Introduction
Urothelial carcinoma (UC) of the urinary bladder is
a relevant social problem with almost 67,160 novel cases diagnosed
each year and accounts for ~13,750 cancer-associated mortalities
each year in the United States (1).
Mhawech-Fauceglia et al (2)
suggested that the pathology of bladder carcinoma was a difficult
to fully elucidate due to the complex oncogenic pathways involved
and the inconsistent clinical behaviour of the disease.
Numerous previous studies have identified potential
molecular markers for bladder carcinoma in an effort to fully
elucidate the cellular mechanisms involved in its pathogenesis and
development. Consequently, the prediction of tumour biological
potential may help to select patients for treatment, thus improving
the survival rates and quality of life of these patients (3–5). Tumour
genome analysis has been used to obtain information regarding the
natural history of UC (6). Of note,
the loss of heterozygosis (LOH) on chromosome (Chr) 18 was reported
to be the initial genetic event in bladder cancer (7).
As tumour-suppressor genes, including the DCC
(18q21.3) and DPC4 (18q21.1) genes were identified on Chr 18q
(8), it was suggested that somatic
alterations on this chromosome may be a critical step for bladder
carcinogenesis. In addition, it was reported that loss or
inactivation of the SMAD4/DPC4 gene may be involved in the onset of
various types of cancer, while LOH of 18q21.1 was demonstrated to
be associated with a poor prognosis in bladder carcinoma patients
(8). The distal section of Chr 18
(18q21-q23) was considered to be a potential locus of numerous
crucial genes for the pathogenesis and progression of bladder
cancer. A previous study highlighted the role of LOH analysis of
Chr 18 for enhancing the prediction of recurrence in patients with
low-grade non-muscle-invasive bladder cancer (4); however, further studies are required.
Notably, the D18S51, MBP LW and MBP H loci are located on the long
arm of Chr 18, where a number of tumor suppressor genes, including
DCC and DPC4, are located (6).
The present study aimed to evaluate LOH on Chr 18 in
patients with muscle-invasive urothelial bladder cell carcinomas in
order to determine whether there is an association between LOH on
Chr 18 and tumour stage. In addition, the present study aimed to
investigate whether LOH on Chr 18 has a role in predicting the
clinical outcome of patients with muscle-invasive urothelial
bladder cancer.
Materials and methods
Study design
LOH on Chr 18 was investigated in muscle-invasive UC
of the urinary bladder and the findings were then correlated with
patient follow-up data. A total of 34 consecutive patients were
recruited for the present prospective study who underwent a
transurethral resection of bladder tumour (TURBT) at the Department
of Urology, University of Florence (Florence, Italy), between
January and December 2002. All patients enrolled in the present
study were diagnosed with muscle-invasive urothelial bladder cancer
[MIBC; ≥primary tumour stage (pT)2, according to the European
Association of Urology guidelines] and were able to comply with the
follow-up schedules. Patients were excluded if they had a history
of UC of the upper urinary tract, prostate cancer or other urologic
cancer; in addition, patients with associated carcinoma in situ
were omitted from the present study. All patients with other
urologic diseases were also excluded, as were all patients lost to
follow-up. All selected patients provided written informed consent
and the study was approved by the research ethics committee of the
University of Florence. The present study was conducted in
accordance with the latest version of the Declaration of Helsinki
(2008) and in line with Good Clinical Practice guidelines (9).
Specimen collection, histological and
molecular analysis
Blood samples were collected from all patients
during clinical evaluation and stored at −80°C until molecular
analysis. Fresh tumour tissue samples were obtained during TURBT
for pathological evaluation and were snap-frozen in liquid nitrogen
in the operating room and stored at −80°C until molecular analysis.
