Introduction
Malignant glioma is the most common malignant tumor
that occurs in the brain (1). The
World Health Organization (WHO) classification system is used for
tumor grading, and the prognosis and management of the disease are
indicated by the tumor classification (2). Tumor resection followed by radiotherapy
and temozolomide treatment is the current standard therapy for
glioma. However, the majority of patients with glioma exhibit tumor
progression within two years of diagnosis (3). Therefore, it is important to develop a
potent prognostic marker and therapeutic target for human
glioma.
Downregulation of oxidative phosphorylation in
combination with the activation of aerobic glycolysis is a hallmark
for numerous types of human cancer (4–6).
H+-adenosine triphosphate (ATP) synthase acts as a
critical marker for energy metabolism and cell fate. ATPase
inhibitory factor 1 (IF1) is a heat-stable protein in mammals that
is mainly located within the mitochondrial matrix (7). IF1 has been considered to be an
inhibitor for the activity of the mitochondrial H+-ATP
synthase (8). Elevated IF1 expression
is observed in a number of human cancers, including colon (9), lung (10),
breast (10) and ovarian cancers
(10) and hepatocellular carcinoma
(HCC) (11). A previous study have
reported that reciprocal activation between IF1 and nuclear factor
(NF)-κB promoted HCC angiogenesis and metastasis (11). In colon cancer cells, IF1 promoted
aerobic glycolysis and reactive oxygen species-mediated signaling
pathway to enhance cell proliferation and cell survival (9). Furthermore, IF1 has been considered to
be an independent prognostic marker for human cancer (11). However, the clinical significance of
IF1 and its role in glioma metastasis have been insufficiently
investigated.
In the present study, IF1 expression was detected in
human glioma tissues using immunohistochemical staining and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
association between IF1 expression and the clinicopathological
features of glioma were systemically analyzed. Furthermore, the
role of IF1 in the migration and invasion of glioma cells was
investigated to confirm the effect of IF1 on the initiation and
development of human glioma.
Materials and methods
Clinical samples
A total of 86 paraffin-embedded glioma and 20 normal
brain (NB) tissue samples were obtained from the Fifth Affiliated
Hospital of Zhengzhou University (Zhengzhou, Henan, China) between
January 2008 and December 2010. All samples were used subsequent to
obtaining informed consent from patients. The demographical
features and clinicopathological data of the patients are reported
in Table I. The diagnosis of all
specimens was pathologically confirmed and the tissues were
classified according to the WHO criteria. The Zhengzhou University
Ethics Committee approved all protocols, which were in accordance
with the Declaration of Helsinki (12).
 | Table I.Association between
clinicopathological characteristics of glioma patients and
expression of the IF1 protein (n=86). |
Table I.
Association between
clinicopathological characteristics of glioma patients and
expression of the IF1 protein (n=86).
|
|
| IF1 protein
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Total, n | Present, n | Absent, n | P-value |
|---|
| Age (years) |
|
|
|
|
|
<50 | 40 | 29 | 11 | 0.468 |
| ≥50 | 46 | 30 | 16 |
|
| Gender |
|
|
|
|
| Male | 55 | 38 | 17 | 0.897 |
|
Female | 31 | 21 | 10 |
|
| Histological
type |
|
|
|
|
|
Astrocytic tumors | 62 | 42 | 20 | 0.153 |
|
Oligodendrogial tumors | 9 | 7 | 2 |
|
|
Oligoastrocytic tumors | 15 | 10 | 5 |
|
| WHO grade |
|
|
|
|
| I+II | 26 | 12 | 14 | 0.003 |
|
III+IV | 60 | 47 | 13 |
|
Immunohistochemical staining
Immunohistochemistry with streptavidin peroxidase
conjugate was performed using formalin-fixed paraffin-embedded
sections. The sections underwent dewaxing, rehydration, antigen
retrieval, endogenous peroxidase activity blocking and goat serum
blocking, and the sections were then incubated with the mouse
anti-human IF1 monoclonal antibody (clone no., 5E2D7; catalog no.,
ab110277; Abcam, Cambridge, MA, USA) at 4°C overnight.
SP-conjugated secondary antibody and 3,3′-diaminobenzidine were
used for the staining of sections. According to the percentage of
positive tumor cells, IF1 expression was classified as absent
(<10%) or present (≥10%) (13).
