Introduction
Plants have been used for millennia in traditional
medicine to treat diseases and to supplement various nutrients
(1–3).
Recently, studies showing that extracts from various types of
plants are useful in the medical industry has encouraged the
development of novel types of natural products that are effective
in various types of diseases, and which may function as antitumor,
antioxidant, antiobesity and antimicrobial molecules (3).
The widely cultivated and fast-growing Moringa
oleifera (also known as Moringa or drumstick tree) is
cultivated in tropical and sub-tropical locales, such as the
sub-Himalayan region, Oceania, Latin America, Africa and Asia.
M. oleifera has been regarded as a ‘miracle tree’, as it is
a significant source of fats, proteins, β-carotene, vitamin C,
iron, potassium and other nutrients, and is also effective in the
treatment of numerous diseases (4–13). The
flowers, roots, leaves and bark of M. oleifera have long
been used by the public as nutritional supplements and foods, as
well as in the manufacture of perfume, skin oil and other products
(12,14–19).
Certain parts of M. oleifera (leaf, stem and root) have been
demonstrated to produce various biological activities, including
antiatherosclerotic (20),
immune-boosting (21),
anticardiovascular disease (22),
antiviral (1,23–25),
antioxidant (2,26–28),
antimicrobial (27),
anti-inflammatory (29) and
tumor-suppressive effects (30). Due
to its long history of usage and various biological effects, M.
oleifera has long been the subject of research interest. A
previous study reported on the therapeutic potential of the
water-soluble extract from M. oleifera leaves (MOL) in the
treatment of various types of cancers, including lung, breast and
skin cancers (30).
The majority of studies that describe the
preparation of bioactive compounds from natural plants have
utilized solvent extraction, in which the water insoluble extracts
have been typically obtained using methanol and ethanol, in
addition to hot water and buffers (1,26,27,31,32.
Solvents are widely used, as they allow for effective extraction of
a broad range of different phytochemicals, such as bioactive but
water insoluble phenolic compounds found in plants. However, these
water insoluble compounds may be difficult to orally administer to
human patients, and additional efforts to enhance the
bioavailability or absorption rates are often required (26). This solvent extraction technique has
also been used to extract useful compounds from M. oleifera
by a number of research groups; however, they have not reported on
the effects of the water-soluble extract of M. oleifera. In
our previous study, the effectiveness of a cold water (4°C)-soluble
extract of MOL in lung cancer prevention was reported (30).
Generally, anticancer drug candidates have bulky
hydrophobic groups within their chemical structures that render
them water-insoluble and may lead to formulation problems and
serious therapeutic challenges, including serious complications
such as embolism and respiratory system failure due to the
precipitation of the drug in the case of intravenous administration
(33–35). For these reasons, increasing the water
solubility of anticancer agents and developing soluble bioactive
compounds with strong anticancer activities is vital. Compared with
parenteral injections, the continued development of oral anticancer
therapies has been fueled by their ease of administration, lack of
requirement for hospitalization or clinic visits, acceptable
disease outcomes, patient satisfaction, reduced interference in
work and social activities, and the paradigm shift that views
cancer as a chronic condition. However, orally administered drugs
may be degraded by metabolic processes prior to arriving at their
target sites (36–38). To address this disadvantage, the
development of oral medications with high bioavailability has
received much attention in anticancer therapy research.
In the present study, a water-soluble MOL extract
was prepared and its anticancer activity was tested against
hepatocellular carcinoma cells. Furthermore, its efficacy as an
orally administered therapeutic agent in mice with lung and liver
cancer was investigated.
Materials and methods
Sample preparation
The leaves of M. oleifera (MOL), cultivated
in Chiangmai, Thailand, were purchased from Moringa Korea Co.
(Milyang, Gyeongsangnam, South Korea). The dried MOL (150 mg) were
suspended in 1 ml of cold water (4°C), vigorously and continuously
vortexed for 30 sec, and allowed to stand in the refrigerator (4°C)
for 10 min. After another vigorous vortexing of the MOL extract for
1 min, the water soluble supernatants were collected by
centrifugation (13,000 × g for 10 min, twice) and then
membrane-filtered (0.2-µm filter). The filtrated MOL extracts were
lyophilized at −50°C for 2 days using a freeze dryer (FD5505;
Ilshin Biobase Co., Ltd., Seoul, Korea) then stored at −20°C. For
the experiments, the lyophilized MOL extracts were resuspended in
distilled water (DW) at a final concentration of 20 mg/ml of
protein.
Cell cultivation
Human non-small cell lung cancer A549 and human
hepatocellular carcinoma HepG2 cells were purchased from the
American Type Culture Collection (Manassas, VA, USA). The cells
were seeded at an initial density of 1×105 cells in a
6-well plate containing RPMI-1640 medium (for A549) or DMEM (for
HepG2) (GE Healthcare, Pittsburgh, PA, USA) supplemented with 10%
fetal bovine serum (GE Healthcare) and 1% penicillin-streptomycin,
and incubated at 37°C.
Flow cytometric analysis
Prior to MOL extract treatment, the HepG2 cells
(1×105) were seeded in a 6-well culture plate and were
incubated for 1 day. Following another 2-day incubation, the cells
were collected, fixed with 70% ethanol at 4°C for 2 h and stained
with propidium iodide (PI; 50 µg/ml) for 30 min at room
temperature. A FACScan system (EPICS XL Flow Cytometry, Beckman
Coulter Counter; Beckman Coulter, Inc., Indianapolis, IN, USA) was
used to measure the DNA content. The proportion of cells in each
cell cycle stage was determined using the Phoenix Multicycler
Software (Phoenix Flow System, San Diego, CA, USA) and the numbers
in the images indicate the percentages of the total that were
sub-G1 cells.
Cell proliferation assay (MTT assay)
and colony forming assay
A cell proliferation assay using tetrazolium salt
(MTT) was performed to measure the viability of the HepG2 cells
(24). The cells were adjusted to a
density of 3×103 cells in each well of a 96-well plate
and incubated for 1 day. Various concentrations of MOL extracts
(0–200 µg/ml) were added, and the cells were incubated for another
2 days. Cell proliferation was measured by Cell Counting Kit-8
(cat. no. CK04, Dojindo Laboratories, Kumamoto, Japan). The
percentage ratio is the relative proportion of viable cells
compared with the control group (non-MOL extract-treated
group).
Colony-forming assay
Clonogenicity was examined by the colony-forming
assay, as previously described (30).
