Introduction
Bladder cancer (BCa) is one of the most common
urologic malignancies worldwide; with a higher frequency amongst
males than females (1). The two major
environmental risk factors for BCa are high exposure to tobacco
smoke, and occupational exposure to aromatic amines, polycyclic
aromatic hydrocarbons and chlorinated hydrocarbons (2,3).
Furthermore, exposure to ionizing radiation, a variety of lifestyle
choices, occupations, dietary factors, drugs, urologic pathologies,
family history and genetic polymorphisms may increase the risk of
bladder carcinoma (1–3). There are two clinical phenotypes of
bladder carcinoma: Non-invasive and invasive BCa. Non-invasive BCa
includes the papillary carcinoma phenotype (Ta) and flat carcinoma
in situ (cis). Ta bladder carcinoma is superficial and
rarely invades the basement membrane or metastasizes, but is
associated with a high risk of local recurrence; while invasive
bladder carcinoma has a high risk of disease progression, including
muscle invasion and metastasis, and mortality. Ta tumorigenesis
typically occurs via a molecular pathway that is distinct from that
of cis and the invasive cancer phenotype (4). Superficial tumors are generally treated
with transurethral tumor resection and intravesical Bacille
Calmette-Guérin, whereas invasive tumors are aggressively resected
(5). To date, cystoscopy remains the
gold standard for the diagnosis of malignancies of the bladder. In
addition, examination by cystoscopy is required not only for
diagnosis, but also for follow-up. Cytoscopic examination is
repeated at 3-month intervals as no other method currently
available is sufficiently sensitive and specific. However,
cystoscopy is an invasive, relatively costly and uncomfortable
diagnostic method, which may also be inconclusive, particularly in
the diagnosis of cystitis. Therefore, the development of
non-invasive methods for the diagnosis of bladder carcinoma is
urgently required. Urinary or circulating biomarkers represent a
potential area of interest for the early detection and surveillance
of bladder carcinoma due to the high accessibility of samples.
Multiple tumor biomarkers have been investigated in this capacity,
with variable results (6–9).
An essential change that occurs in malignancies is
tissue invasion; however, the extracellular matrix (ECM) provides a
significant barrier to tumor cell invasion. A unique class of
matrix degrading enzymes, the matrix-metalloproteinases (MMPs), are
able to degrade certain components of the ECM and basement
membrane, facilitating tumor cell dissemination. In addition, MMPs
have key functions in the maintenance of a supportive local
environment that promotes tumor cell growth at the primary and
metastatic sites. The ability to degrade type IV collagen, the
major component of the basement membrane, is unique to MMP-2 and
-9. These MMPs are frequently associated with the malignant
phenotype of tumor cells, and their expression has been found to be
elevated in several cases of human tumors exhibiting aggressive
characteristics and low overall survival (10,11). As a
result of their potential and destructive nature, the activities of
the MMPs are highly regulated at various levels, including via
transcriptional control, secretion from cells as inactive
precursors and functional inhibition by the tissue inhibitor of
metalloproteinases (TIMPs), of which there are four: TIMP-1,
TIMP-2, TIMP-3 and TIMP-4 (12).
TIMP-1 binds and inhibits MMPs with 1:1 stoichiometry, binding
MMP-9 in particular; while TIMP-2 inactivates MMP-2 specifically.
Dysregulation of the balance between MMP and TIMP expression has
been suggested to facilitate tumor progression and recurrence in
cancer.
A previous study by our group revealed, by gelatin
zymography, that urinary MMP-2 and -9 expression was correlated
with increased MMP-9 lytic activity in high-grade and
advanced-stage bladder cancer (13).
In addition, a complex of MMP-9 and human neutrophil
gelatinase-associated lipocalin (NGAL) has been detected in the
urine of patients with prostate cancer and BCa (14). NGAL is an acute-phase protein involved
in innate immunity, with a crucial role in intracellular iron
transport (15). NGAL expression is
altered in several benign conditions, including inflammatory,
ischemic and metabolic disorders. Furthermore, NGAL is
overexpressed in numerous types of tumor (15,16), and
its expression is associated with invasive cancer progression
(17,18).
In the present study, urinary and serum levels of
MMP-2, MMP-9, TIMP-1 and TIMP-2, as well as serum concentrations of
NGAL and MMP-9/NGAL complex were evaluated in samples from 41
patients with bladder carcinoma. These levels were compared with
tumor grade and stage in order to verify whether these molecules
may offer potential as non-invasive biomarkers to provide useful
clinical information for bladder cancer disease management.
