Introduction
Head and neck cancer is growing in prevalence in
many regions of the world. Oral and oropharyngeal cancer (excluding
nasopharyngeal cancer) together represent the sixth most common
malignant neoplasm, with an estimated annual incidence of 275,000
cases for oral cancer and 130,300 for oropharyngeal cancer, two
thirds of which occur in developing countries. Approximately 90% of
head and neck cancers are squamous cell carcinomas (1,2).
The carcinogenetic process includes various phases
that are necessary for the development and evolution of the
neoplasm. Cancer is the result of a series of genetic and
epigenetic alterations that occur in multiple steps in a repeated
and interconnected manner influenced by the genetic predisposition
of the individual and by exogenous environmental factors (1,3).
Collectively, these factors result in a series of molecular
alterations, including the inactivation of tumor suppressor genes
and the activation of oncogenes through deletions, specific
mutations, promoted methylation and gene amplification (3,4).
Tumor suppressor genes are implicated in various
cell division processes, including gene expression regulation, cell
cycle control, apoptosis programming and genome stability (5). The loss of activity of these genes
results in an inability to respond to the control mechanisms that
regulate cell division, resulting in uncontrolled cell
proliferation, the development of neoplasms and their evolution
towards more aggressive processes, from mild or moderate dysplasia
to in situ or invasive carcinoma (5).
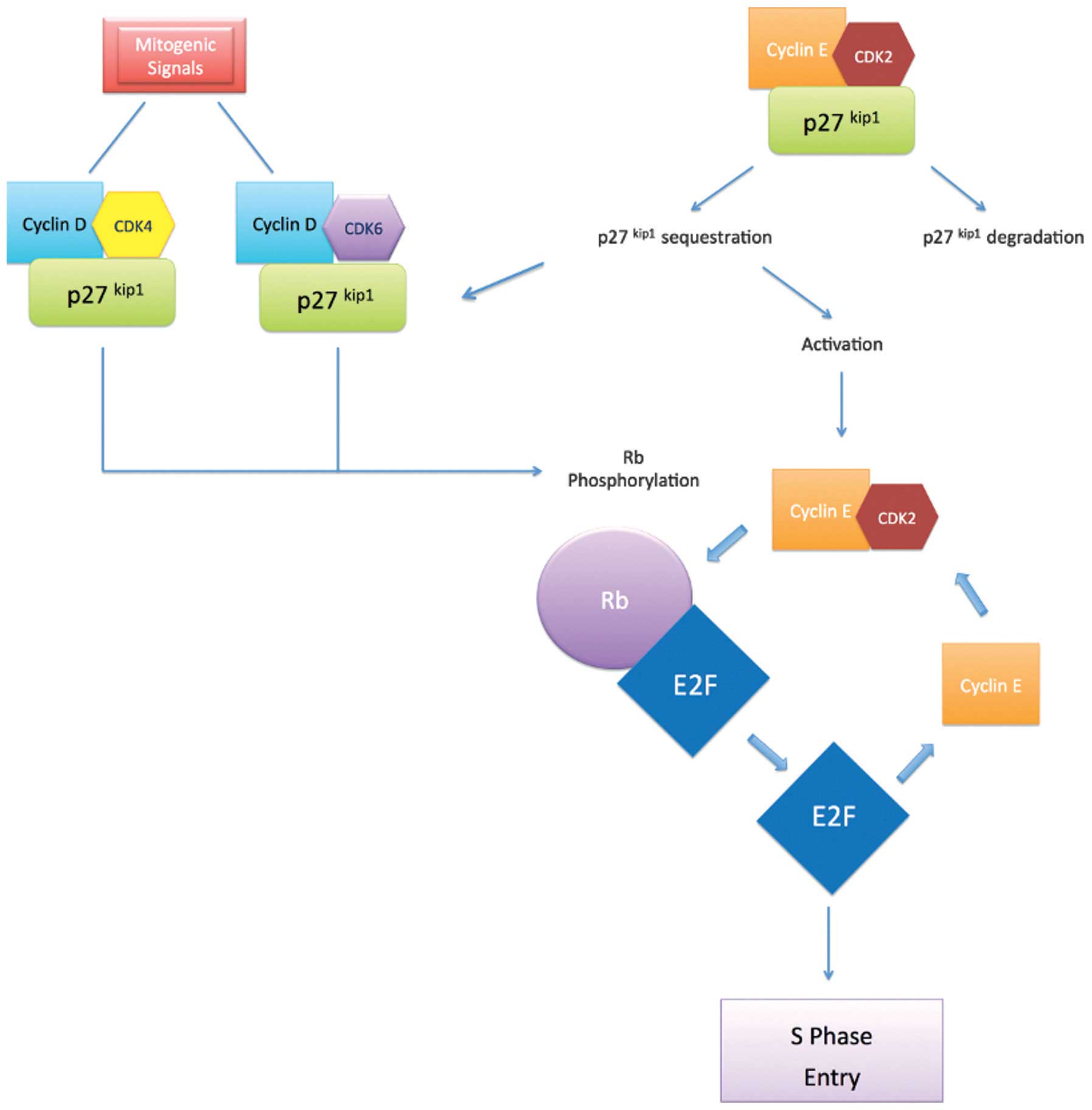
Regulation of the cell cycle is an important factor
in carcinogenesis (6). The cell cycle
is controlled by cyclin-dependent kinases (CDKs), the activity of
which is upregulated by cyclins and downregulated by CDK inhibitors
(CDKIs). CDKs receive signals promoting or inhibiting cell
division, thereby coordinating the progress of the cell cycle
(6). For example, the transition from
G1-S phase is regulated by the activity of cyclin G1/CDK complexes
composed of CDK4 and CDK6, which are activated upon association
with cyclin D, and CDK2, which is activated upon binding with
cyclin E (6). This activity is
essential for transition of the restriction point at the late stage
of G1 (6,7).
The activity of these CDK enzymes is restricted by
the inhibitory action of two major groups of CDKIs: The INK4
family, which comprises the inhibitors p15INK4B,
p16INK4A, p18INK4C and p19WAF1;
