Introduction
Gastric cancer is the second most prevalent cause of
cancer-associated mortality worldwide (1). However, over the past 50 years, the
total incidence rates of gastric cancer have gradually decreased,
particularly in developed countries. Furthermore, the disease most
commonly occurs within the male population in developing countries,
predominantly East Asia, South America and Eastern Europe (2). Conventional therapeutic strategies for
gastric cancer include surgery, chemotherapy and radiation therapy
(3). However, as gastric cancer has
few symptoms during the early stages, the majority of patients are
typically diagnosed once the cancer has progressed to an advanced
stage. Despite undergoing surgical resection, tumors recur in a
large number of patients, in such cases the median survival time
following cytotoxic chemotherapy is <1 year. Therefore, the
diagnosis and effective treatment of advanced gastric cancer
continues to be a challenge for oncologists (4). Although the use of molecular targeted
therapy has been studied in other common types of solid tumors,
including non-small cell lung cancer and breast cancer, it has yet
to be fully explored in gastric cancer (5).
MET was initially identified as an oncogene
encoding the receptor tyrosine kinase (RTK) for hepatocyte growth
factor. The MET gene has been identified on chromosome
7q21-q31, where it encodes a single precursor that is digested and
glycosylated post-transcriptionally, resulting in an extracellular
α-chain (50-kDa) linked to a transmembrane β-chain (140-kDa) via
disulfide bonds. Oncogenic activation of MET suppresses
apoptosis and promotes cell survival, proliferation, migration and
differentiation, as well as gene transcription and angiogenesis
(6,7).
Gain-of-function mutations in MET are
uncommon in gastric cancer (8), with
MET activation predominantly attributed to gene amplification
(9). A previous used fluorescence
in situ hybridization analysis in order to detect MET
amplification, which was reported to occur in ≤4% of patients with
gastric cancer (10). Various MET
inhibitors have been investigated in clinical trials, which showed
promising initial results indicating that MET may be a potential
therapeutic target for the treatment of gastric cancer (11,12). An
increasing number of pharmaceutical companies are focusing on the
identification of novel small molecular c-MET inhibitors, including
PF2341066 (Pfizer Ltd., Surrey, UK) and ARQ197 (ArQule Inc.,
Woburn, MA, USA) (13,14). However, the identification of
drug-resistant tumors has encouraged the pre-emptive elucidation of
potential mechanisms of clinical resistance. The present study
describes a patient-derived gastric cancer model resistant to a
selective MET inhibitor and attempts to determine the underlying
mechanism.
Materials and methods
Establishment of patient-derived
gastric cancer xenograft models
Female athymic BALB/c nude mice (n=200), aged 6–7
weeks, were purchased from Shanghai Laboratory Animal Centre Co.,
Ltd. (Shanghai, China). Mice were maintained under super-specific
pathogen-free conditions and housed in barrier facilities on a 12 h
light/dark cycle, with food and water provided ad libitum.
All animal experiments were performed in accordance with protocols
approved by the Shandong Tumor Hospital Experimental Animal Care
and Use Committee. Fresh human gastric tumor specimens obtained
from 83 Chinese patients that had undergone surgery were received
from Shandong Tumor Hospital (Jinan, China) by Shandong Tumor
Hospital Experimental Animal Center (Shandong, China) within 1 h of
removal from the patients. The samples were cut into 3×3×3-mm
sections, soaked in 50% Matrigel™ (BD Biosciences, Franklin Lake,
NJ, USA) and subcutaneously implanted into the flank of the nude
mice. The tumors were passaged when the tumor volume reached ~300
mm3. Tumor volumes were calculated using the following
standard formula: Tumor volume = (length × width2)/2.
Written informed consent was obtained from all patients and the
study was approved by the ethics committee of Shandong Tumor
Hospital Experimental Animal Center.
Detection of gene copy number and
expression by microarray in established gastric cancer xenograft
models
The GeneChip® genome-wide human single nucleotide
polymorphism (SNP) 6.0 and human genome U133 plus 2.0 arrays
(Affymetrix, Inc., Santa Clara, CA, USA) were used to analyze the
genomic gene copy number and gene expression levels in all
established patient-derived gastric xenograft tumors, respectively.
MAS5 software (Affymetrix, Inc.) was used to analyze the U133
results. Gene profiling comparison was performed by calculating the
fold change of the copy number and gene expression between these
tumors. The data were processed using the aroma.affymetrix R
package (version 2.13.0; http:www.aroma-project.org/), according to the methods
described by Bengtsson et al (15).
Efficacy studies in gastric cancer
xenograft models with MET amplification and overexpression
Gastric tumors (2-cm diameter) were aseptically
resected from established patient-derived gastric cancer xenografts
with MET amplification and overexpression, then minced into
3×3×3 mm pieces. Host mice were then anesthetized with isoflurane
and a section of tumor was implanted into the left flank of each
mouse. Each gastric model that developed tumors reaching 150–200
mm3 in size were randomized into the following four
treatment groups (10 mice per group): Group 1, once-daily dose with
vehicle by intravenous (i.v.) tail injection; and groups 2, 3 and
4, once-daily dose with 10, 20 and 30 mg/kg PHA665752 by i.v. tail
injection, respectively. PHA665752, a selective MET inhibitor, was
purchased from Selleck Chemicals (Houston, TX, USA). In a
subsequent experiment, the CNGAS028 model was also treated with
vehicle, 15 mg/kg PHA665752, the pan-fibroblast growth factor
receptor (FGFR2) selective inhibitor NVP-BGJ398 (15 mg/kg
once-daily, oral administration; Selleck Chemicals) or 30 mg/kg
PHA665752 in combination with 15 mg/kg NVP-BGJ398, respectively.