Tumour and normal DNA were extracted using the methods previously
described (5). Briefly, the DNA of
each sample underwent digestion with sodium dodecyl sulfate
proteinase K (recombinant; Worthington Biochemical Corporation,
Lakewood, NJ, USA; 55°C overnight), ribonuclease (Multiplex PCR
Kit; Qiagen Spa, Milan, Italy) treatment (2 h at 37°C) and phenol
chloroform (Life Technologies Italia, Monza, Italy) extraction. DNA
was resuspended in 700 ml Tris EDTA (Life Technologies Italia). The
quantity of DNA was evaluated by spectrophotometry optical density
(NanoDrop® 1000UV spectrophotometer; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA), and the samples were diluted to a
concentration of 4 ng/µl for amplification by polymerase chain
reaction (PCR). PCR was performed in a final volume of 15 ml with
10 ng DNA in order to amplify genomic DNA at three specific Chr 18
loci: D18S51, MBP LW and MBP H. The primer sequences were as
follows: Forward, 5′-TTC TTG AGC CCA GAA GGT TA-3′ and reverse,
5′-ATT CTA CCA GCA ACA ACA CAA ATA AAC-3′ for D18S51; forward,
5′-TGG CTA CTT GGG CTA TTG TAA ACG-3′ and reverse, 5′-GGT GGT TCT
GTT CCC TCT ATC TCC-3′ for MBP LW; and forward, 5′-TCC GAG CAG CAG
CCA GCA C-3′ and reverse, 5′-AAG CTC GTC GGA CTC TGA G-3′ for MBP
H. Each primer was fluorescently marked with the dyes 6-FAM, NED
and HEX (Applied Biosystems Life Technologies, Foster City, CA,
USA). Normal and pathological DNA underwent amplification in an
Applied Biosystems® 2720 Thermal Cycler (Life Technologies Italia);
three PCRs were performed per patient. The amplification conditions
of the reactions were a denaturation cycle at 95°C for 10 min, 32
cycles at 95°C for 30 sec, 56°C for D18S51 and 52°C for MBP LW and
MBP H, for 30 sec, and 72°C for 1 min, and final extension at 72°C
for 45 min. DNA quality was assessed by agarose gel and ethidium
bromide staining (Life Technologies Italia). Amplification products
(1 µl) were mixed with 12 µl formamide (molecular biology grade;
Life Technologies Italia) and 0.5µl GeneScan ROX400 (Perkin Elmer
Biosystems, Foster City, CA, USA), denatured at 95°C for 10 min and
then rapidly iced. Up to 48 samples were then electrophoresed in an
ABI Prism® 310 Genetic Analyzer (Life Technologies Italia).
Amplification products (50 µl) were injected electrokinetically in
a 47 cm capillary (Applied Biosystems Life Technologies) filled by
performance optimized polymer 4 (Life Technologies Italia).
Electrophoresis was conducted at 15 kV for 24 min at 60°C and
electrodes were immersed in ABI Prism® 310 Genetic Analyzer Buffer
(Life Technologies Italia). Numerical data collected during
electrophoresis were analyzed by GeneScan software (version 3.1;
Thermo Fisher Scientific, Inc.) to produce graphic and numerical
results of dimension of amplified DNA fragments. The microsatellite
markers employed in the present study and their locations were
taken from the Genome Database (http:www.ncbi.nlm.nih.gov/genome). The primers for the
MBP gene repeats also amplified two short tandem repeat loci (locus
A and locus B) (10). Microsatellite
sequences observed to be most frequently altered in previous
studies (6,11,12) were
selected for the present study. LOH was characterised as the
complete or almost complete absence of one allele in tumour DNA.
All molecular tests were performed in duplicate using isolated PCR
reactions. All available haematoxylin and eosin-stained slides of
bladder carcinomas were reviewed by one pathologist. Specimens were
pathologically staged according to the 2010 Tumour-Node-Metastasis
classification of the American Joint Committee on Cancer (13) and tumour grade was assigned in line
with the 2004 World Health Organization/International Society of
Urologic Pathology classification (14).
Patients
Following the diagnosis of MIBC, all patients
underwent radical cystectomy with standard bilateral pelvic
lymphadenectomy and urinary diversion. Radical cystectomy was
performed by an expert uro-oncology consultant according to the
procedure described by the International Consultation on Bladder
Cancer (15). The lymph node
dissection performed involved the excision of all lymphatic tissues
surrounding the common iliac, external iliac, internal iliac and
obturator arteries as well as the presacral region. Following
cystectomy and urinary diversion, all patients underwent follow-up
examinations, which were performed according to the European
Association of Urology guidelines (16). In brief, chest x-rays and abdominal
ultrasound were required every 3 months, computerised tomography of
the abdomen every 6 months and bone scan and excretory urography
every 12 months. Additional examinations were required for
symptomatic disease (16).
Concomitant urethrectomy was performed only in patients who had
preoperative histologically proven UC of the prostate and/or
urethra in association with MIBC (16).
