
(17). Combined treatment with
tivantinib and gemcitabine is safe and tolerable (17). Although these are preliminary results,
the antitumor effect of this combination appears promising.
Crizotinib, another c-Met inhibitor, inhibits the inactivation of
gemcitabine by pancreatic cancer cells (18). These studies indicate that combining
c-Met inhibitors and gemcitabine may present a novel strategy for
the treatment of pancreatic cancer.
The insulin-like growth factor 1 receptor (IGF-1R)
is involved in pancreatic cancer cell proliferation, and its
inhibition suppresses cell proliferation and motility (19,20).
Notably, combined treatment with a c-Met inhibitor and IGF-1R
inhibitor has been shown to suppress the proliferation of
pancreatic cancer cells in vitro and in vivo
(21). Thus, future studies
investigating combined treatment with inhibitors of c-Met, IGF-1R
and the Wnt pathway are required (22).
In conclusion, in the current study SU11274
suppressed the proliferation of pancreatic cancer cells via the
downregulation of cyclin D1. Cell motility was also suppressed
following treatment with SU11274. Thus, c-Met may present a
promising therapeutic target for pancreatic cancer.
References
|
1
|
Wörmann SM and Algül H: Risk factors and
therapeutic targets in pancreatic cancer. Front Oncol. 3:2822013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lennon AM, Wolfgang CL, Canto MI, Klein
AP, Herman JM, Goggins M, Fishman EK, Kamel I, Weiss MJ, Diaz LA,
et al: The early detection of pancreatic cancer: What will it take
to diagnose and treat curable pancreatic neoplasia? Cancer Res.
74:3381–3389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gresham GK, Wells GA, Gill S, Cameron C
and Jonker DJ: Chemotherapy regimens for advanced pancreatic
cancer: A systematic review and network meta-analysis. BMC Cancer.
14:4712014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Organ SL and Tsao MS: An overview of the
c-MET signaling pathway. Ther Adv Med Oncol. 3 (Suppl):S7–S19.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goetsch L, Caussanel V and Corvaia N:
Biological significance and targeting of c-Met tyrosine kinase
receptor in cancer. Front Biosci (Landmark Ed). 18:454–473. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu GH, Huang C, Qiu ZJ, Liu J, Zhang ZH,
Zhao N, Feng ZZ and Lv XH: Expression and prognostic significance
of CD151, c-Met, and integrin alpha3/alpha6 in pancreatic ductal
adenocarcinoma. Dig Dis Sci. 56:1090–1098. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vilá MR, Nakamura T and Real FX:
Hepatocyte growth factor is a potent mitogen for normal human
pancreas cells in vitro. Lab Invest. 73:409–418. 1995.PubMed/NCBI
|
|
8
|
Li C, Wu JJ, Hynes M, Dosch J, Sarkar B,
Welling TH, Pasca di Magliano M and Simeone DM: c-Met is a marker
of pancreatic cancer stem cells and therapeutic target.
Gastroenterology. 141:2218–2227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin H, Yang R, Zheng Z, Romero M, Ross J,
Bou-Reslan H, Carano RA, Kasman I, Mai E, Young J, et al: MetMAb,
the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic
pancreatic tumor growth and improves survival. Cancer Res.
68:4360–4368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mughal A, Aslam HM, Sheikh A, Khan AM and
Saleem S: c-Met inhibitors. Infect Agent Cancer. 8:132013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gardian K, Janczewska S and Durlik M:
Microenvironment elements involved in the development of pancreatic
cancer tumor. Gastroenterol Res Pract. 2012:5856742012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inagaki Y, Qi F, Gao J, Qu X, Hasegawa K,
Sugawara Y, Tang W and Kokudo N: Effect of c-Met inhibitor SU11274
on hepatocellular carcinoma cell growth. Biosci Trends. 5:52–56.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yasui T, Ohuchida K, Zhao M, Onimaru M,
Egami T, Fujita H, Ohtsuka T, Mizumoto K and Tanaka M: Tumor-stroma
interactions reduce the efficacy of adenoviral therapy through the
HGF-MET pathway. Cancer Sci. 102:484–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan B, VanderLaan PA and Sukhatme VP:
6-Phosphogluconate dehydrogenase regulates tumor cell migration in
vitro by regulating receptor tyrosine kinase c-Met. Biochem Biophys
Res Commun. 439:247–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomizawa M, Kondo F and Kondo Y: Growth
patterns and interstitial invasion of small hepatocellular
carcinoma. Pathol Int. 45:352–358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neesse A, Krug S, Gress TM, Tuveson DA and
Michl P: Emerging concepts in pancreatic cancer medicine: Targeting
the tumor stroma. Onco Targets Ther. 7:33–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pant S, Saleh M, Bendell J, Infante JR,
Jones S, Kurkjian CD, Moore KM, Kazakin J, Abbadessa G, Wang Y, et
al: A phase I dose escalation study of oral c-MET inhibitor
tivantinib (ARQ 197) in combination with gemcitabine in patients
with solid tumors. Ann Oncol. 25:1416–1421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Avan A, Caretti V, Funel N, Galvani E,
Maftouh M, Honeywell RJ, Lagerweij T, Van Tellingen O, Campani D,
Fuchs D, et al: Crizotinib inhibits metabolic inactivation of
gemcitabine in c-Met-driven pancreatic carcinoma. Cancer Res.
73:6745–6756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Insulin-like growth factor-I
receptor in proliferation and motility of pancreatic cancer. World
J Gastroenterol. 16:1854–1858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Insulin-like growth factor I
receptor involvement in proliferation of NOR-P1 cells in serum-free
media. J Cell Biochem. 113:2714–2720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ucar DA, Magis AT, He DH, Lawrence NJ,
Sebti SM, Kurenova E, Zajac-Kaye M, Zhang J and Hochwald SN:
Inhibiting the interaction of cMET and IGF-1R with FAK effectively
reduces growth of pancreatic cancer cells in vitro and in vivo.
Anticancer Agents Med Chem. 13:595–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Frizzled-2: A potential novel
target for molecular pancreatic cancer therapy. Oncol Lett.
7:74–78. 2014.PubMed/NCBI
|