Briefly, the cells were seeded at an initial density of
1×103 HepG2 cells/well in 6-well culture plates,
incubated for 1 day prior to the addition of the MOL extract, and
then incubated for a further 7–14 days. Finally, the cells were
stained with 0.1% crystal violet (Sigma-Aldrich, St. Louis, MO,
USA) and images were captured with a Canon digital camera
(PowerShot S45; Canon, Inc., Seoul, Korea). Representative images
are presented.
Annexin V binding assay
The A549 cells were cultured for 1 day and treated
with MOL extract, after which they were incubated for another 2
days. The Annexin V-fluorescein isothiocyanate (FITC)/PI flow
cytometric assay kit was purchased from Roche Diagnostics (cat. no.
1185877700; Basel, Switzerland) and used to determine the
proportion of apoptotic cells in each MOL-treated group. Briefly,
harvested cells were resuspended in incubation buffer at a
concentration of 104 cells/ml, followed by incubation
with Annexin V-FITC for 20 min and subsequent incubation with PI
for 5 min in the dark. At least 10,000 stained cells from each
sample were analyzed by Cytomics FC 500 (model 175487-FC500;
Beckman Coulter Inc., Indianapolis, IN, USA). Data were analyzed
with CELL Quest software (BD Biosciences, San Jose, CA, USA).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
Apoptosis was also assessed by utilizing the TUNEL
assay to detect DNA strand breaks during apoptosis using an in
situ cell death detection kit (cat. no. 11684795910; Roche,
Basel, Switzerland) according to the manufacturer's instructions.
Briefly, the HepG2 cells were treated with different concentrations
of MOL extracts (0–300 µg/ml) for 2 days, fixed with freshly
prepared 4% paraformaldehyde in phosphate-buffered saline (PBS; pH
7.4) for 20 min, and incubated in permeabilization solution with
0.3% Triton X-100 for 5 min at room temperature. Subsequent to
being washed three times with PBS buffer, the cells were incubated
with TUNEL reaction mixture for 60 min at 37°C and analyzed under a
fluorescence microscope (model nos. BX53F and U-RFL-T; Olympus
Corporation, Tokyo, Japan), through which images of representative
fields were captured. The HepG2 cells were also fixed in 4%
paraformaldehyde and incubated in 0.5 µg/ml
4,6-diamidino-2-phenylindole solution for 30 min in the dark at
room temperature for counterstaining.
Western blot analysis
Prior to adding the MOL extract, the HepG2 cells
(1×105) were seeded in a 6-well culture plate for 1 day.
After 2 days of additional incubation, the proteins were collected
and their concentrations were determined by the Bradford method
(Protein Assay Dye Reagent Concentrate, cat. no. 500-0006; Bio-Rad
Laboratories, Hercules, CA, USA). Proteins were then loaded in
equal amounts (20 µg) onto an 8–12% SDS polyacrylamide gel and
stained with Coomassie Brilliant Blue. The antibodies [polyclonal
rabbit anti-β-actin, cat. no. 4967; polyclonal rabbit
anti-poly(ADP-ribose) polymerase (PARP), cat. no. 9542; monoclonal
rabbit B-cell lymphoma-extra large (Bcl-xL), cat. no. 2764;
polyclonal rabbit caspase-3, cat. no. 9662; and polyclonal rabbit
anti-cleaved caspase-3, cat. no. 9661] for western blot analysis
were purchased from Cell Signaling Technology Inc. (Danvers, MA,
USA).
Hollow fiber assay (HFA)
An HFA was performed following the standard
procedures set by the National Cancer Institute (NCI; Bethesda, MD,
USA; https://dtp.cancer.gov/branches/btb/hfa.html)
(39–42). Briefly, the two tumor cell lines, A549
and HepG2, were cultured in 75-cm2 culture flasks. The
media were flushed into 1-mm (internal diameter) polyvinylidene
fluoride hollow fibers (HFs) with a molecular weight cutoff of 500
kDa (Spectrum Laboratories, Houston, TX, USA) and then the HFs were
loaded with cells (5×105 cells/ml). Each HF was
heat-sealed with a hot smooth-jawed needle holder every 1.5 cm
along its length and cut into segments with 2-mm tails for ease of
handling; at the highest seeding density, each HF was 1.5 cm long.
Each HF segment contained ~1×105 cells. The fibers were
placed into 6-well culture plates containing cell culture medium
and were incubated for 1 day prior to surgical implantation in
6-week-old male immunodeficient nude mice (Harlan Laboratories,
Horst, Netherlands). All mice were housed under 12-h light-dark
cycles in an air-conditioned room with unrestricted access to water
and food (Purina 5001 Rodent Chow; Purina, St. Louis, MO, USA). The
mice were anesthetized with Zoletil (tiletamine/zolazepam; Virbac
Korea Co., Ltd., Seoul, South Korea) and Rompun (xylazine; Bayer,
Seoul, South Korea), and then HFs were implanted at subcutaneous
sites and the incisions closed using skin staples. The mice were
orally administered different concentrations of MOL extract, or
were intravenously injected with doxorubicin (cat no. D1515-10MG;
Sigma-Adrich) as a control, starting on day 3 following the fiber
implantation (each dose of MOL extract and doxorubicin were
administered daily for 4 days). After 7 days, the fibers were
collected and subjected to the trypan blue exclusion assay.
Internationally recognized guidelines on animal welfare were
followed and the experiments were approved by the Gachon University
Institutional Animal Care and Use Committee (approval no.
GIACUC-L2014001).
Statistical analysis
Statistical analysis was performed using SigmaPlot
software (version 7.0; Systat Software, Inc., San Jose, CA, USA). A
Students's t-test was used to analyze differences in cell
viability. P<0.05 indicated a statistically significant
difference.