and the Cip/Kip family, which comprises p21Cip1
(8), p27Kip1 and
p57Kip2. The Cip/Kip family inhibits cyclin/CDK
complexes, including cyclin D/CDK4, cyclin D/CDK2, cyclin E/CDK2
and cyclin A/CDK2 (Fig. 1) (9,10).
First discovered in 1993, p27Kip1
exhibits a unique responsiveness pattern to a wide range of
mitogenic and antimitogenic signals, making it notably different
from the other two members of the CDKI Cip/Kip family. For example,
p27 can inhibit directly without mediators cyclin D and CDK4/6
(11). Although p27 mutations are
rare in human tumors, decreased p27 expression has been associated
with survival rate, tumor size, histological differentiation and
the presence of lymph node metastasis in patients with various
types of cancer (12–15). The mechanism by which p27 is silenced
remains unclear: Whilst its expression is regulated
transcriptionally and translationally, its levels are predominantly
regulated by ubiquitin-dependent proteolysis mechanisms (16).
The aim of the current study is to provide a
literature review on the association between p27 expression and the
clinical and pathological aspects of HNSCC, the expression of CDKIs
of the Cip/Kip family and cyclins.
p27 expression in HNSCC
The Cochrane database, MEDLINE and EMBASE were
searched on January 27, 2014, using the following keywords: ‘p27
oral squamous cell carcinoma’, ‘p27 head and neck squamous cell
carcinoma’ and ‘p27 mouth neoplasms’. From this search, 29 studies
of p27 expression in HNSCC were identified, the results of which
varied widely (17–45). Different methodologies were used to
determine the immunohistochemical expression of p27: Certain
studies used a quantitative analysis of the percentage of stained
cells, whereas the vast majority used a semi-quantitative analysis,
although with different degrees of cell and sample staining. The
various antibodies used were also applied at different
concentrations. In order to compare the results, Table I shows the percentage of tumors with
positive expression as reported by various studies; different
definitions of positivity have been used, depending on the
methodology used in each case. A wide variability was observed,
with a range of 3–89%. By contrast, the difference in positivity
rate between studies that employed a quantitative analysis was
markedly lower, with the p27 score varying from 10±10 to 56.4±16.2%
(Table II).
 | Table I.Semi-quantitative analysis of
immunohistochemical p27Kip1 expression in HNSCC. |
Table I.