Statistical analysis
As the null hypothesis, LOH on Chr 18 was assumed to
have no impact on survival rate in patients with MIBC. The
significance of all parameters was assessed using the Fisher's
exact test and chi-square test, where P<0.05 was considered to
indicate a significant difference between values. The 95%
confidence intervals (CIs) were calculated for the probability of
survival for the Kaplan-Meier estimates. Mann-Whitney tests were
also used in order to compare the means of the different
parameters. Univariate and multivariate relative risk was
calculated using Cox proportional hazards regression analysis. SPSS
11.5 for Apple-Macintosh (SPSS, Inc., Chicago, IL, USA) was used to
perform all statistical analyses.
Artificial Neural Network (ANN) analysis was
employed in order to enhance the standard statistical analysis, as
previously described (17,18). In brief, a neural network was used in
order to predict the outcome of patients with MIBC, according to
the findings of the univariate and multivariate analyses. The
following factors were the input parameters (input neuron) for ANN
analysis: Age, gender, number of lesions, diameter of lesions, LOH
on Chr 18, stage and grade. ANN setup was performed using the
commercially available NeuralWorks Predict software (2005;
NeuralWare Inc., Carnegie, PA, USA).
Results
Patient and tumour
characteristics
A total of 34 patients were enrolled in the present
study, 32 of which were male and 2 were female (mean age,
69.9±8.7). The patient and tumour characteristics are detailed in
Table I.
 | Table I.Summary of clinical and
histopathological patient data. |
Table I.
Summary of clinical and
histopathological patient data.
| A, Patient
characteristics |
|
|---|
|
|---|
| Characteristic | n (%) |
|---|
| No. of patients | 34 |
| Mean age ± standard
deviation | 69.9±8.7 |
| Gender |
|
| Male | 32 (94.1) |
|
Female | 2 (5.9) |
|
| B, Patient
anamnestic, pathological and clinical data |
|
|
| Characteristic | n (%) |
|
| No. of
recurrences/year |
|
| 1 | 16 (47.1) |
| 2 | 9 (26.5) |
| ≥3 | 9 (26.5) |
| No. of lesions |
|
| 1 | 21 (61.8) |
| 2 | 7 (20.6) |
| ≥3 | 6 (17.6) |
| Diameter of lesion
(if multiple, diameter of the largest) |
|
| <3
cm | 24 (70.6) |
| ≥3
cm | 10 (29.4) |
| Stage |
|
| pT2 | 19 (55.9) |
| pT3a | 7 (20.6) |
| pT3b | 5 (14.7) |
| pT4 | 3 (8.8) |
| Grade |
|
| G3 | 34 (100) |
| Lymph node
involvement |
|
| N0 | 28 (82.3) |
| N1 | 6 (17.7) |
| N2 | 0 (0.0) |
| N3 | 0 (0.0) |
| Previous intravesical
therapy | 34 (100) |
| Cigarette
smokers | 18 (52.9) |
| Charlson
comorbidities index |
|
|
<2 | 26 (76.5) |
| 2 | 6 (17.6) |
| ≥3 | 2 (5.9) |
| Urinary
diversion |
|
| Cutaneous
ureterostomy | 11 (32.4) |
| Ileal
conduit | 6 (17.6) |
|
Neobladder | 17 (50.0) |
| Mean no. of lymph
nodes removed (range) | 15.1 (11–23) |
| Adjuvant
chemotherapy | 3 (8.8) |
Molecular results
At the baseline, 18 (52.9%) patients exhibited ≥1
alteration in one of the loci analysed on Chr 18, while 16 (47.1%)
showed no alterations on Chr 18. Out of the 18 patients with
altered loci, the MBP LW locus was altered in 6 (33.3%), MBP H in 7
(38.9%) and D18S51 in 5 (27.8%) patients. In 2 patients the D18S51
locus was singularly altered, in 1 patient alterations occurred on
both MBP H and D18S51 loci and 2 patients exhibited altered MBP LW
and D18S51 loci.
Follow-up results
At a mean follow-up duration of 128.6 months
post-surgery, 11 patients (32.3%) were alive with no evidence of
disease (mean disease-free survival time, 126.6 months; 95% CI,
119.2–129.1 months). In addition, 8 patients were alive with
evidence of tumour recurrence (mean disease-free survival time,
10.8 months; 95% CI, 103.5–115.1 months) and a further 11 patients
succumbed to their disease (mean disease-free survival time, 23.2
months; 95% CI, 19.7–29.1 months) as well as four mortalities from
non-associated causes. Cumulative cancer-specific survival rates of
the whole study group at 1, 3 and 5 years were 88.2, 64.7 and
52.9%, respectively. No correlation was identified between clinical
or pathological factors and the detection of LOH on Chr 18.