RT-qPCR
The IF1 primers used were as follows: Forward,
5′-GGGCCTTCGGAAAGAGAG-3′ and reverse, 5′-TTCAAA GCTGCCAGTTGTTC-3′.
PCR amplification was performed to quantify the expression of IF1
and GAPDH mRNA using a SYBR Premix Ex Taq ii (Perfect Real Time)
kit (Takara Bio, Otsu, Shiga, Japan), as previously described
(14).
Cell lines and transfection
The human glioma U251 and U87 cell lines (Chinese
Academy of Sciences, Shanghai, China) were cultured in complete
Dulbecco's modified Eagle medium (DMEM; Gibco Life Technologies,
Grand Island, NY, USA) containing 10% fetal bovine serum (FBS;
Gibco Life Technologies), 100 U/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in an
incubator with a humidified atmosphere containing 5%
CO2.
Short hairpin (sh)RNA, consisting of IF1 shRNA and
non-targeting (NT) shRNA, was purchased from GeneCopoeia, Inc.
(Rockville, MD, USA). The cells were transfected with the
aforementioned vectors using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA), according to the manufacturer's
instructions.
Western blot analysis
Rabbit anti-human NF-κB p50 polyclonal antibodies
(1:1,000 dilution; catalog no., 06-886; Millipore, Billerica, MA,
USA), mouse anti-human Snai1 monoclonal antibodies (1:1,000
dilution; catalog no., ab117866; Abcam), rabbit anti-human vimentin
polyclonal antibodies (1:1,000 dilution; catalog no., 3932; Cell
Signaling Technology, Danvers, MA, USA), rabbit anti-human
E-cadherin monoclonal antibodies (1:1,000 dilution; catalog no.,
3195; Cell Signaling), mouse anti-human IF1 monoclonal antibodies
(1:1,000 dilution; catalog no., ab110277; Abcam) and mouse
anti-human anti-GAPDH monoclonal antibodies (1:5,000 dilution;
catalog no., G8140-01V; US Biological, Salem, MA, USA) were used
for the immunoblotting assay. Horseradish peroxidase-conjugated
sheep anti-mouse secondary antibody (Bio-Rad Laboratories,
Hercules, CA, USA) was used at a 1:1,000-1:5,000 dilution and
detected by a western blotting Luminol reagent (catalog no.,
sc-2048; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) (14).
Transwell cell migration and invasion
assays
Transwell cell migration assays were performed on 12
well plates with 8-µm BioCoat control inserts (Becton-Dickinson,
Franklin Lakes, NJ, USA). In total, 1–2×105 U251 or U87
cells transfected with IF1 or NT shRNA were suspended in 500 µl
serum-free DMEM and then seeded in the upper well of a Transwell
chamber. DMEM (750 µl) supplemented with 10% FBS was added to the
lower well. Subsequent to completion, the membranes were removed,
the cells on the side facing the upper well were wiped with a
cotton swab, and the adherent cells on the undersurface of the
insert were stained using crystal violet. At least six
representative images of each well were captured and the number of
cells were counted using ImageJ software. The BioCoat Matrigel
invasion chamber (Becton Dickinson Labware) was used for Transwell
cell invasion assays and the following protocols were the same as
the Transwell cell migration assays. The experiments were performed
in triplicate (15).
Statistical analysis
The results were expressed as the mean ± standard
error of the mean. The data was analyzed using the SPSS statistical
package for Windows version 13 (SPSS, Chicago, IL, USA) or GraphPad
Prism 5 software (GraphPad Software, Inc., San Diego, CA, USA).