Semi-quantitative analysis of
immunohistochemical p27Kip1 expression in HNSCC.
| Author, year
(reference) | HNSCC cases, n | Location | Definition of
positivity, % | Positive cases,
% | Antibody
dilution |
|---|
| Kudo et al,
1998 (17) | 70 | Oral | 30 | 13 | Transduction
1:100 |
| Fujieda et
al, 1999 (18) | 60 |
Oral/oropharynx | 40 | 30 | Oncogene |
| Ito et al,
1999 (20) | 43 | Oral | 30 | 14 | Transduction
1:500 |
| Mineta et
al, 1999 (21) | 94 | Tongue | 50 | 26 | Transduction
1:100 |
| Schoelch et
al, 1999 (22) | 20 |
Oral/oropharynx | 33 | 75 | Novocastra
1:25 |
| Venkatesan et
al, 1999 (24) | 35 |
Oral/oropharynx | 11 | 77 | Transduction
1:1,000 |
| Kudo et al,
2000 (25) | 17 | Oral | 30 | 59 | Transduction
1:100 |
| Kapranos et
al, 2001 (26) | 31 | Oral | 10 | 65 | Santa Cruz
1:50 |
| Kudo et al,
2001 (27) | 37 | Oral |
5 | 49 | Transduction
1:100 |
| Harada et
al, 2002 (28) | 81 | Oral | 50 | 63 | Novocastra
1:100 |
| Kuo et al,
2002 (29) | 63 | Oral | 11 | 25 | Transduction
1:500 |
| Shintani et
al, 2002 (30) | 117 | Oral |
5 |
64.1 | Dakopatts
1:1,000 |
| Choi et al,
2003 (32) | 30 | Orala |
5 |
36.6 | Neo Markers
1:100 |
| Shintani et
al, 2003 (33) | 75 | Oral | 25 |
26.6 | Transduction
1:100 |
| Kitajima et
al, 2004 (35) | 63 | Oral | 30 | 51 | Transduction
1:100 |
| Rodolico et
al, 2004 (36) | 95 | Lower lip |
19.7 | 70 | Transduction
1:1,200 |
| Rodolico et
al, 2005 (37) | 97 | Lower lip | 20 | 78 | Transduction
1:1,200 |
| Filies et
al, 2007 (38) | 189 | Oral |
1 | 20 | Pharmingen
1:1,000 |
| Queiroz et
al, 2010 (39) | 34 | Oral | 50 |
3 | Santa Cruz
1:100 |
| Martín-Ezquera
et al, 2011 (40) | 49 | Oral |
5 | 38 | BD Biosciences
1:100 |
| Gao et al,
2012 (41) | 206 | Oral | 10 |
60.2 | Zhongshan 1:30 |
| Monteiro et
al, 2012 (42) | 51 | Oral | 25 | 89 | Novocastra
1:20 |
| Perisanidis et
al, 2012 (43) | 111 |
Oral/oropharynx | 50 | 69 | Neo Markers |
| Canzonieri et
al, 2012 (44) | 25 |
Oropharynx/hypopharynx/larynx |
5 | 25 | Transduction
1:100 |
 | Table II.Quantitative analysis of
immunohistochemical p27Kip1 expression in HNSCC. |
Table II.
Quantitative analysis of
immunohistochemical p27Kip1 expression in HNSCC.