Patients and methods
Patients
A total of 41 patients were enrolled at the
Departmtent of Urology, University of Naples (Naples, Italy)
between May 2010 and 2011. Samples of first morning urine and
peripheral venous blood were collected prior to surgical or other
therapeutic interventions. Standard clinical laboratory criteria
and histopathological investigations were used to diagnose and
confirm the tumor type of each patient. Patient ages ranged from 40
to 86 years (mean, 71.1±9.3 years; median, 72 years), and in total,
there were 4 females and 37 males. The tumors were classified by
grade and stage according to pTNM classifications (19). All patients included provided written
informed consent and the study was approved by the ethics committee
of the University of Naples. A total of 40 age-matched, normal,
healthy laboratory volunteers were recruited as controls and
provided their permission verbally. Healthy volunteers exhibited no
sign of illness and their basic laboratory parameter values were
confirmed to be within reference limits.
Urine sample preparation
The MultistixCombur test (Roche Diagnostics GmbH,
Mannheim, Germany) was used to examine urine samples prior to
analysis, and any urine samples that tested positive for leukocytes
were excluded due to the presence of confounding leukocyte
gelatinases. Microscopic hematuria, which was present in the
majority of cancer samples, was not quantified; however, samples
exhibiting macroscopic hematuria were excluded. The samples were
frozen immediately following collection, and stored at −20°C prior
to analysis. For further analysis, samples were thawed and 15-ml
aliquots of each sample were centrifuged at 1,000 × g for 10 min at
4°C. The supernatant was collected and the levels of MMP-2, MMP-9,
TIMP-1 and TIMP-2 were determined by immunoassay.
Serum
Peripheral venous blood samples were collected in
vacutainers and allowed to clot for 30 min at room temperature,
prior to centrifugation at 1,600 × g for 10 min at 4°C. The samples
were then divided into aliquots and stored at −20°C prior to the
determination of MMP-2, MMP-9, TIMP-1, TIMP-2, NGAL and MMP-9/NGAL
complex levels by immunoassay. Each aliquot was used only once, in
order to prevent enzyme activation due to the freeze-thawing
processes.
Measurement of MMP-2, MMP-9, TIMP-1,
TIMP-2, NGAL and MMP-9/NGAL levels
MMP-2 and -9, as well as TIMP-1 and -2 levels were
detected by ELISA using commercial kits (Biotrak Cell Proliferation
ELISA System) obtained from GE Healthcare Life Sciences (Chalfont,
UK). These assays were based on a two-site sandwich format,
utilizing two antibodies directed against various epitopes of each
molecule. The assay for MMP-2 detected pro-MMP-2 (free pro-MMP-2
and pro-MMP-2 complexed with TIMP-2), but not the active form of
MMP-2. The assay for MMP-9 recognized pro-MMP-9 (free pro-MMP-9 and
pro-MMP-9 complexed with TIMP-1). The assay for TIMP-1 detected
free TIMP-1 and TIMP-1 complexed with MMP-9. The assay for TIMP-2
detected free TIMP-2 and TIMP-2 complexed with MMP-2. NGAL and
MMP-9/NGAL complex levels were determined with a solid-phase
immunoassay, using a commercial kit (Quantikine) from R&D
Systems (Minneapolis, MN, USA). The NGAL assay used two monoclonal
antibodies specific for two epitopes of lipocalin 2. The MMP-9/NGAL
complex assay used monoclonal antibodies against recombinant human
NGAL, and was therefore unable to detect recombinant human MMP-9 or
NGAL in their free forms. All assays were conducted according to
the manufacturer's instructions.
Statistical analysis
Descriptive statistics are provided as the mean ±
standard deviation, or as the median [range] in the case of
quantitative variables; and as frequencies (percentage) in the case
of qualitative variables. Student's t-test and the Mann-Whitney U
test were used for comparisons of numeric variables between groups.
Prognostic validity of the various biomarkers was evaluated by
receiver operating curve (ROC) curve analysis. The diagnostic
accuracy was measured using the area under the ROC curve (AUC) and
the corresponding 95% confidence interval (CI).
For all analyses, two-sided tests were used, and
P<0.05 was considered to indicate a statistically significant
difference.
All statistical analyses were performed using
statistical computing environment R software (version 3.01; R
Foundation for Statistical Computing, Vienna, Austria).
Results
During a 1-year period, urine and serum samples from
a total of 41 patients with bladder cancer were evaluated, and the
histopathological characteristics are listed in Table I.
 | Table I.Clinical characteristic of 41
patients. |
Table I.