All treatments were continued for 21 days and the mice were
sacrificed by CO2 inhalation 2 h after the last
treatment.
Western blot analysis
The tumor tissues were resected 2 h following the
final treatment with PHA665752 or/and NVP-BGJ398 on day 21 of the
efficacy studies. The tumor tissues were then homogenized and lysed
in cell lysis buffer (Bio-Rad Laboratories, Hercules, CA, USA)
containing phosphatase inhibitor cocktail and proteinase inhibitor
cocktail (Sigma-Aldrich, St. Louis, MO, USA), and the protein
concentrations were determined using the bicinchoninic acid protein
assay kit (Pierce Biotechnology, Inc. Rockford, IL, USA).
Subsequently, equal quantities of protein (30 µg) were separated by
sodium dodecyl sulfate/polyacrylamide gel electrophoresis on 8%
gels, blotted on polyvinylidene difluoride membranes (Invitrogen
Life Technologies, Inc., Carlsbad, CA, USA), then probed with
monoclonal phosphorylated (p)-MET (1:1,000; cat. no. 3126),
polyclonal p-FGFR2 (1:1,000; cat no. af3285; R&D Systems, Inc.,
Minneapolis, MN, USA), monoclonal MET (1:1,000; cat. no. 4560) and
monoclonal FGFR2 (1:1,000; cat. no. 11835) rabbit anti-human
antibodies. Subsequently, the membranes were incubated with goat
anti-rabbit horseradish peroxidase-conjugated secondary antibodies
(1:1,000; cat. no. 7074) and detected by chemiluminescence. Gel
Doc™ XR+ (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used
to visualize the western blots. All antibodies were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA), unless
otherwise stated.
Statistical analysis
All data are presented as the mean ± standard
deviation for the indicated number of independently performed