| Author, year
(reference) | HNSCC cases, n | Location | p27 score, % | Antibody
dilution |
|---|
| Jordan et
al, 1998 (19) |
8 | Oral | 28.7±5.1 | Transduction
1:500 |
| Fujieda et
al, 1999 (18) | 60 |
Oral/oropharynx | 31.1±30 | Oncogene |
| Saito et al,
1999 (23) | 44 | Oral | 24±6 | Transduction
1:5,000 |
| Kapranos et
al, 2001 (26) | 31 | Oral | 56.4±16.2 | Santa Cruz
1:50 |
| Kuo et al,
2002 (29) | 63 | Oral | 10±10 | Transduction
1:500 |
| Rodolico et
al, 2004 (36) | 95 | Lower lip | 19.7 | Transduction
1:1,200 |
| Rodolico et
al, 2005 (37) | 97 | Lower lip | 26.4 | Transduction
1:1,200 |
| Zhang et al,
2013 (45) | 110 | Oral | 55.46 | Zhongshan |
Thus, differences in expression between the
different working groups may be due to differences in
immunohistochemistry, in addition to population characteristics and
specimen variation. Similarly, it is essential to standardize
semi-quantitative analysis methods to allow methodological
unification of the different studies, and thus enable performance
of systematic reviews and meta-analyses.
Progression of p27 expression
Numerous studies have investigated p27 expression
throughout various phases of HNSCC progression, comparing normal
mucosa with dysplastic lesions, carcinoma in situ, verrucous
carcinoma and SCC (Table III). In
certain of these studies, the comparison was performed
qualitatively on the basis of positive or negative expression.
Thus, Kudo et al (25)
observed that the percentage of tumors with positive expression was
significantly lower in SCC and in lesions with severe dysplasia
compared with that in normal mucosa (P<0.05). Queiroz et
al (39) also found that p27
expression is lower in oral squamous cell carcinoma (OSCC) and in
benign lesions compared with in normal tissue (P<0.05). By
contrast, Schoelch et al (22)
and Shintani et al (33) found
no statistically significant differences. In other studies, a
quantitative analysis of the percentage of stained cells (p27
score) was conducted. Thus, Jordan et al (19) reported that p27 expression in lesions
with moderate dysplasia, severe dysplasia and SCC is lower than
that in normal mucosa (P<0.05), whilst Saito et al
(23) observed lower p27 expression
in verrucous carcinoma compared with in SCC (P<0.001).
Similarly, Kuo et al (29)
obtained significantly reduced p27 expression values in SCC
compared with that in dysplastic lesions, and in the latter
compared with in normal mucosa.
 | Table III.Progression of p27Kip1
expression during development of HNSCC as assessed by
semi-quantitative or quantitative analysis. |
Table III.
Progression of p27Kip1
expression during development of HNSCC as assessed by
semi-quantitative or quantitative analysis.
| A,
Semi-quantitative analysis |
|
|
|
|
|
|
|
|
|
|
|---|
|
|---|
|
|
| p27+, % [n] |
|
|---|
|
|
|
|
|
|---|
| Author
(reference) | Definition of
positivity | NM | OBL | MiD | MoD | SD | ISC | VC | HNSCC | P-value |
|---|
| Schoelch et
al (22) | >30% | 100 [8] | 100 [4] | 100 [9] | 93.3 [15] | 57 [7] | 0 [3] | ND | 75 [20] | ND |
| Kudo et al
(25) | >30% | ND | ND | 100 [4] | 100 [7] | 69 [6]a | ND | ND | 18
[17]a |
P<0.05a |
| Shintani et
al (30) | >5% | 100 [20] | ND | 100 [12] | 100 [12] | 92.9 [14] | ND | ND | 64.1 [117] | ND |
| Queiroz et
al (39) | >25% | 81.3 [32] | 66.7
[30]a | ND | ND | ND | ND | ND | 2.9
[34]a |
P<0.05a |
|
| B, Quantitative
analysis. |
|
|
|
|
|
|
|
|
|
|
|
|
|
| p27+, % [n] |
|
|
|
|
|
|
| Author
(reference) |
| NM | OBL | MiD | MoD | SD | ISC | VC | HNSCC | P-value |
|
| Jordan et al
(19) |
| 49.6±5.8 [10] | ND | 46.2±7.4 [8] | 33.0±6.5
[11]a | 23.4±2.7
[17]a | ND | ND | 28.7±5.1
[8]a |
P<0.05a |
| Saito et al
(23) |
| 53.0±4.28 [10] | ND | 40.7±7.2 [23] | 32.1±7.19 [23] | 28.4±13.5 [11] | ND | 52.6±15.7 [15] | 24.0±6.0 [44] |
P<0.001b |
| Kuo et al
(29) |
| 65±10
[10]a | ND |
| 32±11
[19]a* |
| ND | ND | 10±10
[63]a |
P<0.05a |
With regard to the age of the patient at the onset
of the neoplasm, no statistically significant differences were
detected. In certain studies, the mean age of patients with tumors
positive and negative for p27 expression was calculated, and no
differences were observed between the two (18,21,28).