Results
Effect of MOL extract on HepG2
hepatocellular carcinoma cells
Dried MOL was extracted with cold DW (4°C) and the
resulting water soluble extract was used in this study. Human
hepatocellular carcinoma HepG2 cells were treated with the MOL
extract for 2 days, and then analyzed by FACScan. As presented in
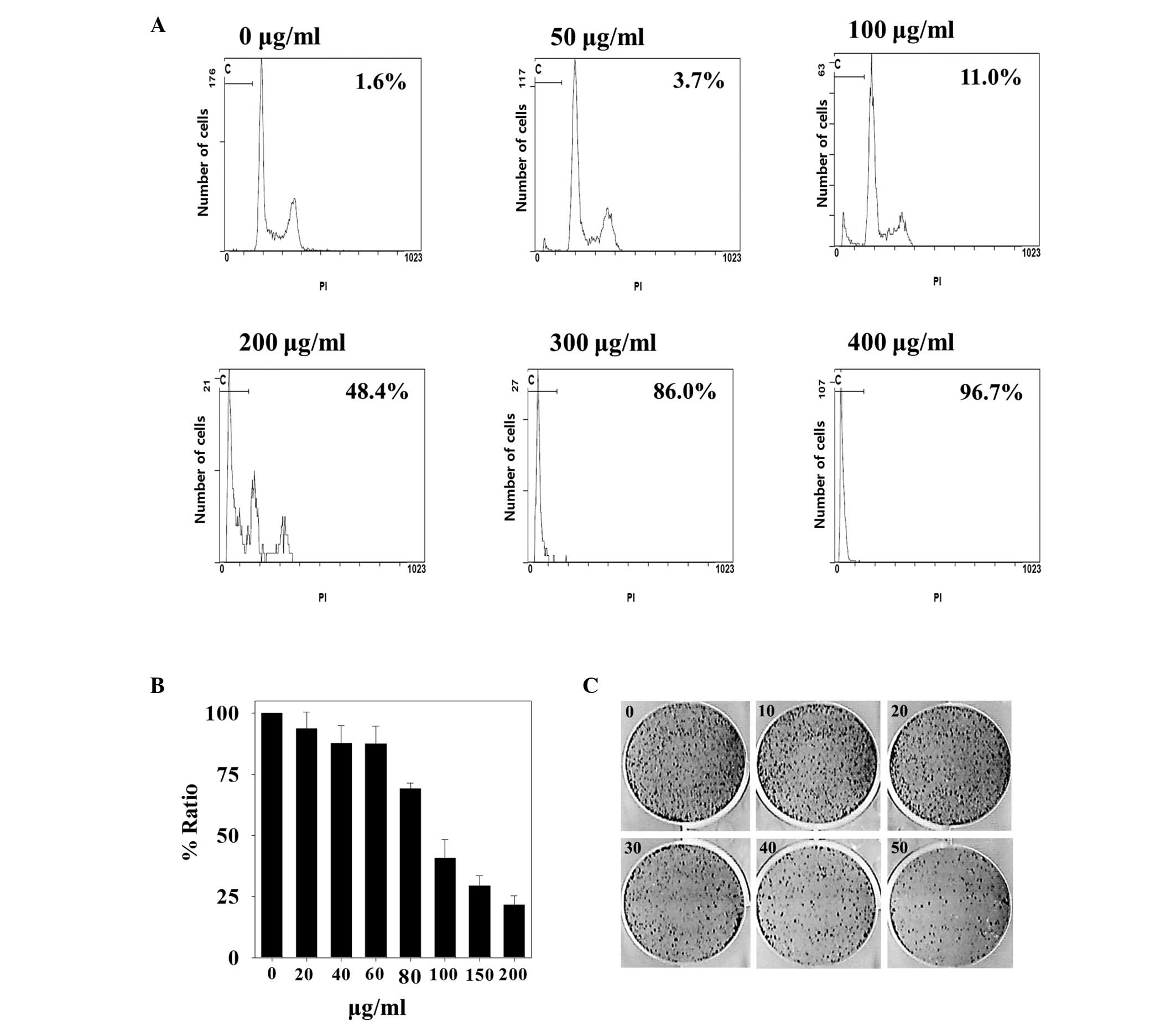
Fig. 1A, cell cycle analysis
demonstrated that MOL at 50, 100, 200, 300 and 400 µg/ml resulted
in a concentration-dependent accumulation of HepG2 cells in the
sub-G1 phase, which indicated apoptotic cells, as compared to the
control (non-MOL extract-treated group). In particular, the HepG2
cells treated with 400 µg/ml of MOL extract showed a sub-G1
fraction of up to 96% compared with the control group.
The cytotoxic inhibitory effect of MOL extract on
HepG2 cell proliferation was further verified using the MTT assay
(Fig. 1B). Cells treated with
different concentrations of MOL extract (0–200 µg/ml) for 2 days
were subjected to the MTT assay. The results demonstrated that cell
proliferation was significantly inhibited in a
concentration-dependent manner by MOL extract (P<0.05), and
treatment with 200 µg/ml of MOL extract produced inhibition ratios
of up to 80%.
The impact of the MOL extract on the clonogenic
growth of the HepG2 cells was also examined (Fig. 1C). The cells were seeded onto 6-well
plates and treated with MOL extract, and then stained with crystal
violet after an incubation period of 7 days. The data showed that
the control cells (not treated with MOL extract) formed colonies
that were uniformly distributed in the plate (0 µg). By contrast,
the cells treated with MOL extract demonstrated an up to 70%
reduction in the number of colonies (50 µg/ml of MOL). In
conclusion, clonogenic growth was inhibited by MOL extract in a
dose-dependent manner.