Clinical characteristic of 41
patients.
| Characteristic | Patients, n (%) |
|---|
| Histological
grade |
|
| Low grade
(G1) | 17 (41.5) |
| High
grade (G3) | 24 (58.5) |
| Tumor stage |
|
| Ta | 13 (32.0) |
| T1 | 20 (49.0) |
| T2 or
higher | 8
(19.0) |
| Gender |
|
|
Female | 4
(10.0) |
| Male | 37 (90.0) |
Levels of MMP-2 and -9, as well as their inhibitors
TIMP-1 and -2 were determined in urine samples from patients and
normal controls. The four molecules were undetectable in the urine
samples from all normal controls. By contrast, MMP-2 and -9 were
detected in urine samples from 26 of 41 (63%) and 25 of 41 (61%)
patients with BCa, respectively. In patients with detectable MMP-2
and -9, the median urinary MMP-2 concentration was 1.27 ng/ml, and
the median urinary MMP-9 concentration was 1.30 ng/ml. By contrast,
TIMP-1 and TIMP-2 were detectable in the majority of urine samples
from patients with BCa analyzed (95 and 83%, respectively), and the
median values were 5.48 and 3.13 ng/ml, respectively. The specimens
were divided into histological groups according to grade, low grade
(G1) and high grade (G3); and according to T category, Ta, T1 and
T2 or higher (Tables II and III).
 | Table II.Descriptive statistics of measured
molecules in urine samples of 41 patients with bladder
carcinoma. |
Table II.
Descriptive statistics of measured
molecules in urine samples of 41 patients with bladder
carcinoma.
|
| Grade |
| Stage |
|---|
|
|
|
|
|
|---|
| Parameter | Low | High | P-value | Ta | T1 | P-value | ≤T1 | >T1 | P-value |
|---|
| Age,
yearsa | 68.65±11.61 | 72.79±7.11 | 0.354 | 72.85±7.78 | 69.05±10.72 | 0.328 | 70.55±9.75 | 73.25±7.56 | 0.633 |
| MMP-2
ng/mlb | 0.81 (0.33–4.92) | 1.43 (0.47–2.34) | 0.470 | 1.05 (0.33–1.81) | 1.39 (0.47–4.92) | 0.821 | 1.22 (0.33–4.92) | 1.41 (0.98–2.05) | 0.521 |
| MMP-9
ng/mlb | 1.30
(0.03–15.90) | 1.92
(0.05–21.30) | 0.461 | 0.96
(0.03–15.90) | 1.16
(0.03–21.30) | 0.485 | 1.16
(0.03–21.30) | 8.12 (0.1–10.30) | 0.680 |
| TIMP-1
ng/mlb | 1.77
(0.20–46.50) | 7.41
(0.60–139.00) | 0.022 | 1.26
(0.30–13.10) | 6.92
(0.20–139.00) | 0.040 | 4.41
(0.20–139.00) | 7.58
(2.10–44.00) | 0.247 |
| TIMP-2
ng/mlb | 3.02
(0.33–27.15) | 3.23
(0.17–35.60) | 0.868 | 2.08 (0.17–9.66) | 6.04
(0.33–35.60) | 0.074 | 3.66
(0.17–35.60) | 2.38 (1.73–9.56) | 0.624 |
 | Table III.Descriptive statistics of the
expression levels of MMP-2, MMP-9, TIMP-1, TIMP-2, NGAL and
MMP-9/NGAL complex in serum samples from 41 patients with bladder
cancer. |
Table III.
Descriptive statistics of the
expression levels of MMP-2, MMP-9, TIMP-1, TIMP-2, NGAL and
MMP-9/NGAL complex in serum samples from 41 patients with bladder
cancer.
