Introduction
Blood oxygen level-dependent (BOLD) functional
magnetic resonance imaging (MRI), acting via modulation of the
T2*-weighted signal by changes in the ratio of blood paramagnetic
deoxyhemoglobin to diamagnetic oxyhemoglobin, has been widely used
to investigate the neural basis of various brain diseases for ~2
decades (1). In more recent years,
this technique has been extended to evaluate tissue oxygenation in
other organs, including the myocardium (2), kidney (3),
breast (4) and prostate (5). In the liver, BOLD MRI has been used to
investigate tissue oxygenation changes following the administration
of a variety of physiological challenges, such as oxygen, carbon
monoxide, carbogen, ethanol and glucose, in rats (6,7) and humans
(8,9).
Carbogen-challenge BOLD MRI was previously used to investigate
tumor microvessel density (10) and
to assess the early response of liver tumors to chemoembolization
(11) in a rat hepatoma model.
Recently, the feasibility of BOLD MRI studies in the human liver
has been demonstrated in healthy volunteers and 1 patient with
chronic liver disease in a fasting and a postprandial condition
(8) or following oral ingestion of
glucose (9). However, to the best of
our knowledge, the potential of carbogen gas-challenge BOLD MRI to
evaluate hepatic diseases, such as liver fibrosis and tumors, has
not been investigated in the clinical setting. Additionally, no
studies have been published on the feasibility of carbogen
gas-challenge BOLD MRI in evaluating oxygenation changes of liver
tumors, and in assessing therapeutic efficacy and predicting
prognosis in patients with liver tumors. Therefore, the present
study reports the preliminary results of BOLD MRI in assessing the
oxygenation changes of liver tumors after inhaling carbogen, and in
assessing therapeutic efficacy and predicting prognosis of
transarterial chemoembolization (TACE) in patients with
hepatocellular carcinoma (HCC).
Patients and methods
Patients
The present Health Insurance Portability and
Accountability Act-compliant prospective study was approved by the
Institutional Review Board of Jinling Hospital (Nanjing, China) and
all patients provided written informed consent. A total of 25
patients of Jinlong Hospital (22 males and 3 females; median age,
52 years) with HCC were included in this study. In the 25 patients,
HCC was diagnosed by liver resection specimen (n=1), liver biopsy
(n=10) or using updated American Association for the Study of Liver
Diseases criteria (n=14) (12).
MR protocol and image analysis
All scans were performed using a clinical 3T
whole-body MR scanner (Magnetom Trio; Siemens Medical Solutions,
Erlangen, Germany) equipped with eight receiver channels and a
gradient system with a maximum gradient strength of 40 mT/m and a
slew rate of 200 T/m/sec. For signal reception, a combination of
spine coil and body matrix coil was used. All patients first
underwent conventional T1- and T2-weighted imaging for selecting
imaging slice of T2* mapping of the tumor. Two slices with maximal
dimension of the tumor were chosen for the T2* mapping. Next, T2*
mapping with multi-echo gradient-echo sequence with 9 echoes (3.4,
8.0, 12.7, 17.3, 21.9, 26.5, 31.1, 35.5 and 39.2 msec) was acquired
at the end of respiration during the patient's breath hold prior to
and following breathing carbogen (95% O2 and 5%
CO2) for 10 min via a soft face-mask system (Meinuo
Medical Equipment Co., Ltd., Shanghai, China). A continuous gas
flow of 15 l/min was ensured for sufficient gas breathing. Other
imaging parameters were as follows: Time of repetition, 100 msec;
echo train length, 9; intersection spacing, 4.6 msec; flip angle,
30°; number of slices, 2; slice thickness, 3.0 mm; field of view,
27.5×40.0 cm; matrix, 132×192; number of averages, 1; and
bandwidth, 350 Hz/voxel. The scanning time was 9 sec. Of 25
patients, 4 patients underwent follow-up T2* mapping 1 day after
TACE, and 1 patient underwent follow-up T2* mapping one month after
TACE. The slices for T2* mapping were chosen to be as similar as
possible as those prior to TACE.