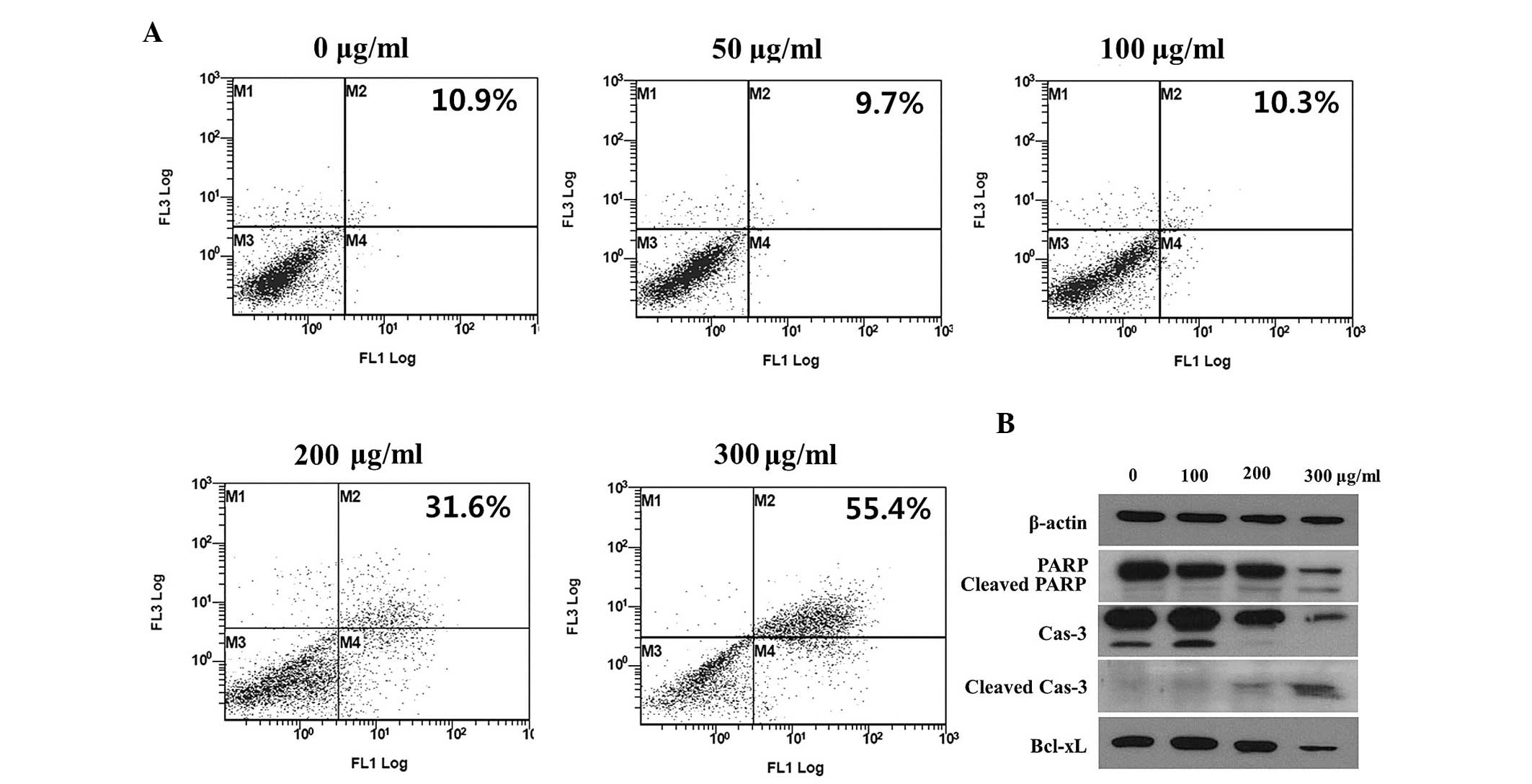
Evaluation of apoptosis
As MOL extract treatment produced an accumulation of
cells at the G0/G1 peak during cell cycle
analysis, as well as a reduction in cell viability and the
inhibition of clonogenic growth, the apoptotic state of the cells
was investigated. In order to detect and quantify apoptosis, the
Annexin V-FITC/PI double staining method was used to stain HepG2
cells treated with MOL extract. The cells were collected following
2 days of treatment with MOL extract at different concentrations
(0–300 µg/ml), then double-stained and subjected to FACS analysis.
The increased dot plots in the late and early apoptotic region
demonstrate a dose-dependent effect (Fig.
2A). Subsequent to MOL extract treatment, ~55.4% of cells were
in either the early (stained only by Annexin V-FITC) or late
(stained by Annexin V-FITC and PI) apoptotic stage. The apoptotic
cell ratio of the cells treated with MOL extract at a concentration
of 300 µg/ml was 5 times higher than that of the control cells.
Apoptosis in the HepG2 cells was further elucidated
by western blotting. As presented in Fig.
2B, the expression of certain apoptotic markers, including
cleaved PARP and cleaved caspase-3, were increased by treatment
with MOL extract. The antiapoptotic Bcl-xL protein was
downregulated by MOL extract treatment, indicating that the MOL
extract inhibited cell proliferation (Fig. 2B).
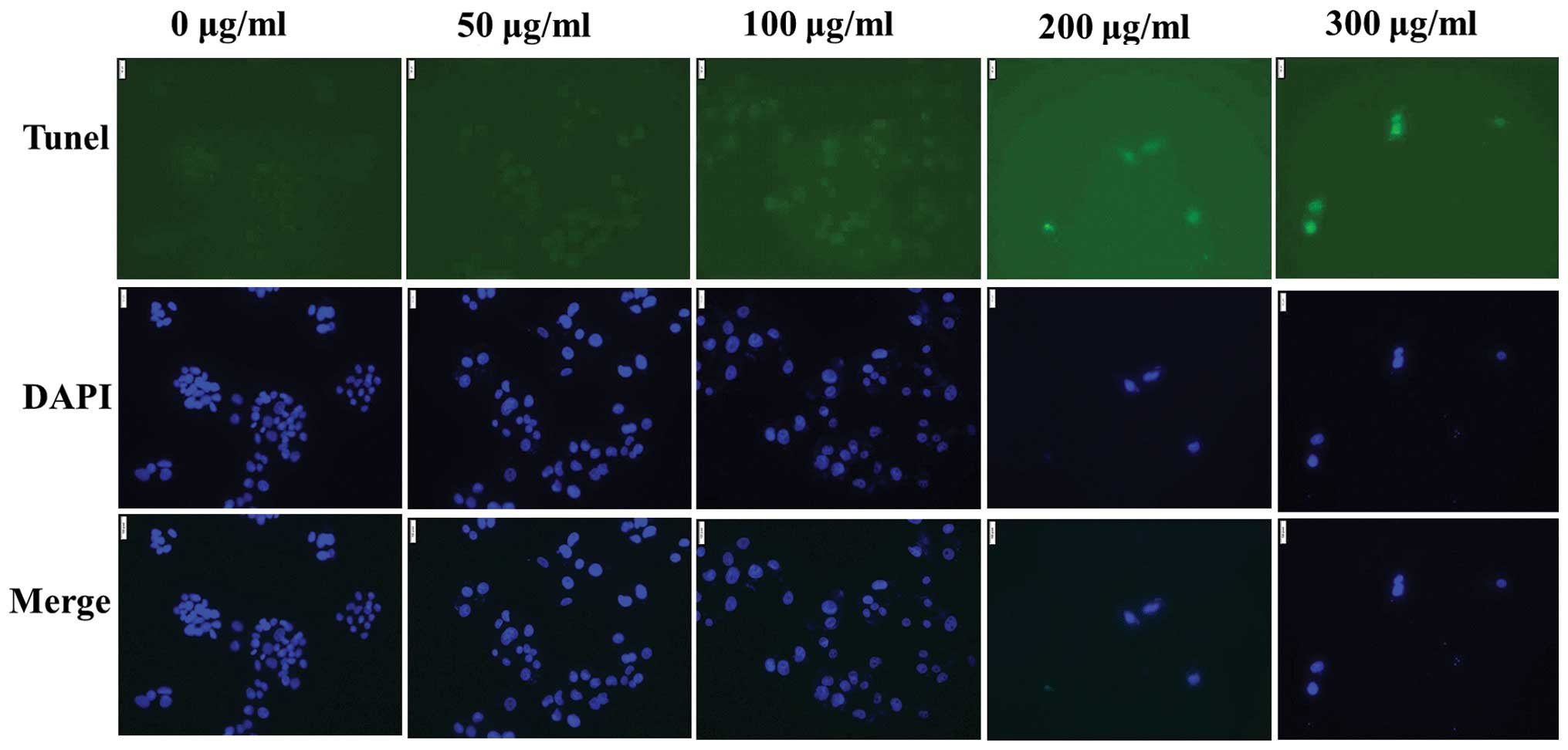
The TUNEL assay is a common method for detecting the
DNA fragmentation that results from apoptotic signaling, which
allows apoptotic cells with DNA fragmentation to be detected by
fluorescence microscopy. In the present study, TUNEL staining was
performed to detect apoptotic morphology alteration in individual
HepG2 cells. The presence of TUNEL-positive cells with fragmented
DNA in their nuclei was indicated by a green fluorescence signal,
indicating that DNA strand breaks had occurred, and that MOL
extract induced apoptosis in the HepG2 cells (Fig. 3).