Pearson's χ2 test, Kaplan-Meier analysis, log-rank test,
multivariate Cox regression analysis or a two-tailed Student's
t-test, when appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of IF1 in glioma and NB
tissues
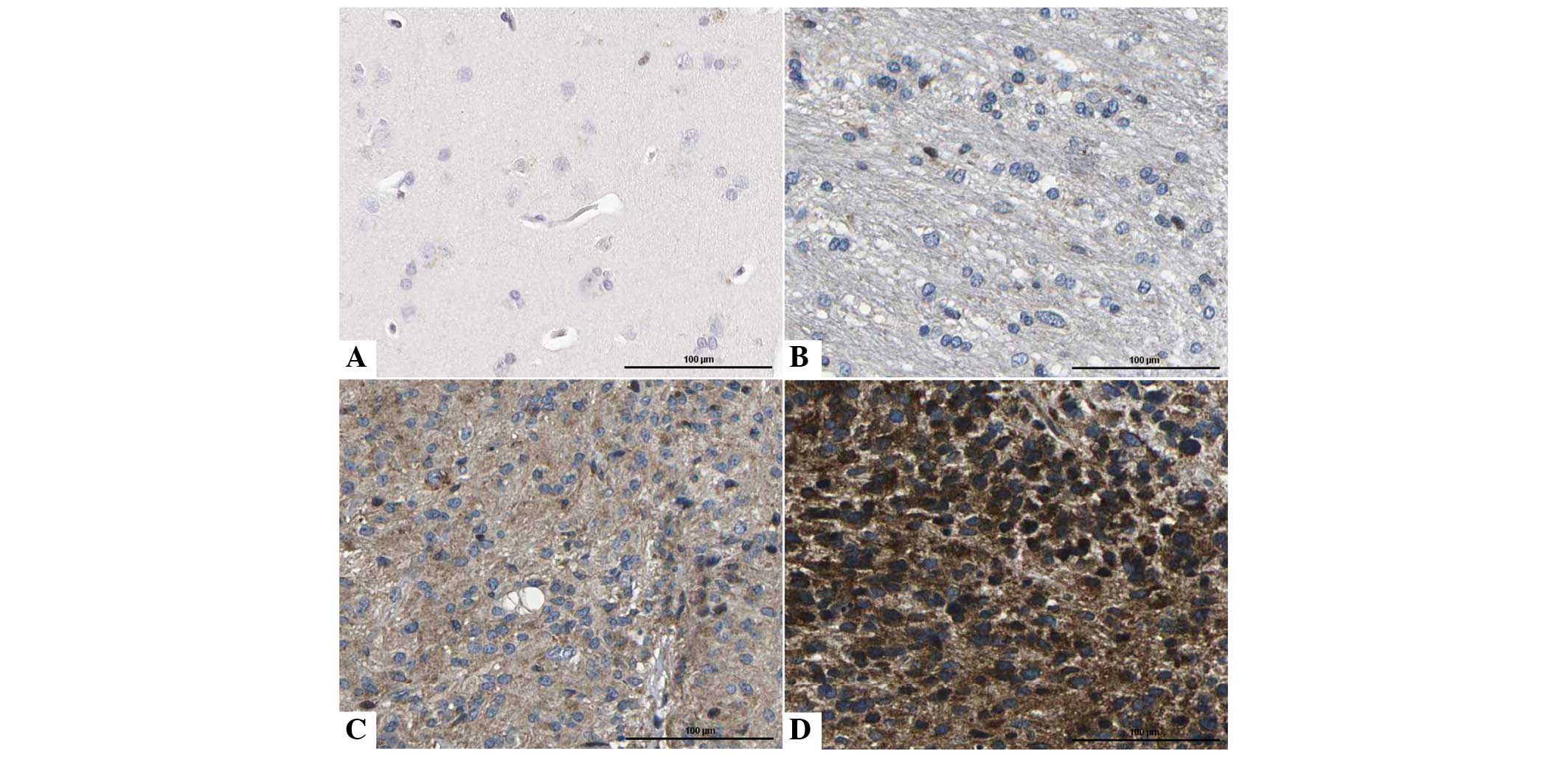
To determine the expression of IF1 in glioma
specimens, the levels of IF1 expression were detected in 86 glioma
and 20 NB tissue specimens using immunohistochemical staining. IF1
expression was considered as either absent or present. The current
data indicated that IF1 expression was present in 68.6% (59/86) of
glioma tissues, while only 20.0% (4/20) of NB tissues exhibited a
signal for IF1 expression (P<0.05; Fig. 1). Furthermore, qPCR was performed to
determine the levels of IF1 mRNA in glioma tissues (n=20) and NB
tissues (n=20). Quantitative analysis indicated that the level of
IF1 mRNA in glioma tissues was significantly increased compared
with the level in NB tissues (P<0.05).