For all T2* mapping analyses, either pre-TACE data
or post-TACE data, T2* and R2* (1/T2*, in sec−1,
reflecting oxygenation in tissue) values of the whole tumor, the
solid region of the tumor and the adjacent liver parenchyma were
measured from T2* maps collected prior to and following carbogen
breathing. For the whole tumor, two regions of interest (ROI),
including the whole tumor, regardless of tumor necrosis, at the two
slices of T2* maps were drawn manually; for the solid region of the
tumor, two ROIs were placed in the solid region of the tumor with
the marked T2* changes prior to and following carbogen breathing in
two slices of the T2* maps. For the adjacent liver parenchyma to
the tumor, three ROIs were chosen with the size of ~1
cm2 for each slice of the T2* maps; their average value
was used for the final analysis. ∆R2* was calculated using the
following formula: ∆R2* = R2*air - R2*carb, where R2*air is the R2*
measurement acquired at steady-state room air
multiple-gradient-echo MR imaging and R2*carb is the R2*
measurement acquired at steady-state carbogen
multiple-gradient-echo MR imaging (7).
TACE procedures
The TACE technique in this study has been widely
used in clinical setting. Briefly, the right common femoral artery
was punctured with an 18-gauge single-wall needle using the
Seldinger technique. A 5-F vascular sheath was placed into the
right common femoral artery. With fluoroscopic guidance, a 4-F glide
catheter (Shanghai Medical Equipment Co., Ltd., Shanghai, China)
was advanced over the guide wire into the desired hepatic artery
branch, depending on the tumor location. Chemoembolization drugs
were mixed in a 2:1 ratio of epirubicin (40 mg; Haizheng
Pharmaceutical Factory, Taizhou, Zhejiang, China), oxalipatin (200
mg; Qilu Pharmaceutical Factory, Jinan, China), fluorouracil (1,000
g; Shanghai Xudong Haipu Pharmacy Co., Ltd., Shanghai, China) and
non-ionic contrast material (30 ml; Ultravist; Schering, Berlin,
Germany) to iodized oil (15 ml; Shanghai Xudong Haipu Pharmacy Co.,
Ltd.), the dose of which depended on the tumor size. Subsequent to
slow effusion of the chemoembolization drugs and gelatin sponge
particles (1 mm3; Wuhan Jiuzhou Medical Equipment Co.,
Ltd., Wuhan, Hubei, China), complete embolization of the feeding
hepatic artery was achieved. The TACE procedures were performed by
an interventional radiologist with 20 years of experience.
Statistical analysis
Statistical analysis was performed with SPSS version
17.0 (SPSS Inc., Chicago, IL, USA). A paired t-test or independent
sample Student's t-test was used to assess the difference in R2*
and ∆R2* measurements in the whole tumor, the solid region of the
tumor and the adjacent liver parenchyma prior to and following
carbogen breathing. P<0.05 was used to indicate a statistically
significant difference.
Results
R2* measurements for patients with
liver tumors
A total of 25 patients with 25 tumor lesions on T2*
mapping images were included into the final analysis. These tumors
ranged in size from 2.3–18.2 cm. R2* (in sec−1) and ΔR2*
(in sec−1) values of the whole tumor, the solid region
of the tumor and the adjacent liver parenchyma prior to and
following carbogen breathing are shown in Tables I and II. As shown in Table I, no significant difference was found
in the R2* value of the adjacent liver parenchyma prior to carbogen
breathing and following carbogen breathing (P>0.05). There was
no difference in the R2* value of the whole tumor prior to and
following carbogen breathing (P>0.05). For the solid region of
the tumor, the R2* value of room air breathing was higher than that
following carbogen breathing (P<0.05), which indicated increased
oxygenation following carbogen breathing compared with prior to
carbogen breathing. Following carbogen gas breathing, 2 patients
exhibited marked T2* changes; however, the T2* changes were
heterogeneous for these tumors with response to carbogen breathing.