|
| Grade |
| Stage |
|---|
|
|
|
|
|
|---|
| Expression | Low | High | P-value | Ta | T1 | P-value | ≤T1 | >T1 | P-value |
|---|
| MMP-2 ng/ml | 1152 (755–1851) | 1109 (820–1679) | 0.922 | 1115 (795–1851) | 1172 (755–1679) | 0.870 | 1120 (755–1851) | 1052 (820–1520) | 0.600 |
| MMP-9 ng/ml | 312 (87–595) | 263 (44–741) | 0.398 | 312 (105–595) | 288 (44–741) | 0.567 | 306 (44–741) | 232 (116–457) | 0.273 |
| TIMP-1 ng/ml | 164 (72–456) | 141 (78–1364) | 0.911 | 163 (72–419) | 127 (72–1364) | 0.683 | 136 (72–1364) | 159 (109–246) | 0.513 |
| TIMP-2 ng/ml | 196 (98–591) | 167 (119–307) | 0.117 | 178 (118–405) | 178 (98–591) | 0.906 | 178 (98–591) | 174 (120–242) | 0.741 |
| NGAL ng/ml | 33 (10–126) | 26 (7.2–90.2) | 0.569 | 33 (7–126) | 34 (8–90) | 0.764 | 33 (7–126) | 16 (11–39) | 0.029 |
| MMP-9/NGAL
ng/ml | 174 (60–520) | 130 (49–666) | 0.475 | 197 (49–520) | 157 (49–666) | 0.920 | 174 (49–666) | 113 (82–214) | 0.195 |
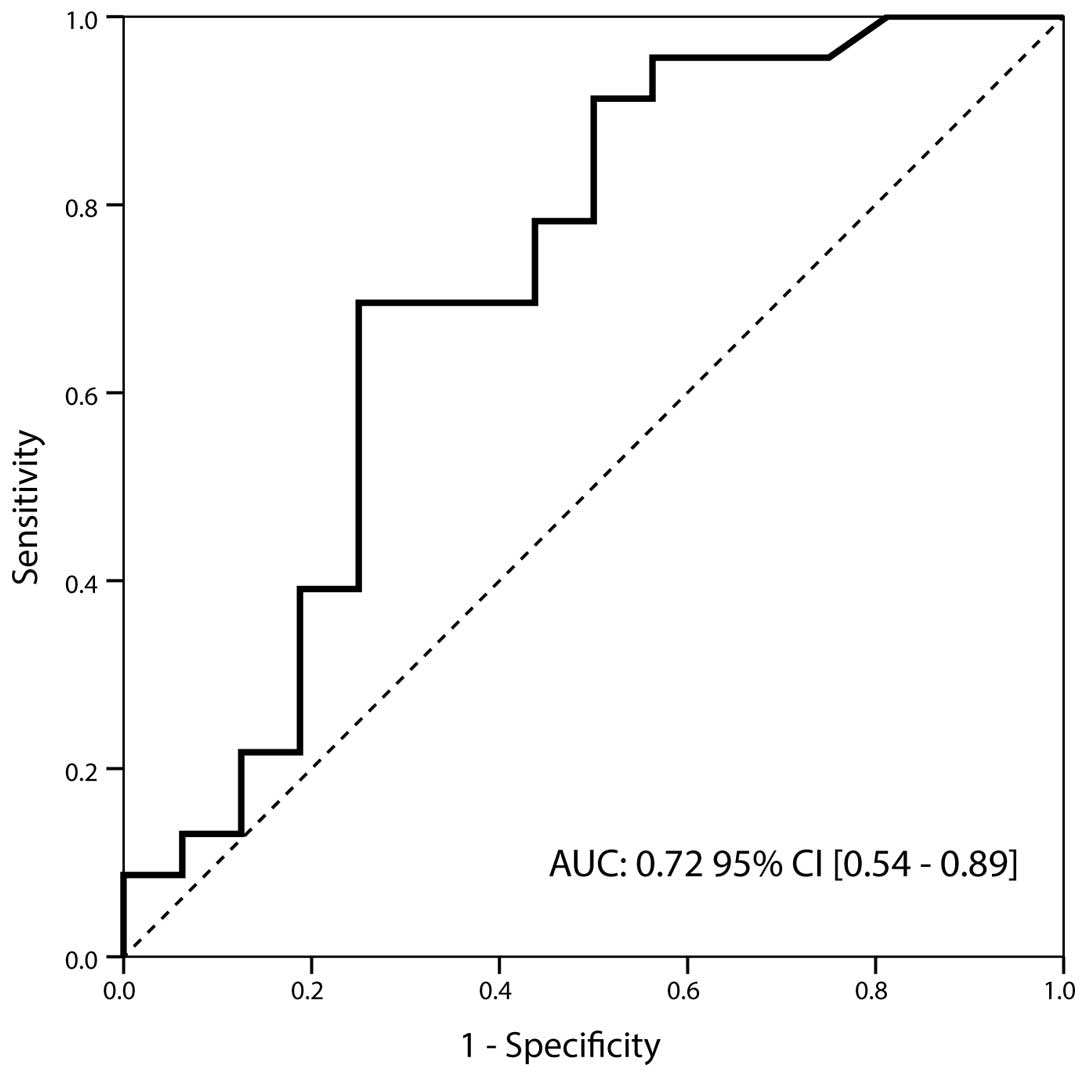
As shown in Table II,
urinary TIMP-1 values were significantly higher in the G3 group
than those of the low-grade specimens (7.41 [0.6–139] ng/ml vs.