Clinical significance of IF1 in
glioma
To investigate the clinical significance of IF1 in
glioma, the association between IF1 expression and
clinicopathological parameters in glioma was investigated. As
reported in Table I, the clinical
association analysis performed using Pearson's χ2 test
indicated that the presence of IF1 expression was evidently
associated with an advanced clinical stage (P=0.003). Furthermore,
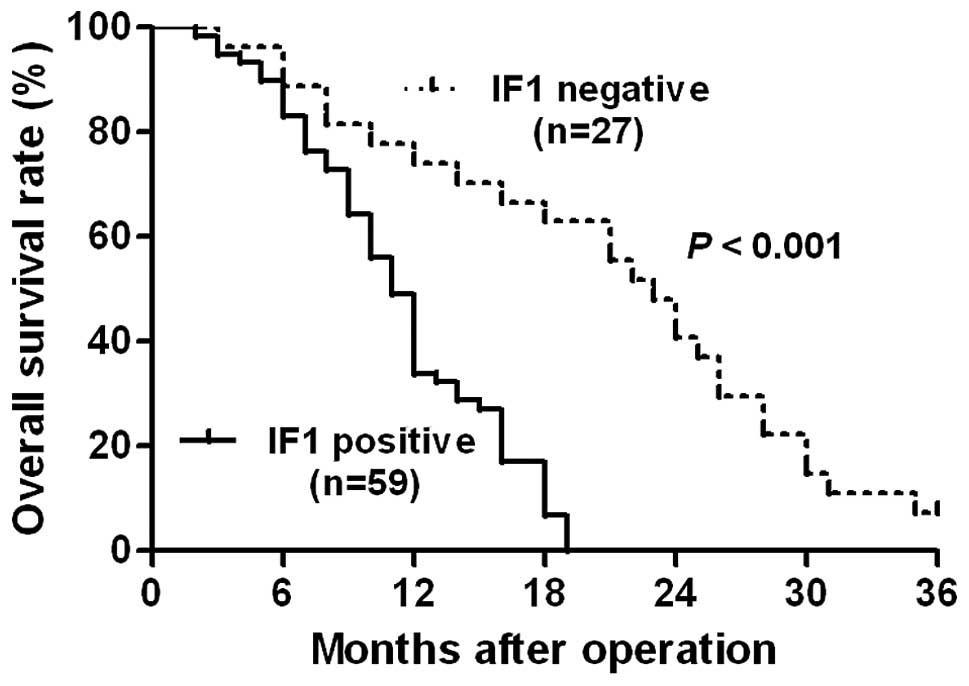
Kaplan-Meier analysis indicated that tumors with IF1 positive
expression were associated with a worse overall survival rate in
glioma patients (P<0.05; Fig. 2).
Notably, IF1 expression was an independent factor for predicting
the overall survival rate of patients with glioma (P=0.018;
Table II). These data indicate that
IF1 may act as a potent biomarker for predicting the prognosis of
glioma patients.
 | Table II.Multivariate Cox regression analysis
of the overall survival time of glioma patients. |
Table II.
Multivariate Cox regression analysis
of the overall survival time of glioma patients.
| Variables | HR | 95% CI | P-value |
|---|