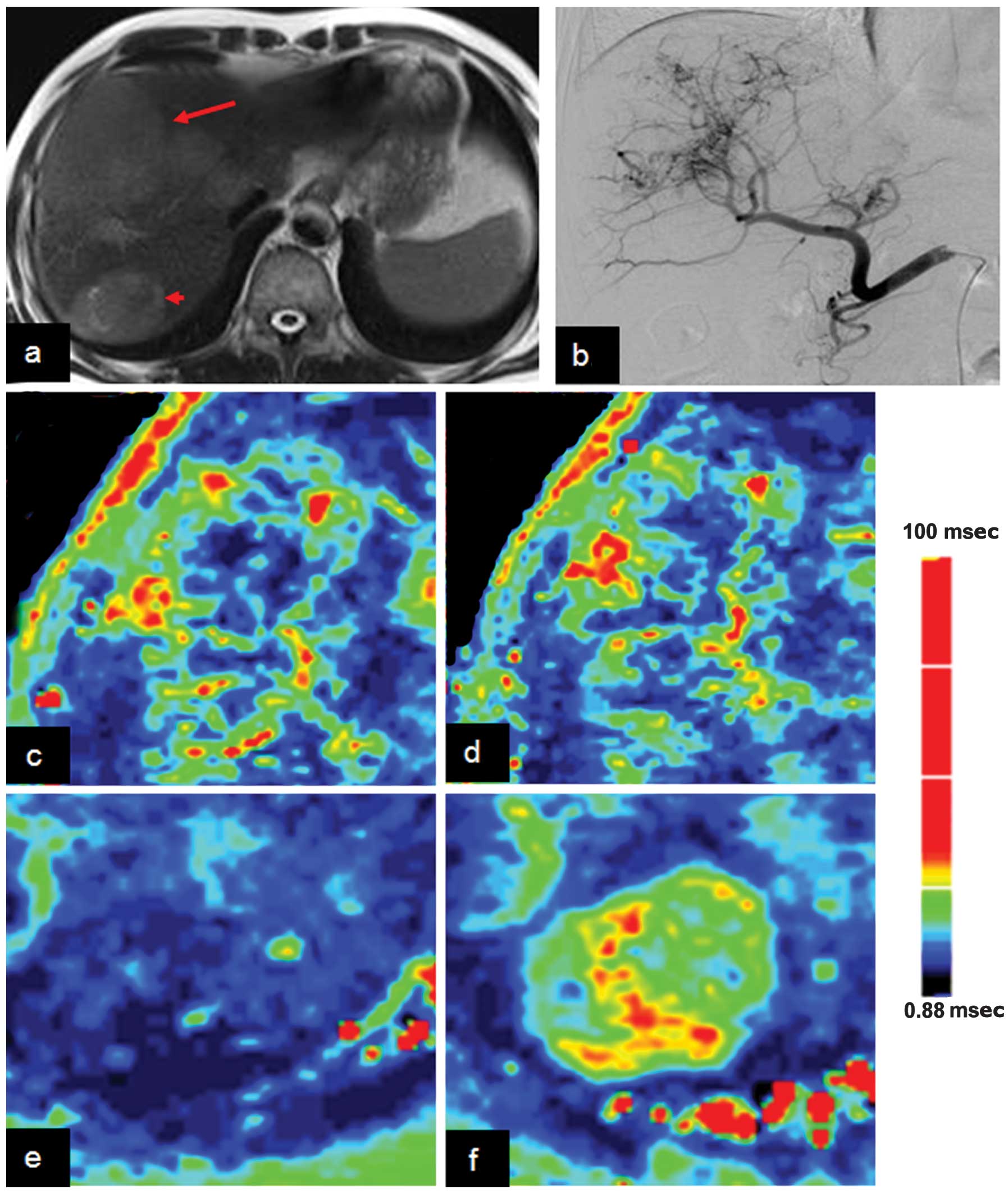
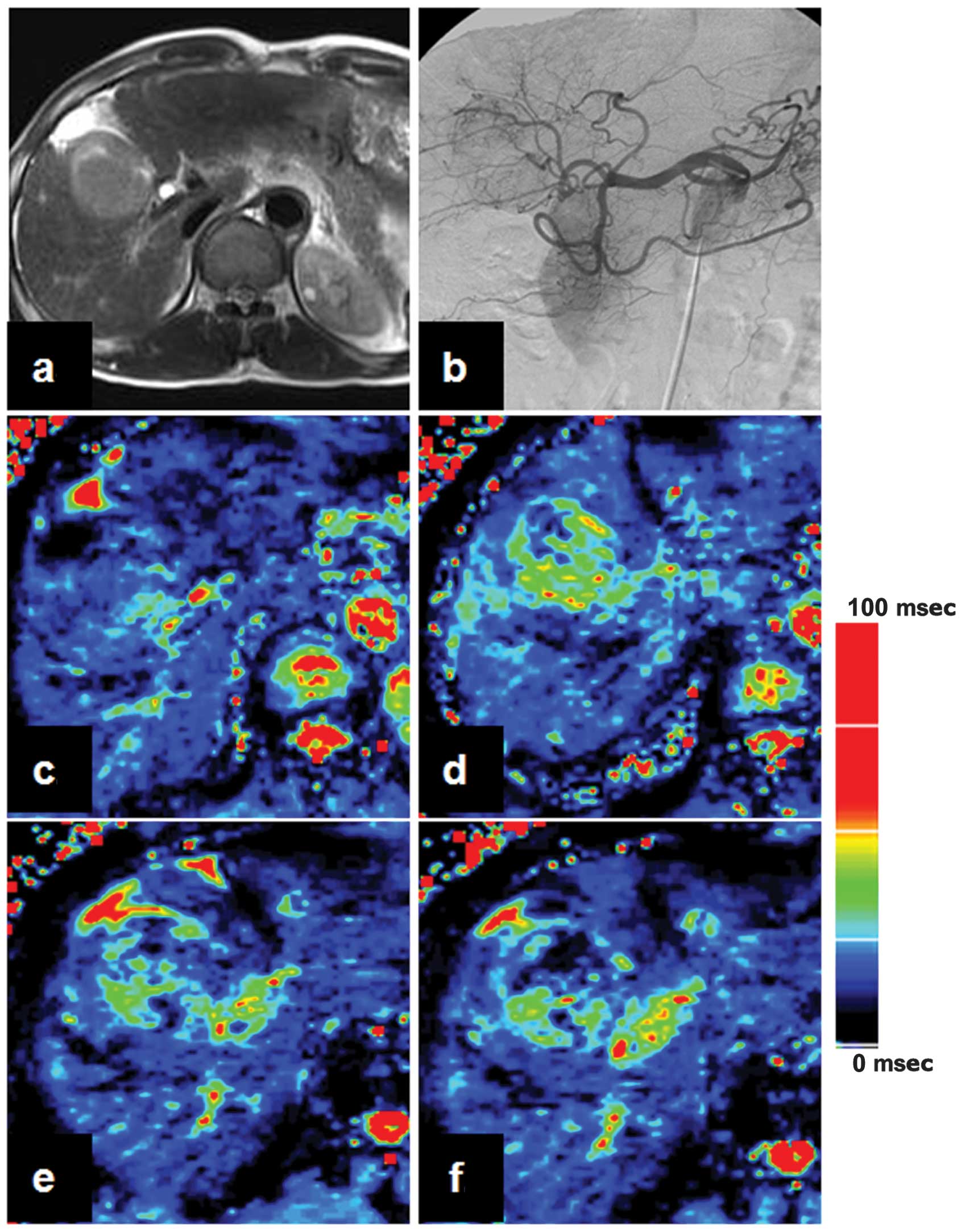
Representative cases are shown in Figs.
1 and 2. As shown in Table II, the R2*air and R2*carb values of
the tumor were lower than those of the adjacent liver parenchyma
(P<0.05). There was no significant difference in ∆R2* between
the tumor and the adjacent liver parenchyma (P>0.05).
 | Table I.Quantitative measurements of liver
tissue and tumor prior to and following carbogen breathing. |
Table I.