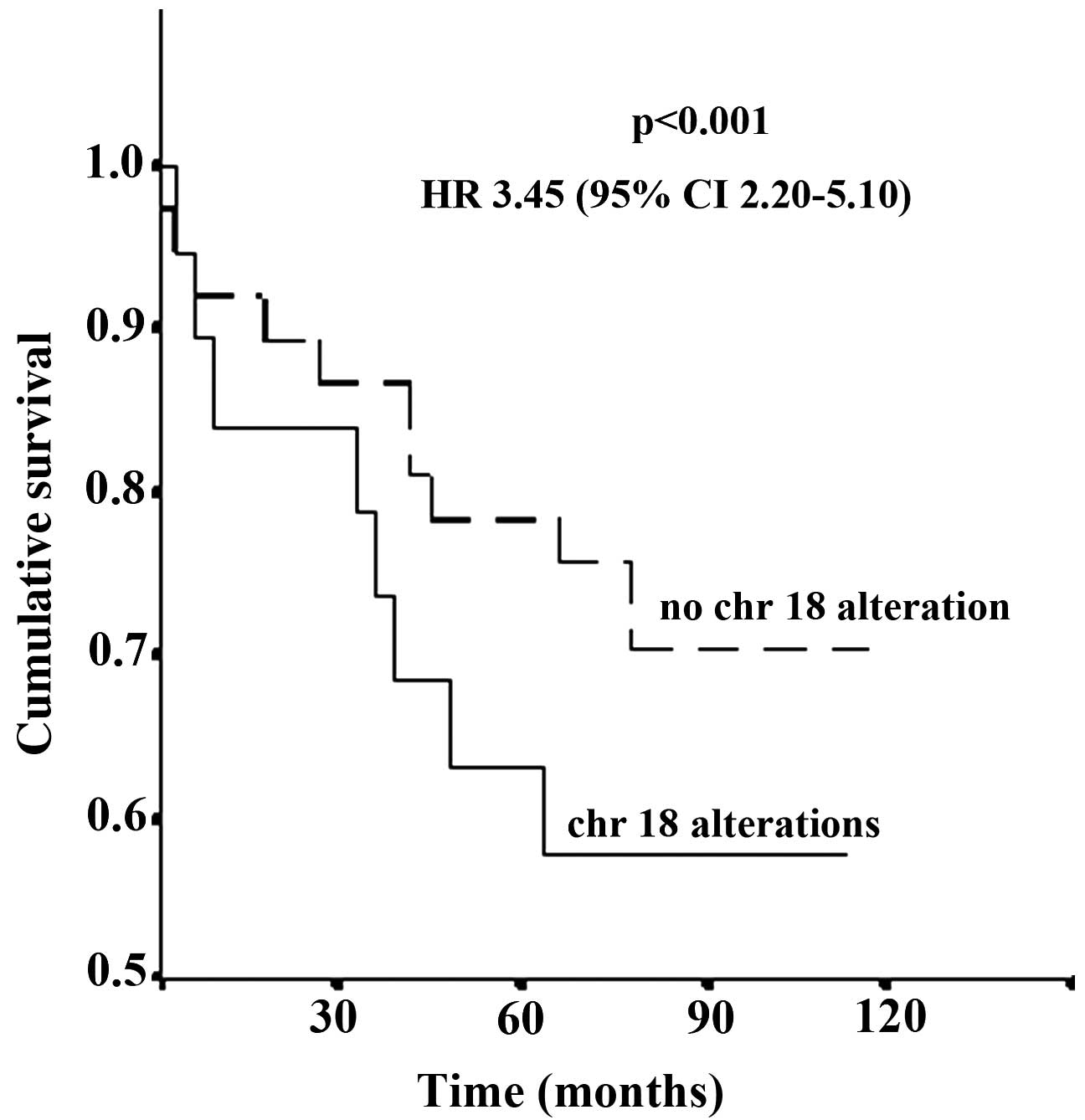
Kaplan-Meier analysis revealed a significant correlation between
patient status at follow-up and LOH on Chr 18 [hazard ratio (HR),
3.45; 95% CI, 2.20–5.10; P<0.001] (Fig. 1). The comparison of microsatellite
analysis with follow-up data identified significant correlations
between altered D18S51 (P<0.001) or MBP H (HR, 2.61; 95% CI,
1.80–3.12; P<0.001) loci and patient status at follow-up.