experiments. Statistical analyses were conducted using GraphPad
InStat software (version 5.0; GraphPad Software, Inc., San Diego,
CA, USA). Student's t tests were performed and P<0.05 was
considered to indicate a statistically significant difference.
Results
MET gene amplification and expression
in Chinese patient-derived gastric cancer models
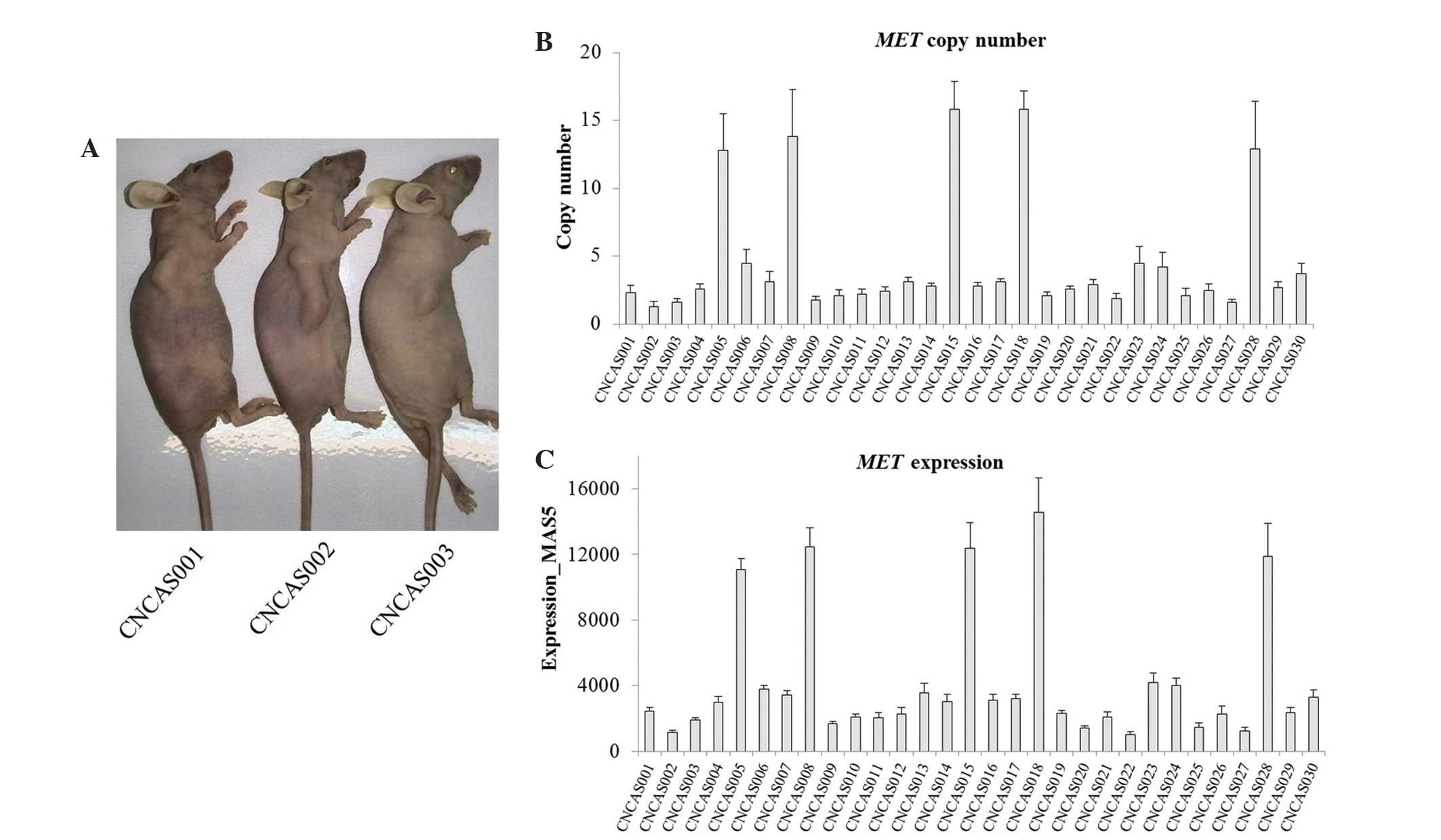
Chinese patient-derived gastric cancer models (n=30)
were established from 83 gastric cancer specimens. The established
models were termed CNGAS001-030. The CNGAS001, CNGAS002 and
CNGAS003 mouse models are indicated in Fig. 1A. Microarray data from the SNP 6.0 and
U133 plus 2.0 gene chips were used to analyze the genomic gene copy
number and gene expression levels of all established models,
respectively. The microarray data demonstrated that MET was
highly amplified and expressed in 16.7% (5/30) of the Chinese
gastric cancer xenograft models (Fig. 1B
and C). From the results, it was observed that MET
amplification was positively correlated with MET
overexpression in the xenograft models.
High amplification and overexpression
of MET predicts response to PHA665752 in patient-derived gastric
cancer models
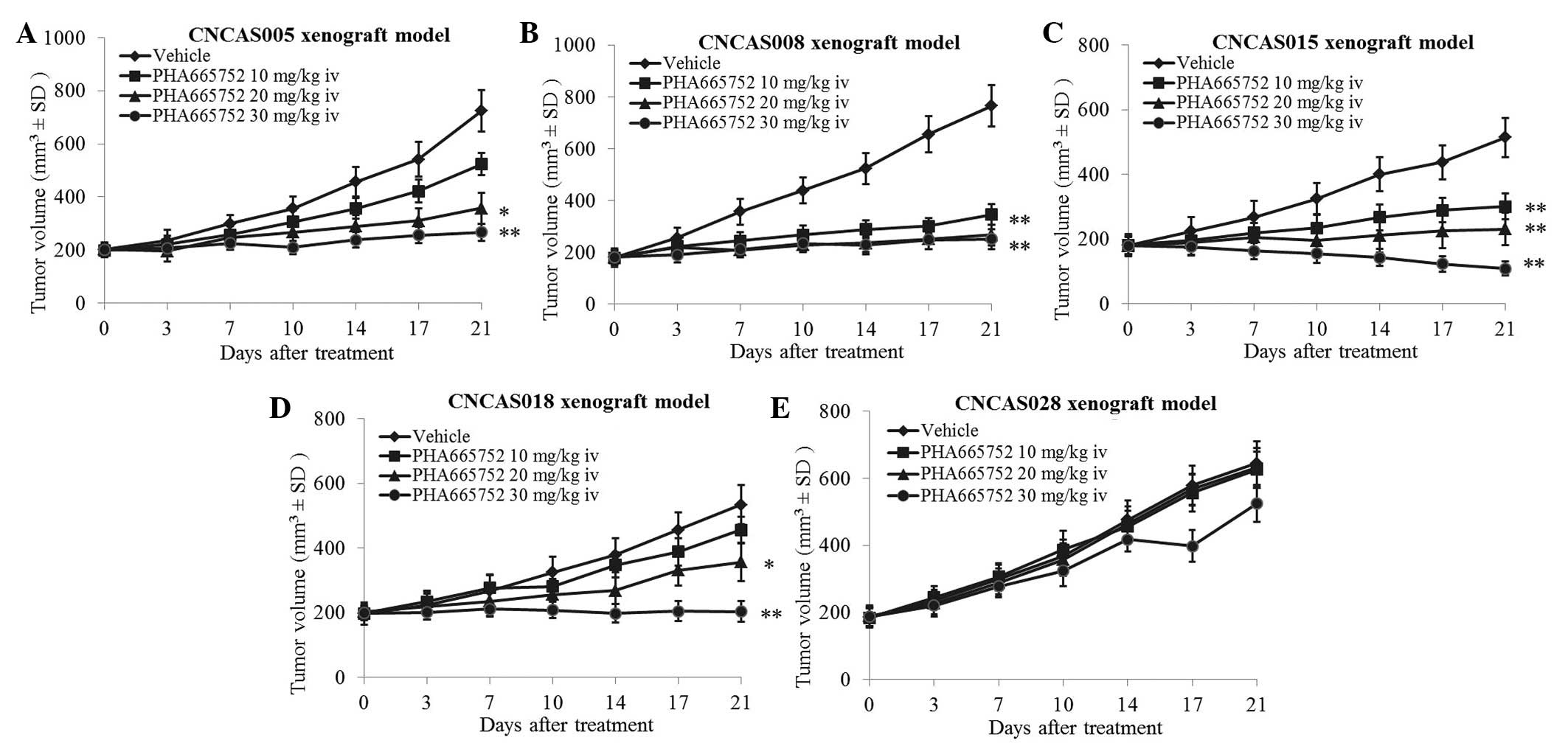
The present study analyzed the efficacy PHA665752 in
the patient-derived gastric xenograft models with high MET
amplification and overexpression (CNCAS005, CNCAS008, CNCAS015,
CNCAS018 and CNCAS028). The results demonstrated that four gastric
cancer xenograft models were significantly sensitive to PHA665752
treatment (P<0.05). However, the CNCAS028 model was resistant to
PHA665752 (Fig. 2A–E; 30 mg/kg i.v.