| Age | 2.113 | 0.888–5.030 | 0.091 |
| Gender | 0.749 | 0.339–1.652 | 0.474 |
| Histological
type | 1.846 | 0.694–4.908 | 0.219 |
| WHO grade | 3.576 |
1.251–10.211 | 0.017 |
| IF1 expression | 0.253 | 0.081–0.790 | 0.018 |
IF1 knockdown inhibits glioma cell
migration and invasion
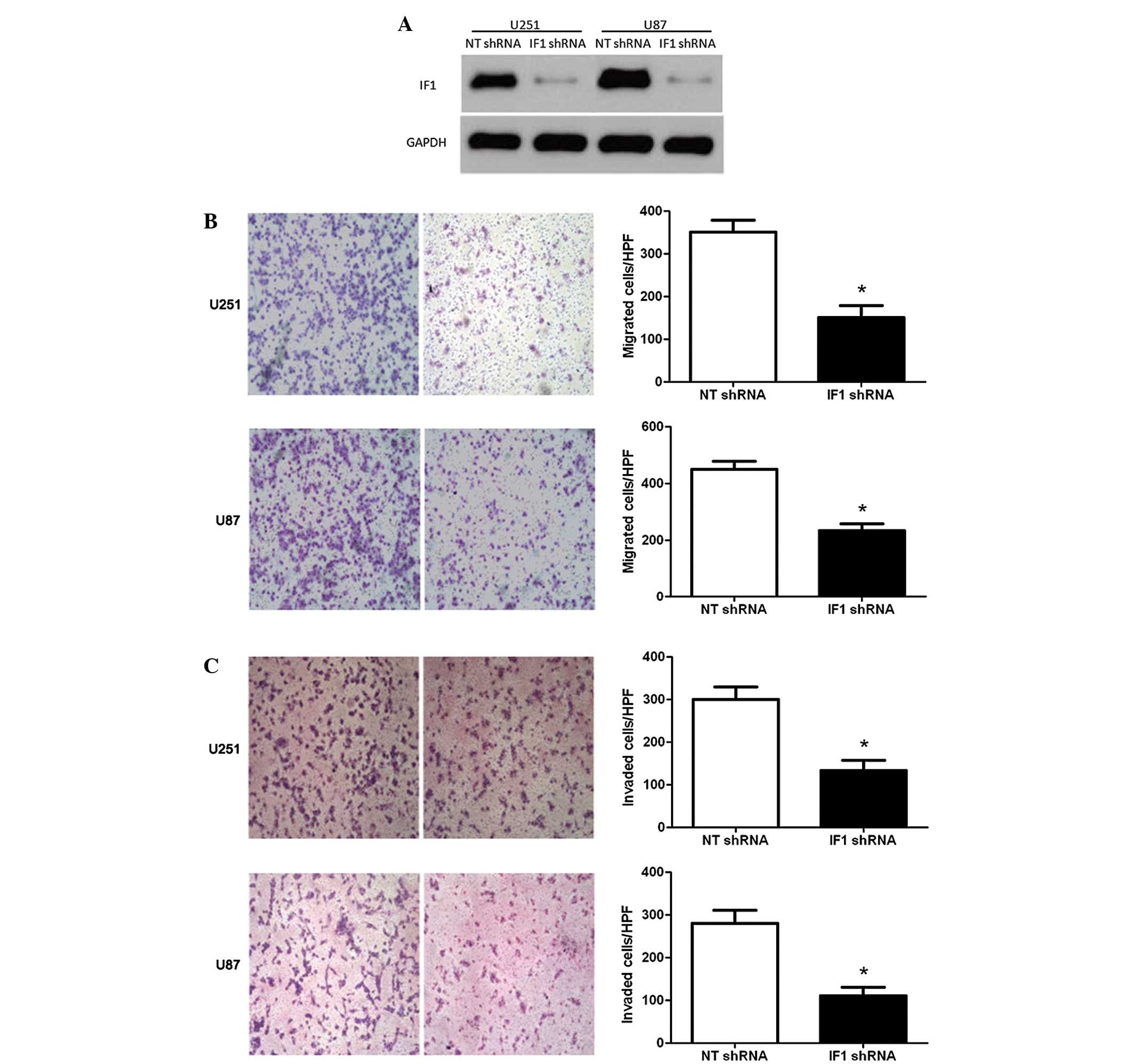
To confirm the role of IF1 in glioma, U251 and U87
cells were transduced with NT or IF1 shRNA and then subjected to
Transwell assays for cell migration and invasion. As measured by
western blotting, the level of IF1 protein was significantly
downregulated by specific shRNA in U251 and U87 cells (P<0.05;
Fig. 3A). Transwell assays were
performed to determine the effect of altering IF1 levels on tumor
cell migration. IF1 knockdown resulted in a significant reduction
of U251 and U87 cell migration (P<0.05; Fig. 3B). Furthermore, as determined by
Transwell assays, the number of invasive U251 and U87 cells was
prominently decreased subsequent to IF1 knockdown (P<0.05;
Fig. 3C). Thus, IF1 may exert a
pro-metastatic effect by promoting cell migration and invasion in
glioma.