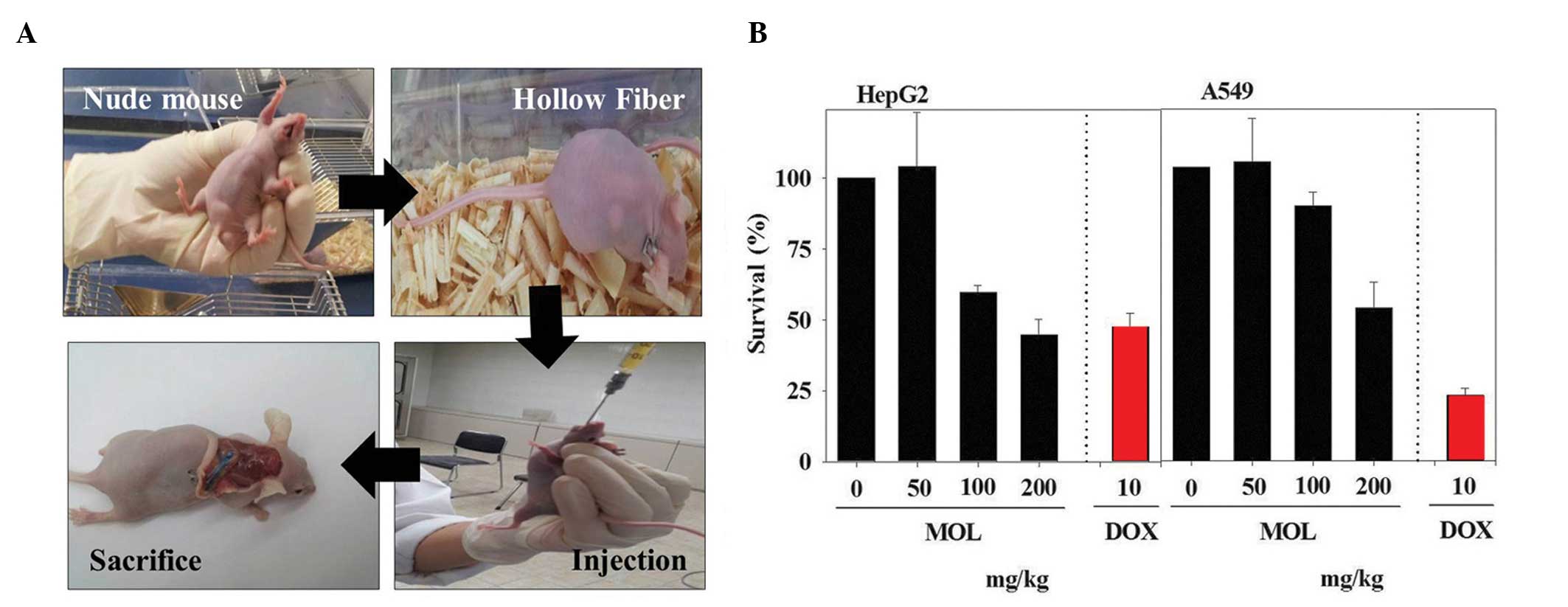
HFA
Considering the in vitro results
demonstrating that the MOL extract inhibited cancer cell
proliferation, an in vivo study was crucial to evaluate
whether MOL inhibits cancer cell progression. To test this
hypothesis, nude mice were implanted with hollow fibers filled with
HepG2 or A549 cells. After 2 days, different concentrations of MOL
extract were orally administered to the MOL-treated mice, and
doxorubicin was intravenously administered to the control mice. On
day 5 after administration, the animals were sacrificed and the
fibers were collected, and the trypan blue exclusion assay was
performed (Fig. 4A). A gradual
reduction in cell viability was observed for the HepG2 cells, while
a similar kinetic profile was observed for the A549 cells (Fig. 4B). Notably, the effective
concentrations of MOL extract in the HFA assay, which inhibited
cancer cell proliferation, were similar to those that were
effective in the in vitro cell-based assay (Fig. 1B) (30),
indicating that MOL extract was effectively delivered to the target
site via oral administration. As compared to that observed in A549
cells, significantly stronger cytotoxicity was produced by MOL
extract in the HepG2 cells, and the rate of cytotoxicity in these
cells was higher compared with that produced by doxorubicin
(control) treatment (P<0.05). Studies involving the
administration of higher concentrations of MOL should now be
performed.
Discussion
M. oleifera, of the monogeneric family
Moringaceae, is distributed worldwide and has been known by a
number of different names, including the horseradish tree
(English), Soanjna (Hindi), Shobhanjana (Sanskrit), Saragvo
(Gujarati), Sajna (Bengali), Munanga (Telugu) and Murangai (Tamil)
(43). For millennia, various parts
of M. oleifera have been used as nutritional supplements (as
a rich source of vitamins A and C, iron, calcium, and protein)
(44,45), and for their antibiotic (46–49),
antihyperlipidemic (50),
antidiabetic (51,52), wound healing (53) and antiulcer properties (54). The edible parts of the plant have also
been employed traditionally for skin diseases, rheumatism, anemia,
cholera and other ailments (53).
Previous studies have shown that M. oleifera has potential
anticancer activities (44,54,55) In
particular, it was previously demonstrated that the water-soluble
fraction of MOL induced apoptosis in lung cancer cells, and is
therefore a novel type of potential anticancer candidate compound
(30).