PHA665752 treatment group: P=0.008, P=0.006, P=0.004, P=0.007 and
P=0.125, for the CNCAS005, 008, 015, 018 and CNCAS028 xenograft
models, respectively).
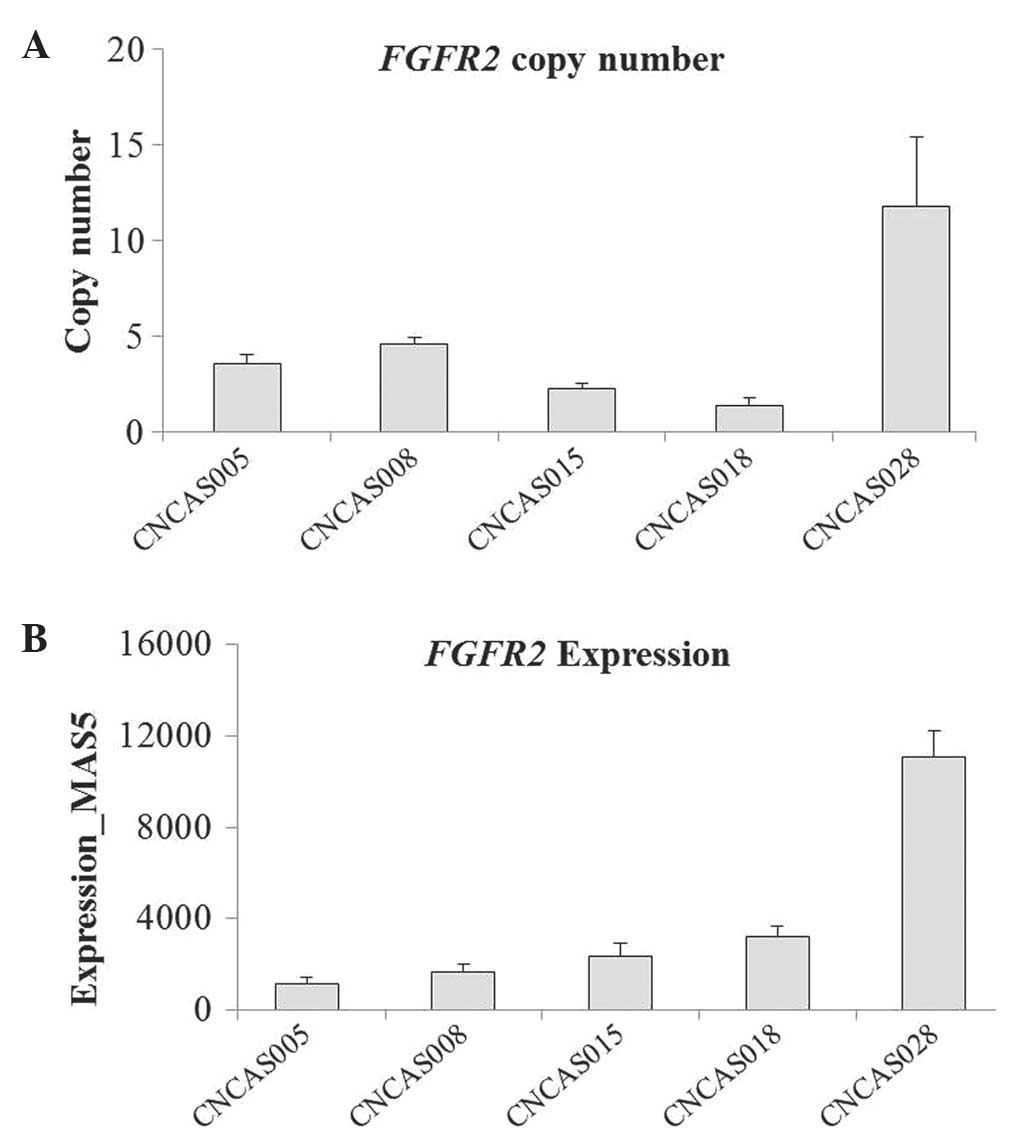
High FGFR2 amplification and
expression in the CNCAS028 model
As indicated in Fig.