IF1 may promote glioma metastasis
through the NF-κB/Snai1 pathway
A previous study has reported that IF1 promotes HCC
metastasis and angiogenesis through the NF-κB/Snai1 and vascular
endothelial growth factor pathway (11). To investigate the potential signaling
pathways involved in IF1 induced glioma cell migration and
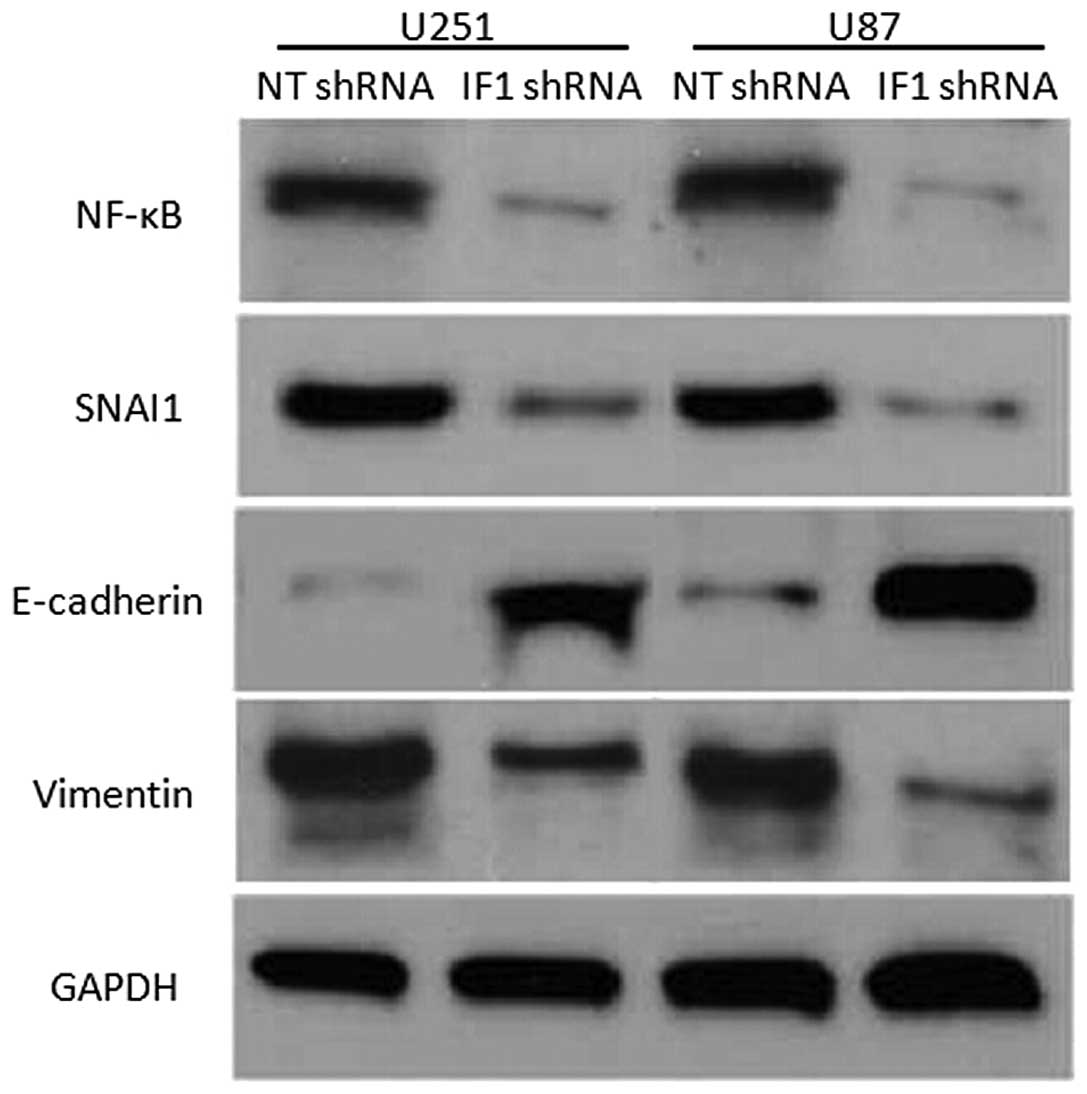
invasion, U251 and U87 cells that were transduced with NT shRNA or
IF1 shRNA were subjected to western blot analysis for the
expression of NF-κB, Snai1, E-cadherin and vimentin. Notably, IF1
knockdown inhibited NF-κB, Snai1 and vimentin expression, but
upregulated E-cadherin expression (Fig.
4). Previous studies reported that NF-κB transcriptionally
regulated Snai1 expression and subsequently promoted
epithelial-mesenchymal transition (EMT). Thus, the present results
indicate that IF1 may promote glioma metastasis via the NF-κB/Snai1
signaling pathway.
Discussion
IF1 specifically inhibits the ATP-hydrolyzing
activity of F1F0-ATP synthase, without impacting the synthesis of
ATP during oxidative phosphorylation (16,17).