Solvent extraction methods have been widely utilized
to obtain extracts from plants, as solvents such as methanol and
ethanol effectively extract polar and non-polar bioactive compounds
(1,26,27,31,32.
However, non-polar compounds are not well absorbed in the body,
although they are bioactive. In contrast to the solvent extraction
method, compounds obtained via water extraction are often more
easily absorbed into the body, although the extraction efficiency
and the range of extracted compounds are normally inferior to those
that result from the solvent extraction method. Despite these
disadvantages, water extraction methods are considered, as the
resulting extracts are easily absorbed and offer the possibility of
oral administration.
In previous studies, a cold water-soluble extract
was prepared from MOL and its anticancer activity was confirmed in
human lung cancer A549 cells. In the present study, as a part of an
ongoing effort, the anticancer activity of MOL extract against
human hepatocellular carcinoma HepG2 cells was investigated to
evaluate its potential as an oral medication for lung and liver
cancer treatment. The results of the present study showed that MOL
extract was effective in the HepG2 cells, in addition to the A549
lung cancer cells. In addition, the oral administration of MOL was
effective in the mice with either lung or liver cancer cells,
indicating that the cold water MOL extract could be a potential
oral anticancer candidate agent.
Recent preclinical studies of anticancer drugs using
mice have tended to use parenteral administration methods,
including the intravenous or subcutaneous routes. However, for
clinical studies, these drugs require further study in order to
increase the absorption efficiency, decrease the possible
side-effects and reduce the inconvenience to patients. In
particular, subcutaneous injection, utilized by a number of studies
in the field of anticancer drug development, does not target
particular types of cancer, and is thus a poor predictor of the
efficacy of its clinical utility, although the candidate agents
administered by this method may show efficacy in lab-scale
experiments. The present study examined whether a water-soluble MOL
extract may be used as an oral medication. As presented in Fig. 4, the oral administration of MOL
extract greatly inhibited cancer cell proliferation, indicating
that the MOL extract used in the present study is potentially
suitable as an oral medication against cancer, although the
detailed mechanisms are yet to be elucidated. Future
pharmacogenetic and pharmacokinetic studies must be performed in
order to further evaluate the clinical potential of MOL extract, in
addition to any chronic and acute toxicity.
Although in vitro cell-based assays are
useful for screening drug candidates and examining their mechanisms
of action, further in vivo assays are usually necessary
prior to clinical testing. For this purpose, researchers developed
the traditional xenograft assay, which has been used worldwide.
However, the cost of the traditional xenograft assay is high, and
it takes a substantial period of time due to the large number of
animals required. The in vivo HFA in immunodeficient nude
mice was designed at the NCI to try to bridge the gap between in
vitro cell-based assays and human tumor models; the goal of the
assay was to predict which compounds would be active in a
subsequent xenograft system (56–59). In
the HFA, proliferating cancer cells containing hollow fibers with
pores, which are small enough to retain the cancer cells, but large
enough to permit potential chemotherapeutic drugs to enter, are
transplanted into the peritoneum or under the skin of the host
mice. Next, test drug candidates are administered to the mice, and
the fibers are then retrieved for analysis of any viable cell
masses (56). The HFA is similar to
the xenograft assay, but is a time- and cost-saving method. For
these reasons, the finding that MOL had anticancer activity in the
HFA of the present study supports its potential as a novel
anticancer drug. However, future xenograft mouse assays and other
non-clinical tests will be required prior to clinical testing.
Drug candidates with anticancer activity often have
bulky hydrophobic groups that render them water-insoluble (60). Low water solubility causes a number of
problems in the formulation and administration of anticancer agents
(61), thus, increasing water
solubility and/or developing soluble bioactive compounds with
strong anticancer activity have become areas of interest. Oral
therapies have the advantage of resulting in the persistent
exposure of tumor cells and the tumor environment to the
administered cytotoxic drug, and oral anticancer agents allow
therapeutic drug treatment in the comfort of the patient's home or
in alternative settings, such as retirement homes and
assisted-living or extended-care facilities (28). Therefore, efforts to develop soluble
drugs that can be orally administered have been considered in the
drug discovery processes. However, such a strategy is rarely used
by researchers, as the majority of active compounds are
water-insoluble. For this reason, a number of researchers are using
solvent extraction. Despite this trend, the present study focused
on a novel water-soluble MOL extract in order to overcome the
problems caused by low solubility, and examined its potential as an
oral anticancer drug.
In the present study, the HFA was used to
demonstrate that oral administration of MOL, in addition to
intravenous injection of doxorubicin, markedly inhibited lung and
liver cancer cell proliferation. This significant tumor inhibition
produced by oral administration of MOL may be due to the high
bioavailability of the extract, as the concentration used in the
in vitro cell experiment was similar to that used in the
in vivo mouse experiment. The reasons for the similarity in
the effective concentrations in the cell- and mouse-based assays
are not clear at this time, however, future preclinical trials,
such as pharmacokinetic studies, could uncover them. In the current
study, it was demonstrated that the water soluble MOL extract may
be a novel and promising natural anticancer drug candidate.
Acknowledgements
The present study was supported by funds from the
Korea Atomic Energy Research Institute Creativity Project (grant
no. 527240-14).
References
|
1
|
Khalafalla MM, Abdellatef E, Dafalla HM,
et al: Active principle from Moringa oleifera lam leaves
effective against two leukemias and a hepatocarcinoma. Afr J
Biotechnol. 9:8467–8471. 2010.
|
|
2
|
Iqbal S and Bhanger MI: Effect of season
and production location on antioxidant activity of Moringa
oleifera leaves grown in Pakistan. J Food Compos Anal.
19:544–555. 2006. View Article : Google Scholar
|
|
3
|
Wood M: The book of herbal wisdom: Using
plants as medicine. 1st. North Atlantic Books; Berkeley, CA, USA:
pp. 3741997
|
|
4
|
Oliveira JTA, Silveira SB, Vasconcelos KM,
Cavada BS and Moreira RA: Compositional and nutritional attributes
of seeds from the multiple purpose tree Moringa oleifera
Lamarck. J Sci Food Agric. 79:815–820. 1999. View Article : Google Scholar
|
|
5
|
Fahey JW: Moringa oleifera: a
review of the medical evidence for its nutritional, therapeutic,
and prophylactic properties. Part 1. Trees for Life Journal.