2A, the tumor growth of CNCAS028 was not inhibited by treatment
with 30 mg/kg PHA665752 for 21 days. To explore the mechanism of
the resistance to the selective MET inhibitor, the genome-wide gene
profiles of CNCAS028 were compared with those of the other four
gastric cancer models. It was identified that FGFR2 was
highly amplified and expressed in the CNCAS028 model, whereas
FGFR2 was expressed at a normal level and not amplified in
the PHA665752-sensitive xenografts [normal FGFR2 expression,
copy number <5 and expression level <4,000 (as determined by
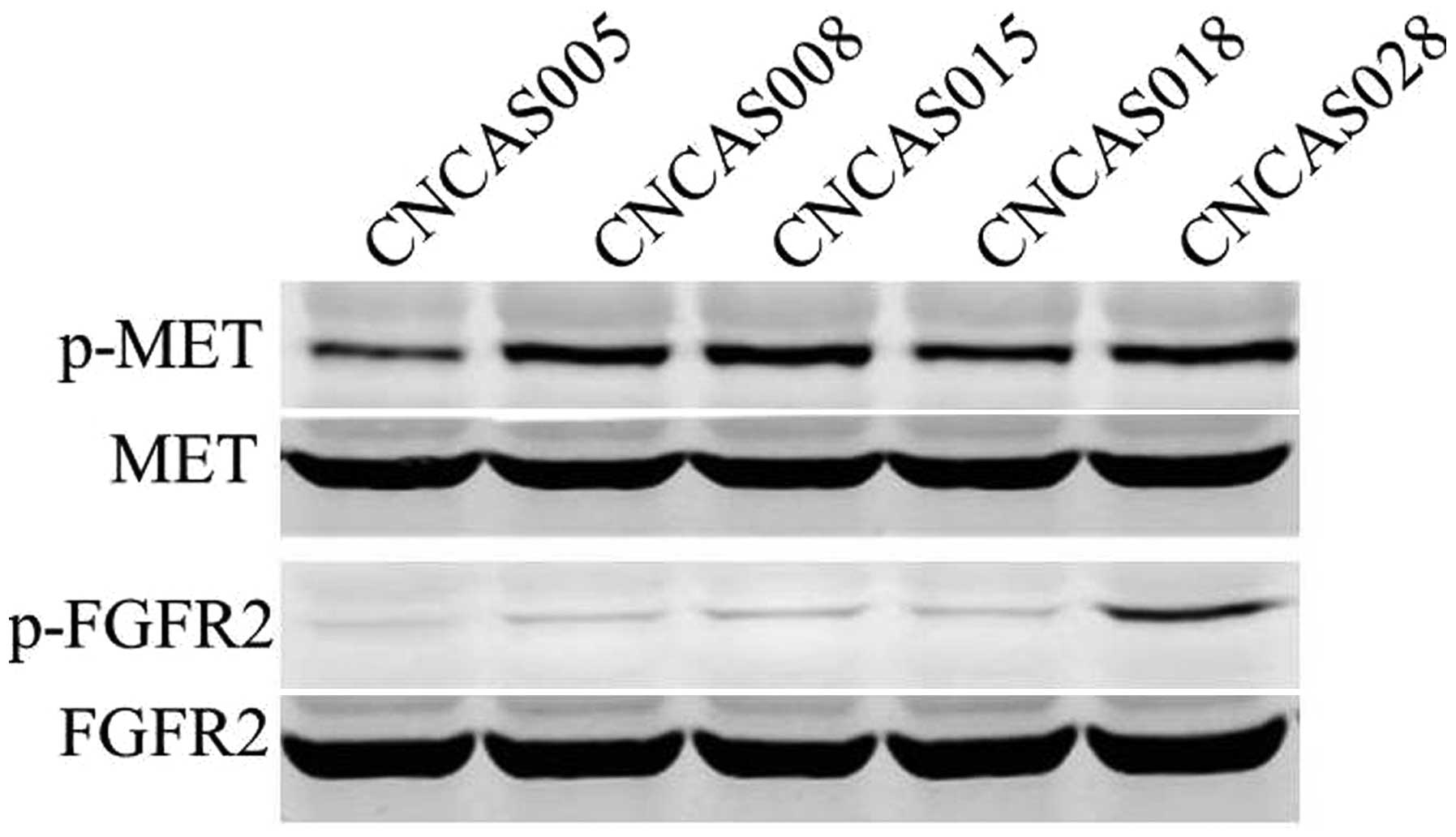
GeneChip®); Fig. 3A and B]. These
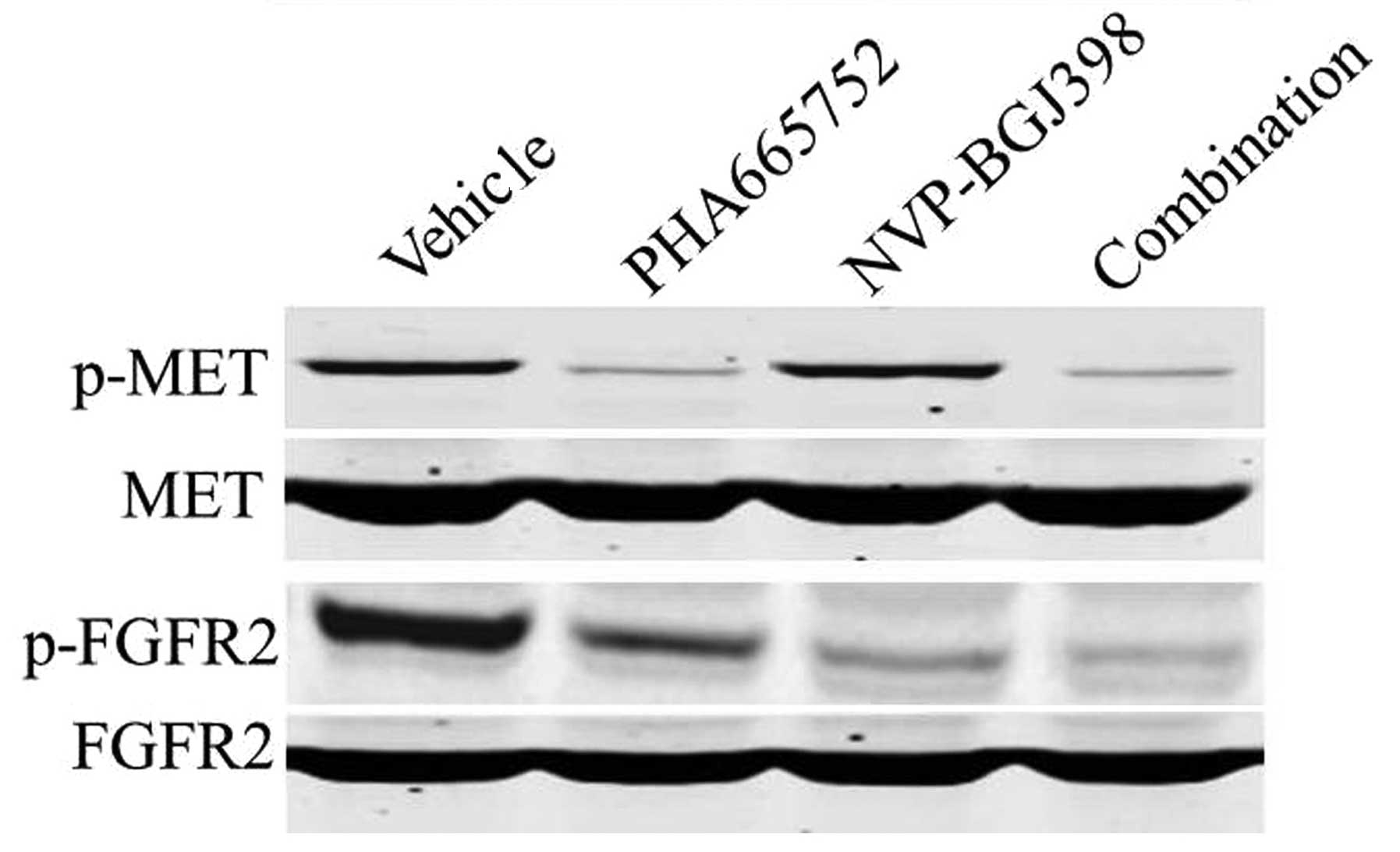
results were confirmed by western blot analysis (Fig. 4).
PHA665752 and NVP-BGJ398 combination
treatment significantly inhibits tumor growth in the CNCAS028
model
To validate the association between FGFR2
amplification and overexpression as well as PHA665752 resistance,
PHA665752 was combined with a selective pan-FGFR2 kinase inhibitor
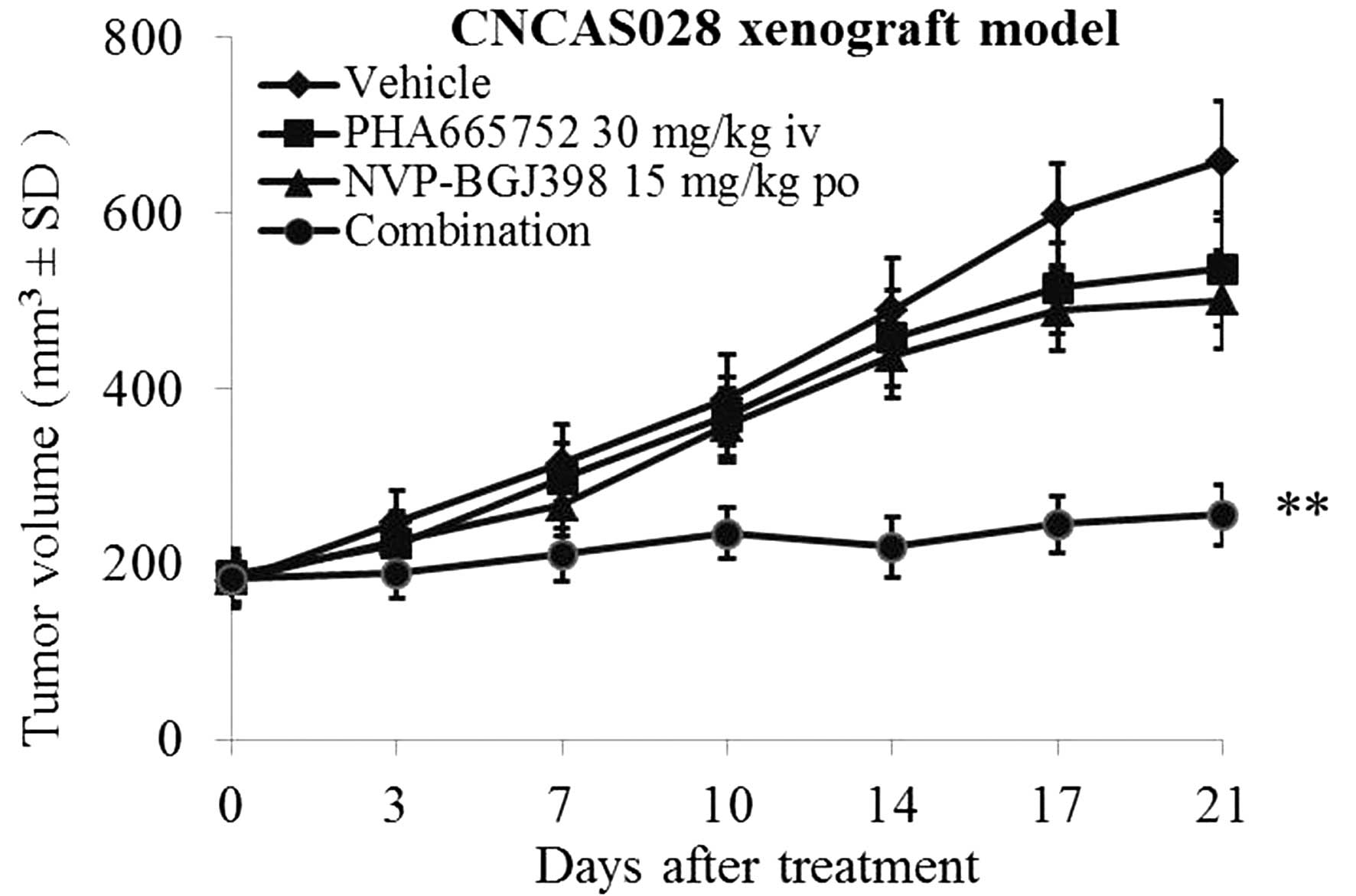
(NVP-BGJ398) to treat the CNCAS028 model (16). As indicated in Fig. 5, treatment with 30 mg/kg PHA665752 was
not able to inhibit tumor growth in the CNCAS028 model.
Furthermore, treatment with 15 mg/kg NVP-BGJ398 only marginally
inhibited tumor growth. By contrast, combined treatment with these
two compounds significantly inhibited tumor growth following 21
days of treatment (P<0.01).
Effect of PHA665752 and/or NVP-BGJ398
on signaling transduction in patient-derived gastric cancer