Quantitative measurements of liver
tissue and tumor prior to and following carbogen breathing.
| Protocols | Whole liver
tumor | Solid region of
tumor | Adjacent liver
tissue |
|---|
| R2*air,
sec−1 |
44.7±15.3
(20.4–76.6) |
39.2±19.6
(12.4–113.6) |
102.6±37.1
(47.2–175.4) |
| R2*carb,
sec−1 |
42.3±15.4
(19.3–81.3) |
31.1±12.8
(9.9–54.1) |
100.6±38.6
(42.6–166.7) |
| ∆R2*,
sec−1 |
2.4±7.8
(−17.5–19.8) |
8.1±14.7
(−9.4–64.9) |
2.0±11.0
(−19.6–22.0) |
 | Table II.Analysis of variance test for the
quantitative measurements of liver tissue and tumor prior to and
following carbogen breathing. |
Table II.
Analysis of variance test for the
quantitative measurements of liver tissue and tumor prior to and
following carbogen breathing.
| Protocols | Whole tumor vs. solid
region of the tumor | Whole tumor vs.
adjacent liver tissue | Solid region of tumor
vs. adjacent liver tissue |
|---|
| R2*air,
sec−1 | 0.441 | <0.001 | <0.001 |
| R2*carb,
sec−1 | 0.110 | <0.001 | <0.001 |
| ∆R2*,
sec−1 | - | - | - |
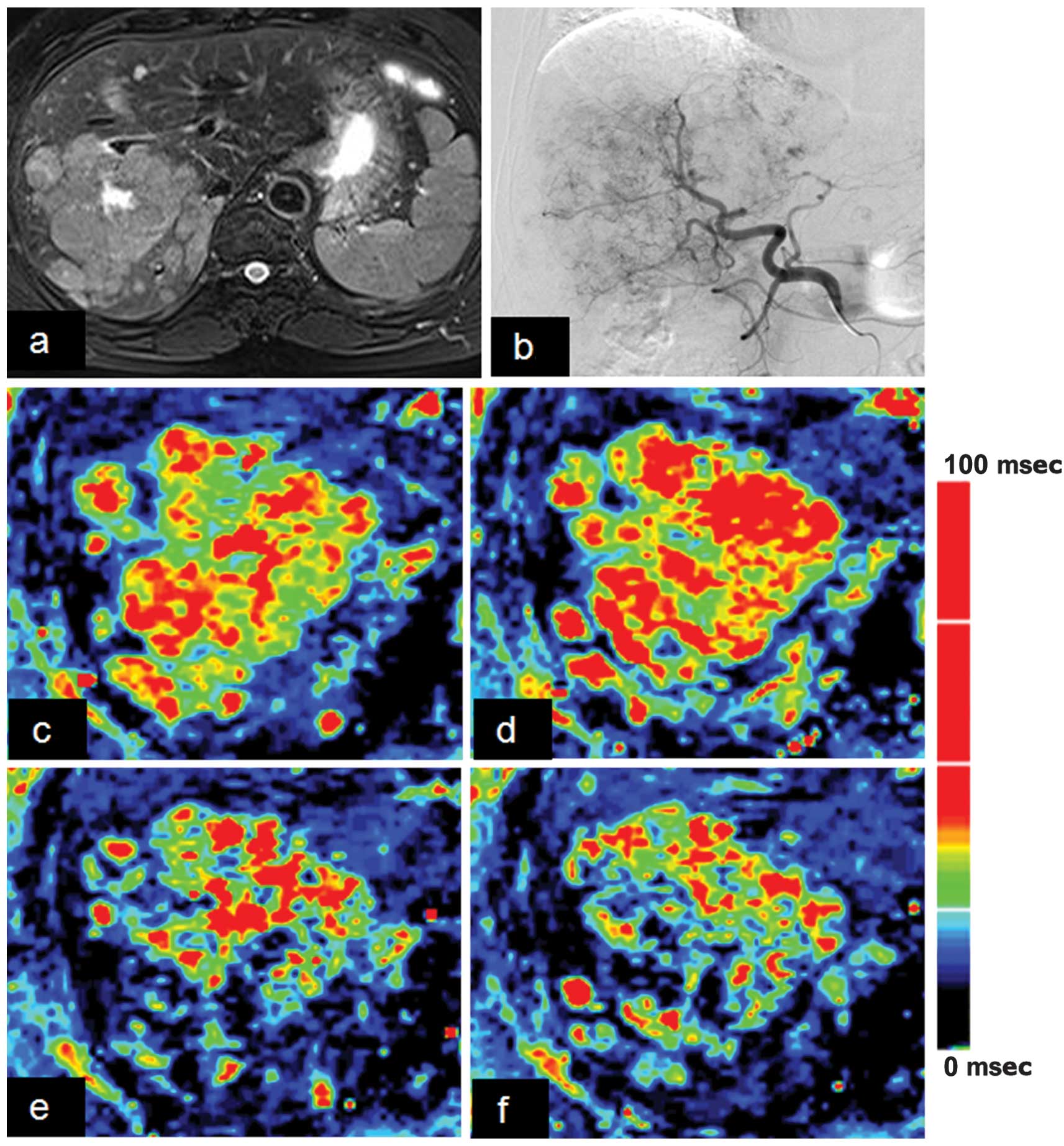
Tumor response
Of the 25 patients, 15 patients with HCC underwent
TACE procedures. Carbogen gas-challenge BOLD MRI was performed in 4
patients with HCC at 1 day (n=4) and 1 patient at 1 month (n=1)
post-TACE procedures; the ∆R2* value prior to TACE (∆R2* range,
0.5–17.8 sec−1 at the whole tumor for the 4 patients)
was significantly lower than that following TACE (∆R2* range, −4.0
− 2.4 sec−1 at the whole tumor for the 5 examinations).
A marked ∆R2* value was recorded in 2 patients at 1 day post-TACE
(∆R2*, 10.2 vs. 2.4 sec−1 and 17.8 vs. −3.4
sec−1 at the whole tumor). A representative case is
shown in Fig. 3.
Discussion
This preliminary study demonstrates that
carbogen-challenge BOLD MRI measurements are feasible in the
clinical setting, and therefore have the potential to serve as a
novel functional biomarker for monitoring the treatment efficacy of
embolic therapies. To the best of our knowledge, this is the first
study to focus on the liver tumor oxygenation changes in humans
using carbogen-challenge BOLD MRI.