Elevated expression of IF1 has been observed in numerous human
cancers (9–11). IF1 has previously been considered to
interact with the canonical NF-κB signaling pathway to promote
tumor progression (9–11). In the present study, the expression of
IF1 was detected in 86 glioma tissues and 20 NB tissues using
immunohistochemical staining. It was found that IF1 protein
expression in the glioma tissues was markedly increased compared
with the expression in the NB tissues. In addition, the results of
RT-qPCR indicated that the difference in IF1 mRNA expression
between the glioma and NB tissues was consistent with IF1 protein
expression. Clinical analysis revealed that the positive expression
of IF1 was evidently associated with an advanced clinical stage of
glioma. Notably, the present data revealed that the presence of IF1
expression conferred a significantly reduced overall survival rate
for patients with glioma. Multivariate Cox regression analysis
indicated that IF1 was an independent factor for predicting the
overall survival of patients with glioma. Overall, the present
results indicate that IF1 may be a potential oncogene and act as a
prognostic biomarker for predicting the survival of glioma
patients.
IF1 has previously been considered to be a crucial
factor in tumorigenesis (9–11). However, the roles of IF1 and the
IF1-mediated signaling pathways in glioma have yet to be
elucidated. In the present study, a novel role of IF1 in glioma was
revealed. IF1 was knocked down in U251 and U87 cells through the
transfection of exogenous shRNA. Transwell cell migration assays
found that IF1 knockdown led to a significant reduction in the cell
migration of U251 and U87 cells. In addition, the Transwell cell
invasion assays revealed that IF1 knockdown decreased the number of
invasive U251 and U87 cells. The present data indicated that IF1
may promote tumor progression by promoting migration and invasion
in glioma cells. An increase in NF-κB signaling is a key tumor
survival mechanism, and promotes processes involved in tumor
metastasis, including EMT, resistance to apoptosis and angiogenesis
(18). EMT is a dynamic and
reversible cellular process that is characterized by the loss of
cell polarity and intracellular junctions and the acquirement of
mesenchymal features, resulting in increased cell migration and
invasion (19). Cancer cells that
undergo EMT lead to tumor metastasis and poor survival in patients
(20). Previous studies have reported
that glioma cells with EMT exhibit enhanced invasion and metastatic
potential (21,22). Furthermore, tumor tissues that were
obtained from glioma patients were used for molecular subtyping,
and the data indicated that tumors with mesenchymal gene
characteristics conferred a reduced overall survival rate and
resistance to treatment in patients, indicating that EMT plays a
key role in the progression of glioma (19). In the present study, IF1 knockdown
inhibited the expression of NF-κB and Snai1, a key regulator of
EMT. Furthermore, IF1 knockdown led to the increased expression of
E-cadherin and reduction of vimentin expression. The current data
suggest that the NF-κB/Snai1 axis may be responsible for
IF1-mediated metastasis in glioma.
In conclusion, the present study found that the
expression of IF1 is elevated in glioma tissues and the presence of
IF1 expression is associated with an advanced clinical stage.
Furthermore, IF1 is an independent prognostic marker for predicting
the overall survival of glioma patients. IF1 knockdown decreases
the number of migratory and invasive glioma cells in glioma tissue.
The NF-κB/Snai1 axis may be involved in the IF1-mediated metastasis
of glioma. Overall, IF1 may be a potential valuable biomarker and
therapeutic target in human glioma.
References
|
1
|
Li PD, Wang XJ, Shan Q, Wu YH and Wang Z:
Evaluation of TAZ expression and its effect on tumor invasion and
metastasis in human glioma. Asian Pac J Trop Med. 7:757–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brat DJ, Scheithauer BW, Fuller GN and
Tihan T: Newly codified glial neoplasms of the 2007 WHO
classification of tumours of the central nervous system:
Angiocentric glioma, pilomyxoid astrocytoma and pituicytoma. Brain
Pathol. 17:319–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hagemann C, Fuchs S, Monoranu CM, Herrmann
P, Smith J, Hohmann T, Grabiec U, Kessler AF, Dehghani F, Löhr M,
et al: Impact of MACC1 on human malignant glioma progression and
patients' unfavorable prognosis. Neuro Oncol. 15:1696–1709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cuezva JM, Ortega AD, Willers I,
Sánchez-Cenizo L, Aldea M and Sánchez-Aragó M: The tumor suppressor
function of mitochondria: Translation into the clinics. Biochim
Biophys Acta. 1792:1145–1158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faccenda D, Tan CH, Seraphim A, Duchen MR
and Campanella M: IF1 limits the apoptotic-signalling cascade by
preventing mitochondrial remodelling. Cell Death Differ.