Similarly, other studies compared the percentage of tumors
positively expressing p27 in younger and older patients (below and
above the mean cohort age, respectively) and also identified no
differences (29,32,41,46).
Regarding gender, no differences in p27 expression
were observed between males and females (21,28,32,41).
The results concerning survival are controversial
(17,18, 21,24–26,28,29,32,38,41,43,45,46).
In the majority of solid tumors, p27 expression silencing is
associated with a decrease in the five-year survival rate. As can
be seen in Table IV, in the case of
HNSCC, the majority of studies have also reported similar findings,
some with statistically significant differences (21,24,27–29,33,38,41).
However, in studies where improved survival rates were observed for
tumors with negative p27 expression, the differences were not
statistically significant (18,32,42,45).
 | Table IV.Analysis of the P-value obtained when
comparing survival rates for tumors with high and low
p27Kip1 expression. |
Table IV.
Analysis of the P-value obtained when
comparing survival rates for tumors with high and low
p27Kip1 expression.
|
|
|
|
| Survival, % |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Author
(reference) | Cases, n | Minimum follow-up,
years | Definition of p27
positivity | Low p27
expression | High p27
expression | P-value | Test used |
|---|
| Kudo et al
(17) | 70 | 5 | 5% | 50 | 80 | <0.05 | Kaplan-Meier curve
(Mantel-Cox Test) |
| Fujieda et
al (18) | 60 | 5 | 40% |
57.8 |
50.7 |
0.69 | Kaplan-Meier curve
(Log-Rank Test) |
| Mineta et al
(21) | 94 | 5 | 50% | 44 | 68 |
0.04 | Kaplan-Meier curve
(Log-Rank Test) |
| Venkatesan et
al (24) | 35 | 2 | 35% | ~20 | ~65 |
0.0001 | Kaplan-Meier curve
(Log-Rank Test) |
| Kudo et al
(25) | 37 | 5 | 5% | ~50 | ~80 | ND | Kaplan-Meier curve
(Mantel-Cox Test) |
| Kapranos et
al (26) | 31 | 5 | 10% |
19.2 | 21 |
0.11 | Kaplan-Meier curve
(Log-Rank Test) |
| Harada et al
(28) | 81 | 5 | 50% |
56.7 |
80.4 |
0.009 | Kaplan-Meier curve
(Log-Rank Test) |
| Kuo et al
(29) | 63 | 1 | 10% | ~65 | ~92 |
0.01587 | Kaplan-Meier curve
(Log-Rank Test) |
| Choi et al
(32) | 30 | 3 | 5% |
63.2a |
36.4b | ND | None |
| Shintani et
al (33) | 75 | 2 | 25% | ~50 | ~75 |
0.027 | Kaplan-Meier curve
(Log-Rank Test) |
| Fillies et
al (38) | 189 | 5 | 1% | ~25 | ~60 |
0.03 | Kaplan-Meier curve
(Log-Rank Test) |
| Gao et al
(41) | 206 | 5 | 10% | ~40 | ~70 |
0.001 | Kaplan-Meier
curve |
| Monteiro et
al (42) | 51 | 3 | 25% |
66.7 |
51.3 |
0.233 | Cox proportional
hazards model |
| Perisanidids et
al (43) | 111 | 5 | 50% |
ND |
ND |
0.41 | Univariate Cox
regression |
| Zhang et al
(45) | 110 | 5 | 2.8c | 73 | 64 |
0.27 | Cox proportional
hazards model |
Relapse rate has been analyzed to a lesser extent.