models
To investigate the effect of PHA665752 and
NVP-BGJ398 on downstream molecules of the phosphoinositide 3-kinase
and RAS signaling pathways, western blot analysis was used to
observe changes in the phosphorylation status and total protein
expression levels of the tumor tissues. The results demonstrated
that all five patient-derived gastric xenograft models highly
expressed p-MET and the CNCAS028 xenograft also highly expressed
p-FGFR2 (Fig. 4). Western blot
analysis also identified that treatment with PHA665752 inhibited
the phosphorylation of MET in all five gastric tumor models. In
addition, the expression of p-FGFR2 was markedly inhibited by
NVP-BGJ398 or combination treatment in the CNCAS028 model (Fig. 6).
Discussion
Gastric and gastroesophageal cancer affect 1 million
individuals worldwide every year and are the second most common
cause of cancer-associated mortality (17). Targeted therapies have been developed
and incorporated into the standard treatment strategies for other
types of solid cancer, such as lung or breast. However, such
therapies (including MET inhibitors) are only now being examined in
the context of gastric and gastroesophageal cancer (18). The current investigations identified
MET gene amplification in 5/30 (16.7%) patient-derived
gastric cancer xenografts. These results indicated the therapeutic
potential of MET inhibitors in gastric cancer. Additional analysis
identified that a selective MET inhibitor (PHA665742) was able to
significantly inhibit tumor growth in 4/5 gastric cancer models