20:686–697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sánchez-Cenizo L, Formentini L, Aldea M,
Ortega AD, García-Huerta P, Sánchez-Aragó M and Cuezva JM:
Up-regulation of the ATPase inhibitory factor 1 (IF1) of the
mitochondrial H+-ATP synthase in human tumors mediates the
metabolic shift of cancer cells to a Warburg phenotype. J Biol
Chem. 285:25308–25313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Formentini L, Sánchez-Aragó M,
Sánchez-Cenizo L and Cuezva JM: The mitochondrial ATPase inhibitory
factor 1 triggers a ROS-mediated retrograde prosurvival and
proliferative response. Mol Cell. 45:731–742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sánchez-Aragó M, Formentini L,
Martinez-Reyes I, García-Bermudez J, Santacatterina F,
Sánchez-Cenizo L, Willers IM, Aldea M, Nájera L, Juarránz A, et al:
Expression, regulation and clinical relevance of the ATPase
inhibitory factor 1 in human cancers. Oncogenesis. 2:e462013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song R, Song H, Liang Y, Yin D, Zhang H,
Zheng T, Wang J, Lu Z, Song X, Pei T, et al: Reciprocal activation
between ATPase inhibitory factor 1 and NF-kappaB drives
hepatocellular carcinoma angiogenesis and metastasis. Hepatology.
60:1659–1673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
World Medical Association, . World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tu K, Li C, Zheng X, Yang W, Yao Y and Liu
Q: Prognostic significance of miR-218 in human hepatocellular
carcinoma and its role in cell growth. Oncol Rep. 32:1571–1577.
2014.PubMed/NCBI
|
|
14
|
Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C,
Yao Y and Liu Q: Fbxw7 is an independent prognostic marker and
induces apoptosis and growth arrest by regulating YAP abundance in
hepatocellular carcinoma. Mol Cancer. 13:1102014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C, Yang W, Zhang J, Zheng X, Yao Y, Tu
K and Liu Q: SREBP-1 has a prognostic role and contributes to
invasion and metastasis in human hepatocellular carcinoma. Int J
Mol Sci. 15:7124–7138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calzia D, Candiani S, Garbarino G, Caicci
F, Ravera S, Bruschi M, Manni L, Morelli A, Traverso CE, Candiano
G, et al: Are rod outer segment ATP-ase and ATP-synthase activity
expression of the same protein? Cell Mol Neurobiol. 33:637–649.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
ZarcoZavala M, Morales-Ríos E,
Mendoza-Hernández G, Ramírez-Silva L, Pérez-Hernández G and
García-Trejo JJ: The ζ subunit of the F1FO-ATP synthase of
α-proteobacteria controls rotation of the nanomotor with a
different structure. FASEB J. 28:2146–2157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katoh M and Katoh M: Integrative genomic
analyses of ZEB2: Transcriptional regulation of ZEB2 based on
SMADs, ETS1, HIF1alpha, POU/OCT and NF-kappaB. Int J Oncol.
34:1737–1742. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhat KP, Salazar KL, Balasubramaniyan V,
Wani K, Heathcock L, Hollingsworth F, James JD, Gumin J, Diefes KL,
Kim SH, et al: The transcriptional coactivator TAZ regulates
mesenchymal differentiation in malignant glioma. Genes Dev.
25:2594–2609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahabir R, Tanino M, Elmansuri A, Wang L,
Kimura T, Itoh T, Ohba Y, Nishihara H, Shirato H, Tsuda M and
Tanaka S: Sustained elevation of Snail promotes glial-mesenchymal
transition after irradiation in malignant glioma. Neuro Oncol.
16:671–685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan YR, Xie Q, Li F, Zhang Y, Ma JW, Xie
SM, Li HY and Zhong XY: Epithelial-to-mesenchymal transition is
involved in BCNU resistance in human glioma cells. Neuropathology.
34:128–134. 2014. View Article : Google Scholar : PubMed/NCBI
|