1.77 [0.2–46.5] ng/ml; P=0.022). The corresponding AUC was 0.72
(95% CI, 0.54–0.89), with a sensitivity of 0.70 and a specificity
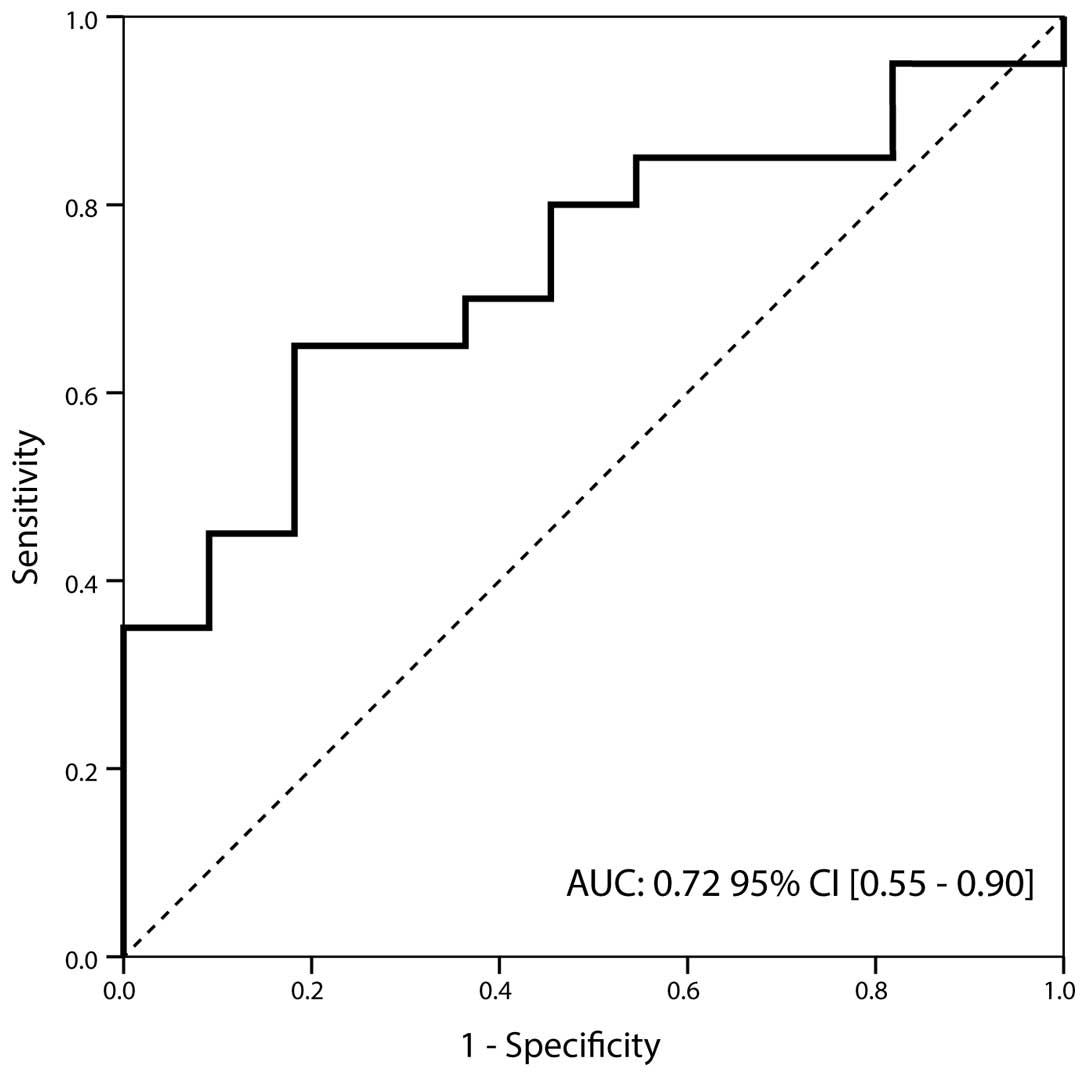
of 0.75 at a cut-off value of 4.95 ng/ml (Fig. 1). Furthermore, TIMP-1 also
demonstrated significantly different expression between Ta and T1
stage specimens (1.26 [0.3–13.1] ng/ml vs. 6.92 [0.2–139] ng/ml;
P=0.040). The corresponding AUC was 0.72 (95% CI, 0.55–0.90) with a
sensitivity and specificity of 0.70 and 0.75 at a cut-off value of
4.95 ng/ml, respectively (Fig. 2).
Conversely, no significant difference was identified in urinary
TIMP-2 concentration between low- and high-grade specimens, or
among various tumor stages.