with MET amplification. These results are consistent with a
previous study, which demonstrated that MET amplification
was associated with the response of the MKN45 gastric cancer cell
line to PHA665752 treatment (19).
Aberrant RTK expression produces growth and survival
signals that are essential for the pathogenesis and progression of
various types of cancer. Furthermore, cancer patients treated with
targeted inhibitors of key oncogenic kinase drivers, including
imatinib, gefitinib and erlotinib, have exhibited promising
clinical outcomes (20). However,
based on the precedence set by agents such as imatinib in chronic
myeloid leukemia and erlotinib in lung adenocarcinoma, inherent
resistance may potentially limit the application of single agent
therapies (21). The elucidation of
novel oncogenic drivers may have extensive implications for
targeted therapy. Corso et al (22) reported that activation of HER family
members in MET-addicted cancer cells, subsequent to MET
inactivation, resulted in increased cell viability in vitro
and recovered tumorigenicity in vivo. In addition, Lee et
al (23) reported that a novel
SND1-BRAF fusion gene exhibited resistance to the MET
inhibitor PF-04217903 in GTL16 cells via RAS/RAF/ERK signaling
pathway activation. By contrast, the current study determined that
a MET-amplified CNCAS028 model was resistant to MET
inhibitor PHA66575 as a result of FGFR2 gene amplification
and overexpression. Inhibition of FGFR2 signaling in this xenograft
model recovered its sensitivity to PHA665752.