1:52005.
|
|
6
|
Fuglie LJ: The Miracle Tree: Moringa
oleifera: Natural Nutrition for the Tropics. Church World
Service, Dakar. Revised in 2001 and published as The Miracle Tree:
The multiple attributes of Moringa. 68–172. 1999.
|
|
7
|
Mukunzi D, Nsor-Atindana J, Xiaoming Z,
Gahungu A, Karangwa E and Mukamurezi G: Comparison of volatile
profile of Moringa oleifera leaves from Rwanda and China
using HS-SPME. Pak J Nutrit. 10:602–608. 2011. View Article : Google Scholar
|
|
8
|
Faizi S, Siddiqui BS, Saleem R, Siddiqui
S, Aftab K and Gilani AH: Fully acetylated carbamate and
hypotensive thiocarbamate glycosides from Moringa oleifera.
Phytochemistry. 38:957–963. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kooltheat N, Sranujit RP, Chumark P, Potup
P, Laytragoon-Lewin N and Usuwanthim K: An ethyl acetate fraction
of Moringa oleifera Lam. Inhibits human macrophage cytokine
production induced by cigarette smoke. Nutrients. 6:697–710. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mahmood KT, Mugal T and Haq IU: Moringa
oleifera: A natural gift-A review. J Pharm Sci Res. 2:775–781.
2010.
|
|
11
|
Anwar F, Latif S, Ashraf M and Gilani AH:
Moringa oleifera: a food plant with multiple medicinal uses.
Phytother Res. 21:17–25. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Olusola ATE: The miracle tree: Moringa
oleifera (drumstick)Archive Vibrant Health with Nature. (Keep
Hope Alive Series). Unijos Consultancy Limited Press; Jos, Nigeria:
pp. 120–136. 2006
|
|
13
|
Ijarotimi OS, Adeoti OA and Ariyo O:
Comparative study on nutrient composition, phytochemical, and
functional characteristics of raw, germinated, and fermented
Moringa oleifera seed flour. Food Sci Nutr. 1:452–463. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moyo B, Oyedemi S, Masika PJ and Muchenje
V: Polyphenolic content and antioxidant properties of Moringa
oleifera leaf extracts and enzymatic activity of liver from
goats supplemented with Moringa oleifera leaves/sunflower
seed cake. Meat Sci. 91:441–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Busani M, Masika PJ, Hugo A, et al:
Nutritional characterization of Moringa (Moringa
oleifera Lam.) leaves. Afr J Biotechnol. 10:12925–12933.
2011.
|
|
16
|
Waldron KW, Parker ML and Smith AC: Plant
cell wall and food quality. J Sci Food Technol. 2:109–110.
2003.
|
|
17
|
Tetteh ONA: Effects of blanching and
dehydration methods on the quality of Moringa leaf powder
used as herbal green tea (unpublished Master's thesis)Kwame Nkrumah
University; Ghana: 2008
|
|
18
|
Fuglie LJ: Combating malnutrition with
MoringaThe Miracle Tree: The Multiple Attributes of
Moringa. Fuglie LJ: CTA Publication; Wageningen, The
Netherlands: pp. 117–136. 2001
|
|
19
|
Ayotunde EO, Fagbenro OA and Adebanyo OT:
Toxicity of aqueous extract of Moringa oleifera seed powder
to Nile tilapia (Oreochromis niloticus) fingerlings. Int Res
J Agric Sci. 1:142–150. 2011.
|
|
20
|
Chumark P, Khunawat P, Sanvarinda Y, et
al: The in vitro and ex vivo antioxidant properties, hypolipidaemic
and antiatherosclerotic activities of water extract of Moringa
oleifera Lam leaves. J Ethnopharmacol. 116:439–446. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyoshi N, Takabayashi S, Osawa T and
Nakamura Y: Benzyl isothiocyanate inhibits excessive superoxide
generation in inflammatory leukocytes: implication for prevention
against inflammation-related carcinogenesis. Carcinogenesis.
25:567–575. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Faizi S, Siddiqui BS, Saleem R, Siddiqui
S, Aftab K and Gilani AH: Isolation and structure elucidation of
new nitrile and mustard oil glycosides from Moringa oleifera
and their effect on blood pressure. J Nat Prod. 57:1256–1261. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Waiyaput W, Payungporn S, Issara-Amphorn J
and Panjaworayan N: Inhibitory effects of crude extracts from some
edible Thai plants against replication of hepatitis B virus and
human liver cancer cells. BMC Complement Altern Med. 12:246–252.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lipipun V, Kurokawa M, Suttisri R,
Taweechotipatr P, Pramyothin P, Hattori M and Shiraki K: Efficacy
of Thai medicinal plant extracts against herpes simplex virus type
1 infection in vitro and in vivo. Antiviral Res. 60:175–180. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murakami A, Kitazono Y, Jiwajinda S,
Koshimizu K and Ohigashi H: Niaziminin, a thiocarbamate from the
leaves of Moringa oleifera, holds a strict structural
requirement for inhibition of tumor-promoter-induced Epstein-Barr
virus activation. Planta Med. 64:319–323. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sultana B, Anwar F and Ashraf M: Effect of
extraction solvent/technique on the antioxidant activity of
selected medicinal plant extracts. Molecules. 14:2167–2180. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar V, Pandey N, Mohan V and Singh RP:
Antibacterial and antioxidant activity of extract of Moringa
oleifera leaves-An in vitro study. Int J Pharm Scis Rev Res.
12:89–94. 2012.
|
|
28
|
Ashok Kumar N and Pari L: Antioxidant
action of Moringa oleifera Lam. (drumstick) against antitubercular
drugs induced lipid peroxidation in rats. J Med Food. 6:255–259.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gupta S Kumar, Kumar B, Srinivasan BP, Nag
TC, Srivastava S, Saxena R and Aggarwal A: Retinoprotective effects
of Moringa oleifera via antioxidant, anti-inflammatory, and
anti-angiogenic mechanisms in streptozotocin-induced diabetic rats.
J Ocul Pharmacol Ther. 29:419–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jung IL: Soluble extract from Moringa
oleifera leaves with a new anticancer activity. PLoS One.
9:e954922014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Budda S, Butryee C, Tuntipopipat S,
Rungsipipat A, Wangnaithum S, Lee JS and Kupradinun P: Suppressive
effects of Moringa oleifera Lam pod against mouse colon
carcinogenesis induced by azoxymethane and dextran sodium sulfate.
Asian Pac J Cancer Prev. 12:3221–3228. 2011.PubMed/NCBI
|
|
32
|
Guevara AP, Vargas C, Sakurai H, et al: An
antitumor promoter from Moringa oleifera Lam. Mutat Res.