The expression of MMP-2 and -9, their inhibitors
TIMP-1 and -2, NGAL and the MMP-9/NGAL complex were determined in
sera samples. These six molecules were detected in the sera of all
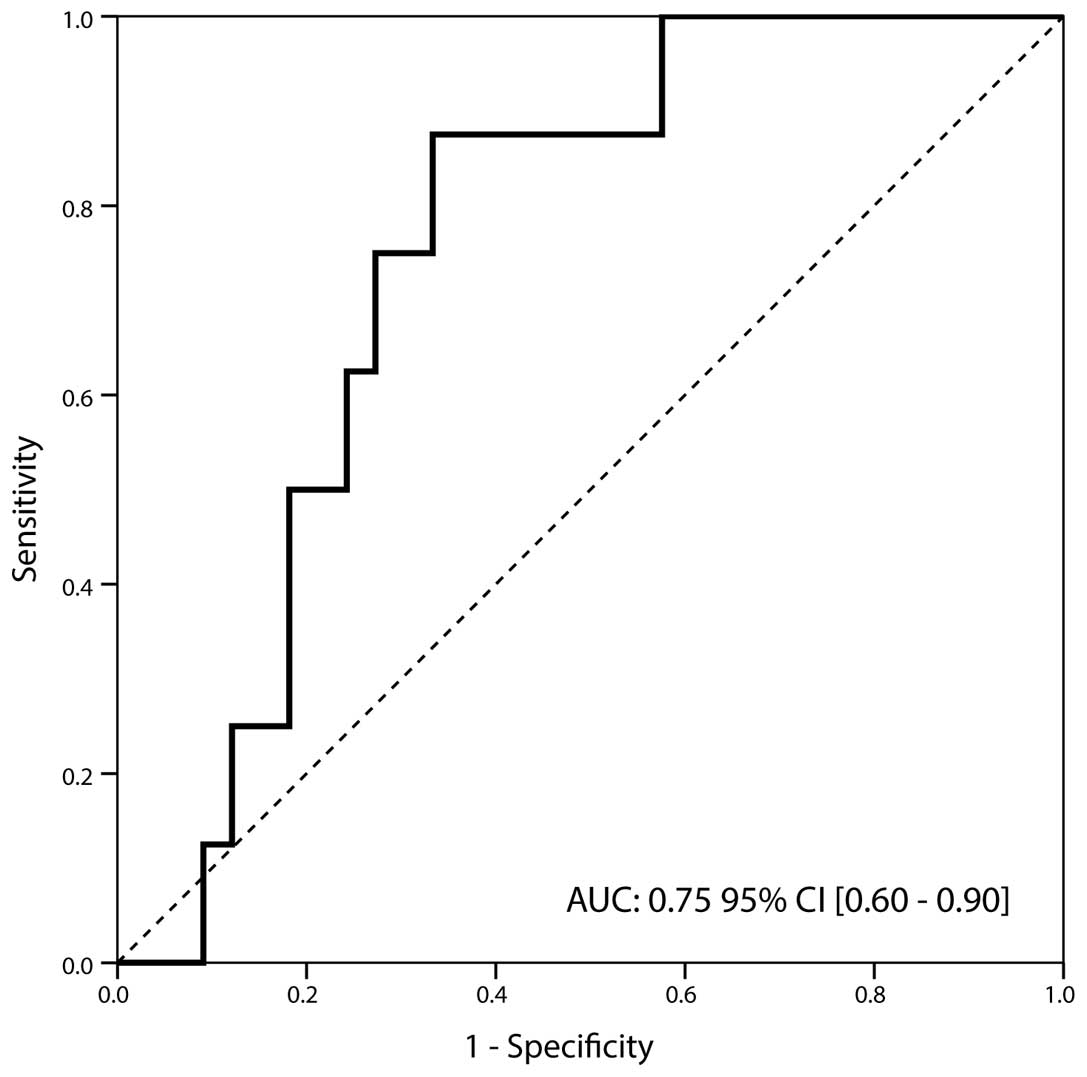
patients studied. In particular, tumors staged as non-muscle
invasive (Ta and T1) exhibited significantly higher NGAL levels
compared with those of muscle invasive BCa (32.8 [7.2–126] ng/ml
vs. 16.2 [11.2–39] ng/ml; P=0.029). The discriminatory ability of
NGAL expression was confirmed by ROC curve analysis that revealed
an AUC of 0.75, (95% CI, 0.60–0.90) and a sensitivity and
specificity of 0.88 and 0.67 at a cut-off value of ≤26.1 ng/ml,
respectively (Fig. 3).