In conclusion, MET and FGFR2 coactivation may
increase resistance to targeted therapy, possibly due to activation
of multiple growth and survival signaling pathways. These findings
indicate that combination therapy with MET and FGFR2 inhibitors may
be a promising strategy to treat inherent resistance to MET
inhibitors in cases of gastric cancer harboring MET and
FGFR2 amplification. Future studies should be performed to
investigate whether similar results could be obtained in an
acquired resistant model.
Acknowledgements
The present authors would like to thank Dr Jianshe
Wu and Dr Yanlin Wu for conducting the bioinformatic analysis of
the GeneChip® genome-wide human single nucleotide polymorphism 6.0
and human genome U133 plus 2.0 array data.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Camargo MC, Anderson WF, King JB, Correa
P, Thomas CC, Rosenberg PS, et al: Divergent trends for gastric
cancer incidence by anatomical subsite in US adults. Gut.
60:1644–1649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamashita K, Sakuramoto S, Nemoto M,
Shibata T, Mieno H, Katada N, et al: Trend in gastric cancer: 35
years of surgical experience in Japan. World J Gastroenterol.
17:3390–3397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meyer HJ and Wilke H: Treatment strategies
in gastric cancer. Dtsch Arztebl Int. 108:698–705. 2011.PubMed/NCBI
|
|
5
|
Morishita A, Gong J and Masaki T:
Targeting receptor tyrosine kinases in gastric cancer. World J
Gastroenterol. 20:4536–4545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maestrini E, Tamagnone L, Longati P,
Cremona O, Gulisano M, Bione S, et al: A family of transmembrane
proteins with homology to the MET-hepatocyte growth factor
receptor. Proc Natl Acad Sci USA. 93:674–678. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sattler M and Salgia R: c-Met and
hepatocyte growth factor: Potential as novel targets in cancer
therapy. Curr Oncol Rep. 9:102–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JH, Han SU, Cho H, Jennings B, Gerrard
B, Dean M, et al: A novel germ line juxtamembrane Met mutation in
human gastric cancer. Oncogene. 19:4947–4953. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakajima M, Sawada H, Yamada Y, Watanabe
A, Tatsumi M, Yamashita J, et al: The prognostic significance of
amplification and overexpression of c-met and c-erb B-2 in human
gastric carcinomas. Cancer. 85:1894–1902. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hara T, Ooi A, Kobayashi M, Mai M,
Yanagihara K and Nakanishi I: Amplification of c-myc, K-sam and
c-met in gastric cancers: detection by fluorescence in situ
hybridization. Lab Invest. 78:1143–1153. 1998.PubMed/NCBI
|
|
11
|
Sierra JR and Tsao MS: c-MET as a
potential therapeutic target and biomarker in cancer. Ther Adv Med
Oncol. 3 Suppl:S21–S35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawakami H, Okamoto I, Arao T, Okamoto W,
Matsumoto K, Taniguchi H, et al: MET amplification as a potential
therapeutic target in gastric cancer. Oncotarget. 4:9–17.
2013.PubMed/NCBI
|
|
13
|
Christensen JG, Zou HY, Arango ME, Li Q,
Lee JH, McDonnell SR, et al: Cytoreductive antitumor activity of
PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and
c-Met, in experimental models of anaplastic large-cell lymphoma.
Mol Cancer Ther. 6:3314–3322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abidoye O, Murukurthy N and Salgia R:
Review of clinic trials: agents targeting c-Met. Rev Recent Clin
Trials. 2:143–147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bengtsson H, Irizarry R, Carvalho B and
Speed TP: Estimation and assessment of raw copy numbers at the
single locus level. Bioinformatics. 24:759–767. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pietrantonio F, Maggi C, Di Bartolomeo M,
Facciorusso MG, Perrone F, Testi A, et al: Gain of ALK gene copy
number may predict lack of benefit from anti-EGFR treatment in
patients with advanced colorectal cancer and RAS-RAF-PI3KCA
wild-type status. PLoS One. 9:e921472014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oshima T and Masuda M: Molecular targeted
agents for gastric and gastroesophageal junction cancer. Surg
Today. 42:313–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Funakoshi Y, Mukohara T, Tomioka H,
Ekyalongo RC, Kataoka Y, Inui Y, et al: Excessive MET signaling
causes acquired resistance and addiction to MET inhibitors in the
MKN45 gastric cancer cell line. Invest New Drugs. 31:1158–1168.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jänne PA, Gray N and Settleman J: Factors
underlying sensitivity of cancers to small-molecule kinase
inhibitors. Nat Rev Drug Discov. 8:709–723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
SeboltLeopold JS and English JM:
Mechanisms of drug inhibition of signalling molecules. Nature.
441:457–462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Corso S, Ghiso E, Cepero V, Sierra JR,
Migliore C, et al: Activation of HER family members in gastric
carcinoma cells mediates resistance to MET inhibition. Mol Cancer.
9:1212010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee NV, Lira ME, Pavlicek A, Ye J, Buckman
D, Bagrodia S, et al: A novel SND1-BRAF fusion confers resistance
to c-Met inhibitor PF-04217903 in GTL16 cells though [corrected]
MAPK activation. PLoS One. 7:e396532012. View Article : Google Scholar : PubMed/NCBI
|