Studies by Perisanidis et al (43) and Monteiro et al (42) analyzed the risk of relapse and
observed no significant differences between tumors with different
p27 expression values. Canzonieri et al (44) observed a lower p27 expression
positivity in tumors with relapse, however, the differences were
not statistically significant. With regard to the time at which
relapse occurred, Venkatesan et al (24) reported that the mean was 324 days in
patients with tumors exhibiting low p27 expression levels,
significantly lower than for patients with high expression
levels.
The presence of lymph node metastasis is a
clinicopathological factor known to be associated with poorer
prognosis. As such, it is important to determine whether any
association exists between p27 silencing and the appearance of
lymph node metastasis. The majority of published studies report
that patients with a positive lymph node metastasis status have
reduced p27 expression levels compared with those without lymph
node metastasis (21,27,33,36); in
studies that reported contrasting results, the differences were not
statistically significant (20,29).
Advanced tumor stages are also associated with
poorer prognosis in HNSCC. Thus, in theory, lower p27 expression
levels should be observed in tumors of more advanced stages.
Indeed, all studies identified in the literature that relate p27
expression with tumor stage report that expression is reduced in
the advanced stages compared with in earlier stages (18,21,27–29,32,33,41).
Furthermore, the majority of studies reported statistically
significant differences (18,21,27,41),
thereby, to some extent, confirming that p27 silencing is important
role in the development of HNSCC. Thus, if p27 expression is
associated with tumor stage, tumor size should also be related.
However, in the few studies that have investigated the correlation
between tumor size and p27 expression, the results obtained were
not consistent with this hypothesis. Mineta et al (21), for example, reported statistically
significant differences for tumor stage, but no such differences
for tumor size. These findings were in accordance with those of
Harada et al (28) and Kuo
et al (29), who observed a
trend in which p27 was overexpressed in smaller tumors.
With regard to tumor differentiation, although
reduced p27 expression may be expected in poorly differentiated
tumors, studies into the correlation between p27 expression and
tumor differentiation indicate a tendency for this gene to be
silenced in poorly differentiated tumors; however, the vast
majority of studies report no statistically significant differences
(20,21,27,29,32,33,41).
Harada et al (28) is the only
study to have identified a significant correlation between
histological grade and decreased p27 expression.
Concerning other clinicopathological factors, such
as smoking habit, alcohol consumption, tumor invasion and tumor
depth, no statistically significant differences indicating any
association with these factors and p27 expression have been
reported in any studies, to the best of our knowledge (17–45).
Thus, regarding the multistep progression of oral
cancer, almost all studies confirm that p27 expression is reduced
as the degree of dysplasia or the differentiation of the tumor
increases.
Correlation between expression of p27 and
genes from the Cip/Kip family
In a similar manner to p27, the other members of the
Cip/Kip family, p21Cip1 and p57Kip2, also
inhibit cyclin/CDK complexes by blocking the cell cycle at the G1-S
phase checkpoint. However, although Fan et al (47) considered p57 expression to be a good
prognostic marker for OSCC, it has received relatively little
attention. Therefore, the current review will focus more on the
correlation between p21 and p27. As is the case for p27, a loss of
p21 expression is observed in HNSCC. However, according to Shintani
et al (30), this occurs
during the initial stages of carcinogenesis and, probably as a
result, the correlation between p21 expression and the
clinicopathological parameters is highly variable, although the
appearance of lymph node metastases is often related to p21
silencing. With regard to the association between p21 and p27
expression, the results in the literature are somewhat unclear.
Whilst Choi et al (32)
reported an inverse correlation (P=0.08) between the two, this was
not related to clinicopathological parameters. Similarly, a study
by Zhang et al (45) revealed
a statistically significant correlation between the expression of
the two genes by way of a Pearson analysis, whereas Fillies et
al (38) obtained different
results, although a different statistical test (Cox regression
model) was used and thus, the results are not comparable. Upon
analyzing tumors of the oral cavity and larynx together, Kapranos
et al (26) also failed to
find a correlation between the expression of these genes, or any
differences in survival rate between the four possible expression
combinations.
Although the results are not entirely convincing, it
appears that the activity of p27 is independent of other cell cycle
regulators of the Cip/Kip family.