Discussion
In BCa, the recurrence of treated tumors and the
progression of tumors to a higher stage and grade represents a
significant risk. Tumor stage and histological grade are strong
prognostic factors and provide information useful for the selection
of appropriate therapeutic modalities. However, clinical staging is
currently imperfect, resulting in significant discrepancies between
clinical and pathological stage (20). A potential strategy for the
improvement of this limitation is the identification of biomarkers
in urine or serum samples, whose levels are indicative of tumor
forms and are able to be used to monitor disease progression. The
current study aimed to detect biomarkers in the urine and serum of
patients with bladder cancer, to differentiate between low- and
high-grade tumors and/or among T stages. MMPs and their inhibitors
have frequently been investigated in human bladder tissues, and
their elevated expression in BCa tissue at the messenger RNA (mRNA)
and protein levels have previously been associated with advanced
tumor stage, grade and decreased survival rate (21). Studies have reported that TIMP-1 is a
multifunctional protein with a variety of functions independent of
MMP inhibition (22). In particular,
it has been demonstrated that TIMP-1 is able to stimulate growth in
a wide range of cell lines, including fibroblasts and epithelial
cells, and is correlated with tumor cell proliferation and
angiogenesis (23). By
immunohistochemical analysis, Miyata et al (24) revealed that TIMP-1 expression was
associated with high T stage and poor prognosis in transitional
cell carcinoma of the upper urinary tract. The results of the
present study demonstrated that urinary TIMP-1 values were able to
differentiate between low- and high-grade BCa, and between Ta and
T1 stage. To the best of our knowledge, there are currently few
publications available regarding the detection of TIMP-1 in urine
for BCa diagnosis, and these have reported variable results. In
particular, Durkan et al (25)
found that urinary TIMP-1 values were significantly higher in
patients with cancer than those of normal volunteers, with higher
values in samples from patients with T2-T4 tumors than those of
patients with cis Ta/T1 tumors; but identified no significant
variation in expression with tumor grade. By contrast, Monier et
al (26) reported that the median
value of urinary TIMP-1 was significantly lower in T1-T4 stage
patients than that observed in Ta stage specimens.
Furthermore, the present study identified an
association between NGAL serum levels and BCa stage.
Muscle-invasive BCa (>T1) was able to be differentiated from
non-muscle invasive BCa (≤T1). Until now, NGAL has been
investigated primarily as an inflammatory factor and marker of
kidney damage (15). However, recent
studies have indicated that NGAL has potential role in cancer
development and that it may have pro-oncogenic or anti-oncogenic
functions. In particular, it has been reported that the NGAL mRNA
transcript and protein levels were higher in bladder cancer tissues
than those in their normal counterparts (27). Accordingly, 50.5% of cases displayed
NGAL transcript levels higher than the 75th percentile
of the ‘normal’ values, suggesting its role as a diagnostic marker
(27). These are in agreement with
the results of previous investigations in which NGAL and MMP-9 were
overexpressed in urothelial bladder carcinomas, suggesting their
role as early diagnostic markers for this tumor type (14). Monier et al (26) found reduced protein levels of NGAL in
urine samples from patients with BCa, suggesting that reduced
levels of this protein may be used as an indicator of tumor
progression. To the best of our knowledge there have been no
previous studies regarding the detection of NGAL in the serum of
patients with bladder carcinomas. Therefore the present association
between sera NGAL and bladder tumor stage has not previously been
reported.
In conclusion, taking these observations together,
it was suggested that the determination of urinary TIMP-1 and serum
NGAL may provide clinicians with additional quantitative and
objective information regarding the differentiation of bladder
tumors. However, due to the small sample size of the present study,
these conclusions may not be transferable to the general population
and therefore require further evaluation to validate their
diagnostic potential.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: GLOBOCAN 2008, Cancer incidence and
mortality worldwide: IARC CancerBase No. 10. Lyon, France.
International Agency for Research on Cancer. 2010.PubMed/NCBI
|
|
2
|
Freedman ND, Silverman DT, Hollenbeck AR,
Schatzkin A and Abnet CC: Association between smoking and risk of
bladder cancer among men and women. JAMA. 306:737–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S and Lotan Y: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu XR: Urothelial tumorigenesis: A tale of
divergent pathways. Nat Rev Cancer. 5:713–725. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Montie JE, Bahnson RR, Cohen SM, Drucker
B, Eisenberger MA, El-Galley R, Herr HW, Hudes GR, Kuzel TM, Lange
PH, et al: National Comprehensive Cancer Network: Bladder
cancer. Clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 1:19–34. 2005.
|
|
6
|
Carlo A, Terracciano D, Mariano A, Oliva
A, D'Armiento M and Macchia V: Role of cytokeratins, nuclear matrix
proteins, Lewis antigen and epidermal growth factor receptor in
human bladder tumors. Int J Oncol. 23:757–762. 2003.PubMed/NCBI
|
|
7
|
Vrooman OP and Witjes JA: Urinary marker
in bladder cancer. Eur Urol. 53:909–916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
VanTilborg AA, Bangma CH and Zwarthoff EC:
Bladder cancer biomarkers and their role in surveillance and
screening. Int J Urol. 16:23–30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shariat SF, Passoni N, Bagrodia A,
Rachakonda V, Xylinas E, Robinson B, Kapur P, Sagalowsky AI and
Lotan Y: Prospective evaluation of a preoperative biomarker panel
for prediction of upstaging at radical cystectomy. BJU Int.