However, no significant correlation was demonstrated between MBP LW
and status at follow-up (P=0.6).
Univariate analysis, multivariate
analysis and artificial ANN results
As determined using univariate analysis, stage,
lymph node status, LOH on Chr 18 and age proved to be significantly
associated with patient survival. According to the multivariate
analysis, the detection of LOH on Chr 18 (HR, 4.08; 95% CI,
2.09–6.61; P=0.001) and stage (≥pT2; HR, 2.23; 95% CI, 1.17–3.46;
P=0.01) were identified as independent prognostic factors in
predicting status at follow-up. ANN analysis identified LOH on Chr
18 and stage as the most powerful variables affecting the output
decision and predicting the natural history of MIBC; these results
were consistent with those obtained by multivariate analysis.
Discussion
Optimal control and management of patients with
bladder cancer is primarily dependent on appropriate risk-group
stratification, which is established according to the correct
assessment of biological and clinical characteristics (19). Numerous previous studies have
identified several sequential genetic events, which were found to
be associated with tumour natural history (8). A novel bladder cancer susceptibility
locus, the urea transporter gene SLC14A1 was identified on Chr 18q
(20,21). In addition, LOH on Chr 18q and 9q was
reported to be able to predict the clinical outcome of patients
with bladder cancer (8).
The present study demonstrated that LOH on Chr 18
was an independent prognostic indicator of survival in MIBC
patients. Three loci were evaluated on the q arm of Chr 18
(18q21-23), where two tumour-suppressor genes, DCC (18q21.3) and
DPC4 (18q21.1), are located (8).
Uchida et al (8) suggested
that Chr 18q was crucial in the development of the malignant
phenotype, particularly in bladder cancer (7,8). Of note,
in the present study, a strong correlation was observed between
altered D18S51 or MBP H loci and patient outcome. A previous study,
which investigated non-muscle-invasive UC, reported no notable
correlation between D18S51 locus and status at follow-up (4), emphasising that LOH on Chr 18q22 (D18S51
locus) may be a late event in bladder tumorigenesis (22). Brewster et al (22) demonstrated that loss of genetic
material on 18q21.3, which includes DCC, was associated with MIBC
disease and was frequently present in tumour recurrences. The
results of the present study confirmed that alterations in MBP LW
and MBP H loci occurred in the early phase of bladder
tumorigenesis, while the D18S51 locus was implicated in a later
phase. Therefore, alteration in the D18S51 locus identified on
tissue samples following TURBT may be considered as a negative
prognostic factor affecting survival in patients with MIBC. A
limitation of the present study is the small number of the patients
analysed, this was due to the time-consuming and labour-intensive,
thus expensive, molecular biology techniques employed. To the best
of our knowledge, the current study was the first to demonstrate
the use of LOH on Chr 18q21-23 in predicting the clinical outcome
of patients with MIBC; however, further studies are required in
order to confirm the present findings.
In conclusion, the present study highlighted the
role of LOH on Chr 18q21-23 in predicting the clinical outcome of
patients with MIBC. In addition, the present study elucidated the
feasibility and utility of Chr 18 LOH as a potential clinical
marker, in conjunction with well-established clinico-pathological
factors, for the development of an effective adjuvant treatment
schedule in MIBC patients.