Correlation between p27 expression and
cyclins
The present study focussed on reviewing the
association between p27 expression and cyclin expression. In
contrast to p27 expression, a number of studies have reported that
cyclins A, D, and E are overexpressed in SCC compared with in
normal mucosa, thereby confirming the cyclin-inhibitory role of
p27. Various studies have also reported that overexpression of
cyclins is associated with tumor stage, histological
differentiation of the tumor and the presence of lymph node
metastases, thereby affecting the prognosis of patients in terms of
survival rate and time to recurrence. Pignataro et al
(48) investigated the correlation
between p27 and cyclin D1 expression in HNSCC, including tumors of
the larynx, and observed an inverse correlation whereby the
majority of tumors that exhibit positive p27 expression also
present negative cyclin D1 expression, and those with positive
cyclin D1 expression exhibit negative p27 expression. The same
study also reported that patients with cyclin D1+/p27- tumors have
a poorer prognosis, whilst those with cyclin D1-/p27+ tumors have a
better prognosis in terms of survival rate (P=0.0015) and time to
recurrence (P=0.0001). Rodolico et al (37) found that tumors overexpressing cyclin
D1 and underexpressing p27 are associated with the appearance of
lymph node metastases in patients with SCCs of the lower lip.
Kuropkat et al (31) believe
that the results obtained in their study, in which no relationship
was identified between cyclin D1 expression and time to recurrence
when p27 expression was also considered, are unreasonable, as both
overexpression or very low expression of cyclin D1 were associated
with shorter time-to-recurrence. Fujieda et al (18) obtained statistically significant
results that contradict those found in the literature and current
theories of cell cycle regulation (12,48,37,31):
The results revealed that tumors with positive cyclin D1 expression
exhibit a higher percentage of cells stained by the p27 antibody
compared with that of tumors with negative cyclin D1 expression
(42.7±31.9 vs. 25.2±29.3%; P=0.001), whereas the majority of
previous studies have demonstrated an inverse correlation between
between cyclin D1 and p27 expression.
Ito et al (20)
reported a strongly significant inverse correlation between cyclin
E and p27 expression (cyclin E+/p27-, 33/42; cyclin E+/p27+, 3/42;
cyclin E-/p27-, 2/42; and cyclin E-/p27+, 4/42; P<0.001) but did
not investigate this association with regard to clinicopathological
factors. Additionally, Jordan et al (19) identified an association between
increased cyclin A expression and reduced p27 expression in
SCC.
Thus, although further confirmation is required, the
literature to date suggests that the expression of CDKIs is
inversely correlated with the expression of cyclins A, D, and E
(12).
Conclusions
A wide variety of tumor behaviors have been reported
at the different sites in the head and neck (21). Thus, in future, more objective
analyses and immunohistochemical expression studies must be
performed at specific anatomical locations, as already undertaken
by a number of research groups (21,36,37).
Various studies of p27 expression in HNSCC have been
performed over the last two decades, and there is now no doubt that
p27 is underexpressed in SCC cells (12,13). p27
may be a promising marker for determining the prognosis of HNSCC,
despite the marked variability of the results obtained. An
association between p27 expression and survival rate, time to
recurrence and tumor stage has been observed (24,48). Based
on the information currently available, it is too early to
recommend the analysis of p27 expression for guiding HNSCC
treatment planning. Although relatively unstudied, the correlation
between p27 expression and other tumor suppressor genes may turn
out to be important for determining the prognosis of HNSCC.
However, further prospective studies and studies involving
standardized laboratory methodologies and statistics that allow
meta-analyses to be performed are required to confirm this
proposal.