Of the physiological challenges used in BOLD MRI,
carbogen (95% O2 and 5% CO2) is widely used
in experimental and clinical studies. Carbogen administration is
known to be safe in humans and has been clinically applied during
radiation therapy in order to raise the oxygen tension and increase
the radiosensitivity of the anoxic region. Compared with using pure
oxygen, incorporating 5% CO2 is believed to counteract
any oxygen-induced vasoconstriction (7). In the present human study, no subjects
had any complaints with regard to the carbogen gas, indicating the
safety of the gas in the clinical setting. In the study, 10-min
carbogen gas breathing was performed rather than the block-designed
scheme reported in our previous experimental study (7). In previous studies (7,8),
block-designed BOLD MRI took a long time (>1 h) to measure the
BOLD response in animals and humans, which limited the technique in
clinical practice. The present preliminary study demonstrated the
feasibility of a steady-state gas challenge to evaluate ∆R2* in
human liver tumors. The T2* and R2* values of the tumor were found
to be statistically different from those of the adjacent liver
parenchyma. Haque et al (9)
reported a statistically significant decrease in R2* (55.8±3.8 to
50.6±0.5 sec−1) following oral ingestion of 75 g glucose
in 6 healthy subjects. Variable BOLD responses of HCC for carbogen
breathing were observed in the present study; the reasons were
complex, but included the fact that the tumors may have adapted to
widely different perfusion environments. Additionally, variation in
tumor microenvironments, including tumor vascularity, blood supply
ratio of the hepatic artery to the portal vein and aerobic
metabolic activity, can result in these variations of ∆R2* value
(10). Thus, further studies are
required.
The ~85% patients of HCC who are diagnosed at an
advanced stage are not candidates for surgical therapy (13). In these patients, the established
treatment protocol is TACE, which has proven survival benefits
compared with best supportive care (14,15).