113:70–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brinckerhoff CE and Matrisian LM: Matrix
metalloproteinases: A tail of a frog that became a prince. Nat Rev
Mol Cell Biol. 3:207–214. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
DiCarlo A, Mariano A, Terracciano D,
Mazzarella C, Galzerano S, Cicalese M, Cecere C and Macchia V:
Gelatinolytic activities (matrix metalloproteinase-2 and-9) and
soluble extracellular domain of Her-2/neu in pleural effusions.
Oncol Rep. 18:425–431. 2007.PubMed/NCBI
|
|
12
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DiCarlo A, Terracciano D, Mariano A and
Macchia V: Urinary gelatinase activities (matrix metalloproteinases
2 and 9) in human bladder tumors. Oncol Rep. 15:1321–1326.
2006.PubMed/NCBI
|
|
14
|
Roy R, Louis G, Loughlin KR, Wiederschain
D, Kilroy SM, Lamb CC, Zurakowski D and Moses MA: Tumor-specific
urinary matrix metalloproteinase fingerprinting: Identification of
high molecular weight urinary matrix metalloproteinase species.
Clin Cancer Res. 14:6610–6617. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chakrabotory S, Kaur S, Guha S and Batra
SK: The multifaceted roles of neutrophil gelatinase associated
lipocalin (NGAL) in inflammation and cancer. Biochem Biophys Acta.
1826:129–169. 2012.PubMed/NCBI
|
|
16
|
DiCarlo A: Evaluation of neutrophil
gelatinase-associated lipocalin (NGAL), matrix metalloproteinase-9
(MMP-9) and their complex MMP-9/NGAL in sera and urine of patients
with kidney tumors. Oncol Lett. 5:1677–1681. 2013.PubMed/NCBI
|
|
17
|
Volpe V, Raia Z, Sanguigno L, Somma D,
Mastrovito P, Moscato F, Mellone S, Leonardi A and Pacifico F: NGAL
controls the metastatic potential of anaplastic thyroid carcinoma
cells. J Endocrinol Metab. 98:228–235. 2013. View Article : Google Scholar
|
|
18
|
Bouchet S and Bauvois B: Neutrophil
gelatinase-associated lipocalin (NGAL), pro-matrix
metalloproteinase-9 (pro-MMP-9) and their complex Pro-MMP-9/NGAL in
leukemias. Cancers (Basel). 6:796–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoboken NJ: Urological tumoursTNM
Classification of Malignant Tumors. Sobin LH, Gospodariwicz M and
Wittekind C: 7th. Wiley-Blackwell; London: pp. 262–265. 2009
|
|
20
|
Shariat SF, Palapattu GS, Karakiewicz PI,
Roger CG, Vazina A, Bastian PJ, Schoenberg MP, Lerner SP,
Sagalowsky AI and Lotan Y: Discrepancy between clinical and
pathological stage: Impact on prognosis after radical cystectomy.
Eur Urol. 51:137–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szarvas T, vom Dorp F, Ergün S and Rübben
H: Matrix metalloproteinases and their clinical relevance in
urinary bladder cancer. Nat Rev Urol. 8:241–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gomez DE, Alonso DF, Yoshiji H and
Thorgeirsson UP: Tissue inhibitors of metalloproteinases:
Structure, regulation and biological functions. Eur J Cell Biol.
74:111–122. 1997.PubMed/NCBI
|
|
23
|
Hayakawa T: Tissue inhibitors of
metalloproteinases and their cell growth-promoting activity. Cell
Struct Funct. 19:109–114. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyata Y, Kanda S, Nomata K, Hayashida Y
and Kanetake H: Expression of metalloproteinase-2,
metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in
transitional cell carcinoma of upper urinary tract: Correlation
with tumor stage and survival. Urology. 63:602–608. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Durkan GC, Nutt JE, Rajjayabun PH, Neal
DE, Lunec J and Mellon JK: Prognostic significance of matrix
metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in
voided urine samples from patients with transitional cell carcinoma
of the bladder. Clinical Cancer Res. 7:3450–3456. 2001.
|
|
26
|
Monier F, Mollier S, Guillot M, Rambeaud
JJ, Morel F and Zaoui P: Urinary release of 72 and 92 kDa
gelatinases, TIMPs, N-GAL and conventional prognostic factors in
urothelial carcinomas. Eur Urol. 42:356–363. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Candido S, Maestro R, Polesel J, Catania
A, Maira F, Signorelli SS, McCubrey JA and Libra M: Role of
neutrophil gelatinase-associated lipocalin (NGAL) in human cancer.
Oncotarget. 5:1576–1594. 2014.PubMed/NCBI
|