Acknowledgements
The authors would like to thank Professor John
Denton, Department of Modern Philology, University of Florence
(Florence, Italy) for manuscript language revision.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2007. CA Cancer J Clin. 57:43–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
MhawechFauceglia P, Cheney RT and
Schwaller J: Genetic alterations in urothelial bladder carcinoma:
An updated review. Cancer. 106:1205–1216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomasini JM and Konety BR: Urinary
markers/cytology: What and when should a urologist use. Urol Clin
North Am. 40:165–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai T, Nesi G, Dal Canto M, Mondaini N,
Piazzini M and Bartoletti R: Prognostic role of loss of
heterozygosity on chromosome 18 in patients with low-risk
nonmuscle-invasive bladder cancer: Results from a prospective
study. J Surg Res. 161:89–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartoletti R, Cai T, Nesi G, Roberta
Girardi L, Baroni G and Dal Canto M: Loss of P16 expression and
chromosome 9p21 LOH in predicting outcome of patients affected by
superficial bladder cancer. J Surg Res. 143:422–427. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartoletti R, Cai T, Dal Canto M, Boddi V,
Nesi G and Piazzini M: Multiplex polymerase chain reaction for
microsatellite analysis of urine sediment cells: A rapid and
inexpensive method for diagnosing and monitoring superficial
transitional bladder cell carcinoma. J Urol. 175:2032–2037. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Knowles MA: What we could do now:
Molecular pathology of bladder cancer. Mol Pathol. 54:215–221.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uchida A, Tachibana M, Miyakawa A,
Nakamura K and Murai M: Microsatellite analysis in multiple
chromosomal regions as a prognostic indicator of primary bladder
cancer. Urol Res. 28:297–303. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Switula D: Principles of good clinical
practice (GCP) in clinical research. Sci Eng Ethics. 6:71–77. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Polymeropoulos MH, Xiao H and Merril CR:
Tetranucleotide repeat polymorphism at the human myelin basic
protein gene (MBP). Hum Mol Genet. 1:6581992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartoletti R, Dal Canto M, Cai T, Piazzini
M, Travaglini F, Gavazzi A and Rizzo M: Early diagnosis and
monitoring of superficial transitional cell carcinoma by
microsatellite analysis on urine sediment. Oncol Rep. 13:531–537.
2005.PubMed/NCBI
|
|
12
|
DalCanto M, Bartoletti R, Travaglini F,
Piazzini M, Lodovichi G, Rizzo M and Selli C: Molecular urinary
sediment analysis in patients with transitional cell bladder
carcinoma. Anticancer Res. 23:5095–5100. 2003.PubMed/NCBI
|
|
13
|
Edge SB, Byrd DR, Compton CC, et al:
American Joint Committee on Cancer Staging Manual. 7th. Springer;
New York, NY: pp. 117–126. 2010
|
|
14
|
Eble JN, Sauter G, Epstein JI and
Sesterhenn IA: World Health Organization Classification of Tumours:
Pathology and Genetics of Tumours of the Urinary System and Male
Genital Organs. IARC Press; Lyon, France: pp. 89–154. 2004
|
|
15
|
Gakis G, Efstathiou J, Lerner SP, et al:
International Consultation on Urologic Disease-European Association
of Urology Consultation on Bladder Cancer 2012: ICUD-EAU
International Consultation on Bladder Cancer 2012: Radical
cystectomy and bladder preservation for muscle-invasive urothelial
carcinoma of the bladder. Eur Urol. 63:45–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stenzl A, Cowan NC, De Santis M, Kuczyk
MA, Merseburger AS, Ribal MJ, Sherif A and Witjes JA: European
Association of Urology (EAU): Treatment of muscle-invasive and
metastatic bladder cancer: Update of the EAU guidelines. Eur Urol.
59:1009–1018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai T, Conti G, Lorenzini M and Bartoletti
R: Artificial intelligences in urological practice: The key to
success? Ann Oncol. 18:6042007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai T, Conti G, Nesi G, et al: Artificial
intelligence for predicting recurrence-free probability of
noninvasive high-grade urothelial bladder cell carcinoma. Oncol
Rep. 18:959–964. 2007.PubMed/NCBI
|
|
19
|
Lee SE and Park MS: Prognostic factors for
survival in patients with transitional cell carcinoma of the
bladder: Evaluation by histopathologic grade, pathologic stage and
flow-cytometric analysis. Eur Urol. 29:193–198. 1996.PubMed/NCBI
|
|
20
|
GarciaClosas M, Ye Y, Rothman N, Figueroa
JD, Malats N, Dinney CP, Chatterjee N, Prokunina Olsson L, Wang Z,
Lin J, et al: A genome-wide association study of bladder cancer
identifies a new susceptibility locus within SLC14A1, a urea
transporter gene on chromosome 18q12.3. Hum Mol Genet.
20:4282–4289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koutros S, Baris D, Fischer A, et al:
Differential urinary specific gravity as a molecular phenotype of
the bladder cancer genetic association in the urea transporter
gene, SLC14A1. Int J Cancer. 133:3008–3013. 2013.PubMed/NCBI
|
|
22
|
Brewster SF, Gingell JC, Browne S and
Brown KW: Loss of heterozygosity on chromosome 18q is associated
with muscle-invasive transitional cell carcinoma of the bladder. Br
J Cancer. 70:697–700. 1994. View Article : Google Scholar : PubMed/NCBI
|