Objective and accurate evaluation of the response of HCC to
treatment is crucial for determining the requirement for repeated
TACE or alternative treatment approaches, and for improving
survival time (16). Multimodality
MRI plays an increasingly important role in assessing the response
of HCC to loco-regional therapy due to its lack of ionizing
radiation, high contrast resolution and the possibility of
performing functional imaging sequences (17). Dynamic contrast-enhanced MRI,
diffusion-weighted imaging, perfusion-weighted imaging and MR
spectroscopy have been used for the assessment of the early
therapeutic response of large HCCs following TACE, each with their
advantages and disadvantages (18).
The present study presented a steady-state carbogen-gas challenge
BOLD MRI method to monitor the response of HCC to treatment in
humans. This steady-state carbogen-gas challenge BOLD MRI scheme is
advantageous to perform in the clinical setting due to a shorter
overall scan time compared with block designed BOLD MRI (10 min vs.
>1 h) (8) and less
contraindication compared with glucose challenge BOLD MRI (9). In the present study, it was found that
TACE resulted in a decreased BOLD response of HCC compared with
untreated HCC due to blocking of the tumor vessels. This indicated
the potential of carbogen gas-challenge BOLD MRI as a biomarker to
monitor treatment efficacy and predict the prognosis of TACE in
patients with HCC. However, further studies in a large cohort are
required to determine whether BOLD MRI has an ability to predict
the prognosis of patients with HCC undergoing TACE. These
preliminary results were supported by previously published
experimental studies. For example, Rhee et al (19) showed that the use of 15-min
oxygen-challenge BOLD MRI to monitor changes in hepatic tumor
oxygenation following embolization was feasible in rabbits. Choi
et al (11) found that
carbogen gas-challenge BOLD MRI could monitor the early response of
liver tumors to chemoembolization in a rat hepatoma model using a
9.4-Tesla scanner. The present findings have also been supported by
a previous clinical study, which showed evidence for a correlation
between tumor oxygenation and treatment outcome (18). Hypoxia is an important factor in
radiotherapy treatment failure and, in clinical studies, has been
associated with poor local tumor control and relapse in numerous
cancer regions (18); a higher level
of oxygen in the tumor tissue may improve the treatment response of
these tumors (19,20).
However, the present study does have certain
limitations. First, the study is only a preliminary report and the
results are limited by a small heterogeneous sample size for liver
tumor patients with variations in the size of the tumors and
treatment options, which may have affected the statistical analysis
of the study. Thus, a large-cohort study is required to further
validate the value of carbogen gas-challenge BOLD MRI in predicting
the liver tumor response to treatment in future. Second,
respiratory motion during two T2* mapping acquisition prior to and
following carbogen breathing can have an effect on T2*
measurements, although as similar slices as possible were kept when
imaging. Breathing instructions and a respiratory navigating or
gating technique should be used carefully. Third, carbogen gas was
arbitrarily used in this study when the optimal challenges remained
underdetermined; further study is required to compare the BOLD
responses during oxygen gas or glucose challenges with those during
carbogen gas challenges. Fourth, T2* maps at two slices of maximal
dimension of the tumor was acquired, which can confer bias for the
evaluation of oxygenation changes of the whole tumor. However,
encompassing the whole tumor would increase the scanning time and
result in longer breath holding time, which would have decreased
the image quality of the T2* maps. Last, the present study adopted
a 10-min carbogen gas breathing scheme rather than a block-designed
scheme, which was similar to that in task-related BOLD MRI in the
brain. However, in our previous experimental study, the overall
scanning time for block-designed BOLD MRI in the liver was too long
to transfer to the clinical setting (7). The duration of carbogen gas breathing
requires optimization in future studies.
In conclusion, the present preliminary study
demonstrated that carbogen gas-challenge BOLD MRI measurements are
feasible in the clinical setting. Carbogen-challenge BOLD MRI
measurements have the potential to serve as a novel functional
biomarker for monitoring the treatment efficacy of embolic
therapies for HCC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81171313) and the Program
for New Century Excellent Talents in University (no. NCET-12-0260).
Partial data was presented in RSNA 2011 (Chicago, USA, November
2011).