440:181–188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dahiru D, Onubiyi JA and Umaru HA:
Phytochemical screening and antiulcerogenic effect of Moringa
oleifera aqueous leaf extract. African J Trad Complement
Alternat Med. 3:70–75. 2006.
|
|
34
|
Peschel W, Sánchez-Rabaneda F, Diekmann W,
et al: An industrial approach in the search of natural antioxidants
from vegetable and fruit wastes. Food Chem. 97:137–150. 2006.
View Article : Google Scholar
|
|
35
|
Siddhuraju P and Becker K: Antioxidant
properties of various solvent extracts of total phenolic
constituents from three different agroclimatic origins of drumstick
tree (Moringa oleifera Lam.) leaves. J Agric Food Chem.
51:2144–2155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hansen MB, Nielsen SE and Berg K:
Re-examination and further development of a precise and rapid dye
method for measuring cell growth/cell kill. J Immunol Methods.
119:203–210. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tani TH, Khodursky A, Blumenthal RM, Brown
PO and Matthews RG: Adaptation to famine: a family of
stationary-phase genes revealed by microarray analysis. Proc Natl
Acad Sci USA. 99:13471–13476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lotito SB and Fraga CG: Catechins delay
lipid oxidation and alpha-tocopherol and beta-carotene depletion
following ascorbate depletion in human plasma. Proc Soc Exp Biol
Med. 225:32–38. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hollingshead MG, Alley MC, Camalier RF,
Abbott BJ, Mayo JG, Malspeis L and Grever MR: In vivo cultivation
of tumor cells in hollow fibers. Life Sci. 57:131–141. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alley MC, Hollingshead MG, Dykes DJ and
Waud WR: Human tumor xenograft models in NCI drug developmentCancer
Drug Discovery and Development: Anticancer Drug Development Guide:
Preclinical Screening, Clinical Trials, and Approval. 72nd. Teicher
BA and Andrews PA: Humana Press Inc; Totowa, NJ, USA: pp. 125–152.
2004
|
|
41
|
Plowman J, Dykes DJ, Hollingshead M,
Simpson-Herren L and Alley MC: Human tumor xenograft models in NCI
drug developmentAnticancer Drug Development Guide: Preclinical
Screening, Clinical Trials, and Approval. Teicher B: Humana Press
Inc; Totowa, NJ, USA: pp. 101–125. 1997, View Article : Google Scholar
|
|
42
|
Mi Q, Pezzuto JM, Farnsworth NR, Wani MC,
Kinghorn AD and Swanson SM: Use of the in vivo hollow fiber assay
in natural products anticancer drug discovery. J Nat Prod.
72:573–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sahoo S, Raghavendra KM and Biswas S:
Identification of a proteinaceous component in the leaf of
Moringa Oleifera lam. with effects on high serum creatinine.
Indian J Pharm Sci. 76:78–81. 2014.PubMed/NCBI
|
|
44
|
Parvathy M and Umamaheshwari A: Cytotoxic
effect of Moringa oleifera leaf extracts on human multiple
myeloma cell lines. Trends Med Res. 2:44–50. 2007. View Article : Google Scholar
|
|
45
|
Khalafalla MM, Abdellatef E, Dafalla HM,
et al: Active principle from Moringa oleifera Lam leaves
effective against two leukemias and a hepatocarcinoma. Afr J
Biotechnol. 9:8467–8471. 2010.
|
|
46
|
Das BR, Kurup PA and Narasimha Rao PL:
Antibiotic principle from Moringa pterygosperma.
Naturwissenschaften. 41:661954. View Article : Google Scholar
|
|
47
|
Kær A, Malver O, El-menshawi B and Reischt
J: Isothiocyanates in myrosinase-treated seed extracts of
Moringa peregrine. Phytochemistry. 18:1485–1487. 1979.
View Article : Google Scholar
|
|
48
|
Dayrit FM, Alcantar AD and Villasenor IM:
Studies on Moringa oleifera seeds, Part I: The antibiotic
compound and its deactivation in aqueous solution. Philipp J Sci.
119:23–32. 1990.
|
|
49
|
Eilert U: Antibiotic principles of seeds
of Moringa oleifera. Indian Med J. 38:1013–1016. 1978.
|
|
50
|
Ghasi S, Nwobodo E and Ofili J:
Hypocholesterolemic effects of crude extract of leaf of Moringa
oleifera Lam. in high fat diet fed wistar rats. J Ethnopharmacol.
69:21–25. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jaiswal D, Kumar Rai P, Kumar A, Mehta S
and Watal G: Effect of Moringa oleifera lam. leaves aqueous
extract therapy on hyperglycemic rats. J Ethnopharmacol.
123:392–396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Edoga C, Njoku O, Amadi E and Okeke J:
Blood sugar lowering effect of Moringa oleifera lam in
albino rats. Int J Sci Technol. 3:88–90. 2013.
|
|
53
|
Muhammad AA, Pauzi NA, Arulselvan P, Abas
F and Fakurazi S: In vitro wound healing potential and
identification of bioactive compounds from Moringa oleifera
Lam. Biomed Res Int. 2013:9745802013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guevara AP, Vargas C, Sakurai H, et al: An
antitumor promoter from Moringa oleifera Lam. Mutat Res.
440:181–188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Murakami A, Kitazono Y, Jiwajinda S,
Koshimizu K and Ohigashi H: Niaziminin, a thiocarbamate from the
leaves of Moringa oleifera, holds a strict structural
requirement for inhibition of tumor-promoter-induced Epstein-Barr
virus activation. Planta Med. 64:319–323. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mi Q, Pezzuto JM, Farnsworth NR, Wani MC,
Kinghorn AD and Swanson SM: Use of the in vivo hollow fiber assay
in natural products anticancer drug discovery. J Nat Prod.
72:573–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Russell WM: The development of the three
Rs concept. Altern Lab Anim. 23:298–304. 1995.PubMed/NCBI
|
|
58
|
Russell WM and Burch RL: The Principles of
Humane Experimental Technique. Methuen; London, England: 1959
|
|
59
|
Suggitt M, Cooper PA, Shnyder SD and Bibby
MC: The hollow fibre model - facilitating anti-cancer pre-clinical
pharmacodynamics and improving animal welfare. Int J Oncol.
29:1493–1499. 2006.PubMed/NCBI
|
|
60
|
Lipinski CA: Drug-like properties and the
causes of poor solubility and poor permeability. J Pharmacol
Toxicol Methods. 44:235–249. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Teicher BA and Andrews PA: Part I: In
vitro methodsAnticancer Drug Development Guide: Preclinical
Screening, Clinical Trials and Approval. 2nd. Humana Press; Totowa,
NJ, USA: pp. 3–78. 2004
|