References
|
1
|
Gore JC: Principles and practice of
functional MRI of the human brain. J Clin Invest. 112:4–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dharmakumar R, Arumana JM, Tang R, Harris
K, Zhang Z and Li D: Assessment of regional myocardial oxygenation
changes in the presence of coronary artery stenosis with balanced
SSFP imaging at 3.0 T: Theory and experimental evaluation in
canines. J Magn Reson Imaging. 27:1037–1045. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haque M, Franklin T and Prasad P: Renal
oxygenation changes during water loading as evaluated by BOLD MRI:
Effect of NOS inhibition. J Magn Reson Imaging. 33:898–901. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
RakowPenner R, Daniel B and Glover GH:
Detecting blood oxygen level-dependent (BOLD) contrast in the
breast. J Magn Reson Imaging. 32:120–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alonzi R, Padhani AR, Maxwell RJ, et al:
Carbogen breathing increases prostate cancer oxygenation: A
translational MRI study in murine xenografts and humans. Brit J
Cancer. 100:644–648. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robinson SP, Rodrigues LM, Griffiths JR
and Stubbs M: Response of hepatoma 9618a and normal liver to host
carbogen and carbon monoxide breathing. Neoplasia. 1:537–543. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin N, Deng J, Chadashvili T, et al:
Carbogen gas-challenge BOLD MR imaging in a rat model of
diethylnitrosamine-induced liver fibrosis. Radiology. 254:129–137.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan Z, Elzibak A, Boylan C and Noseworthy
MD: Blood oxygen level-dependent magnetic resonance imaging of the
human liver: Preliminary results. J Comput Assist Tomogr.
34:523–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haque M, Koktzoglou I, Li W, Carbray J and
Prasad P: Functional MRI of liver using BOLD MRI: Effect of
glucose. J Magn Reson Imaging. 32:988–991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo Y, Jin N, Klein R, et al: Gas
challenge-blood oxygen level-dependent (GC-BOLD) MRI in the rat
Novikoff hepatoma model. Magn Reson Imaging. 30:133–138. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi JW, Kim H, Kim HC, et al: Blood
oxygen level-dependent MRI for evaluation of early response of
liver tumors to chemoembolization: An animal study. Anticancer Res.
33:1887–1892. 2013.PubMed/NCBI
|
|
12
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
MarcosAlvarez A, Jenkins RL, Washburn WK,
et al: Multimodality treatment of hepatocellular carcinoma in a
hepatobiliary specialty center. Arch Surg. 131:292–298. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin B, Wang D, Lewandowski RJ, et al:
Chemoembolization endpoints: Effect on survival among patients with
hepatocellular carcinoma. AJR Am J Roentgenol. 196:919–928. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Llovet JM, Real MI, Montaña X, et al:
Barcelona Liver Cancer Group: Arterial embolisation or
chemoembolisation versus symptomatic treatment in patients with
unresectable hepatocellular carcinoma: A randomised controlled
trial. Lancet. 359:1734–1739. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonekamp S, Shen J, Salibi N, Lai HC,
Geschwind J and Kamel IR: Early response of hepatic malignancies to
locoregional therapy-value of diffusion-weighted magnetic resonance
imaging and proton magnetic resonance spectroscopy. J Comput Assist
Tomogr. 35:167–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee CH, Braga L, de Campos RO and Semelka
RC: Hepatic tumor response evaluation by MRI. NMR Biomed.
24:721–733. 2011.PubMed/NCBI
|
|
18
|
Rudat V, Vanselow B, Wollensack P, et al:
Repeatability and prognostic impact of the pretreatment pO(2)
histography in patients with advanced head and neck cancer.
Radiother Oncol. 57:31–37. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rhee TK, Larson AC, Prasad PV, et al:
Feasibility of blood oxygenation level-dependent MR imaging to
monitor hepatic transcatheter arterial embolization in rabbits. J
Vasc Interv Radiol. 16:1523–1528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schuuring J, Rijpkema M, Bernsen H, et al:
Effect of breathing a hyperoxic hypercapnic gas mixture on the
oxygenation of meningiomas; preliminary results. J Neurooncol.
57:127–132. 2002. View Article : Google Scholar : PubMed/NCBI
|