References
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warnakulasuriya S: Living with oral
cancer: Epidemiology with particular reference to prevalence and
life-style changes that influence survival. Oral Oncol. 46:407–410.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perez-Ordoñez B, Beauchemin M and Jordan
RC: Molecular biology of squamous cell carcinoma of the head and
neck. J Clin Pathol. 59:445–453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pérez-Sayáns M, Somoza-Martin JM,
Barros-Angueira F, Reboiras-Lopez MD, Gándara Rey JM and
Garcia-García A: Genetic and molecular alterations associated with
oral squamous cell cancer (Review). Oncol Rep. 22:1277–1282. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Todd R, Hinds PW, Munger K, Rustgi AK,
Opitz OG, Suliman Y and Wong DT: Cell cycle dysregulation in oral
cancer. Crit Rev Oral Biol Med. 13:51–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patel V, Jakus J, Harris CM, Ensley JF,
Robbins KC and Yeudall WA: Altered expression and activity of G1/S
cyclins and cyclin-dependent kinases characterize squamous cell
carcinomas of the head and neck. Int J Cancer. 73:551–555. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sherr CJ and Roberts JM: Inhibitors of
mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149–1163.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pérez-Sayáns M, Suárez-Peñaranda JM,
Gayoso-Diz P, Barros-Angueira F, Gándara-Rey JM and García-García
A: p16 (INK4a)/CDKN2 expression and its relationship with oral
squamous cell carcinoma is our current knowledge enough? Cancer
Lett. 306:134–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pérez-Sayáns M, Suárez-Peñaranda JM,
Gayoso-Diz P, et al: The role of p21Waf1/CIP1 as a Cip/Kip type
cell-cycle regulator in oral squamous cell carcinoma (Review). Med
Oral Patol Oral Cir Bucal. 18:e219–e225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Toyoshima H and Hunter T: p27, a novel
inhibitor of G1 cyclin-Cdk protein kinase activity, is related to
p21. Cell. 78:67–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Migita T, Oda Y, Naito S and Tsuneyoshi M:
Low expression of p27 (Kip1) is associated with tumor size and poor
prognosis in patients with renal cell carcinoma. Cancer.
94:973–979. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhuang Y, Yin HT, Yin XL, Wang J and Zhang
DP: High p27 expression is associated with a better prognosis in
east Asian non-small cell lung cancer patients. Clin Chim Acta.
412:2228–2231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nozoe T, Oyama T, Takenoyama M, Hanagiri
T, Sugio K and Yasumoto K: Significance of immunohistochemical
expression of p27 and involucrin as the marker of cellular
differentiation of squamous cell carcinoma of the esophagus.
Oncology. 71:402–410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kouvaraki M, Gorgoulis VG, Rassidakis GZ,
Liodis P, Markopoulos C, Gogas J and Kittas C: High expression
levels of p27 correlate with lymph node status in a subset of
advanced invasive breast carcinomas: Relation to E-cadherin
alterations, proliferative activity and ploidy of the tumors.
Cancer. 94:2454–2465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kudo Y, Kitajima S, Ogawa I, Miyauchi M
and Takata T: Down-regulation of Cdk inhibitor p27 in oral squamous
cell carcinoma. Oral Oncol. 41:105–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kudo Y, Takata T, Yasui W, Ogawa I,
Miyauchi M, Takekoshi T, Tahara E and Nikai H: Reduced expression
of cyclin-dependent kinase inhibitor p27Kip1 is an indicator of
malignant behavior in oral squamous cell carcinoma. Cancer.
83:2447–2455. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujieda S, Inuzuka M, Tanaka N, Sunaga H,
Fan GK, Ito T, Sugimoto C, Tsuzuki H and Saito H: Expression of p27
is associated with Bax expression and spontaneous apoptosis in oral
and oropharyngeal carcinoma. Int J Cancer. 84:315–320. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jordan RC, Bradley G and Slingerland J:
Reduced levels of the cell-cycle inhibitor p27Kip1 in epithelial
dysplasia and carcinoma of the oral cavity. Am J Pathol.
152:585–590. 1998.PubMed/NCBI
|
|
20
|
Ito R, Yasui W, Ogawa Y, Toyosawa S,
Tahara E and Ijuhin N: Reduced expression of cyclin-dependent
kinase inhibitor p27 (Kip1) in oral malignant tumors. Pathobiology.
67:169–173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mineta H, Miura K, Suzuki I, Takebayashi
S, Amano H, Araki K, Harada H, Ichimura K, Wennerberg JP and Dictor
MR: Low p27 expression correlates with poor prognosis for patients
with oral tongue squamous cell carcinoma. Cancer. 85:1011–1017.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schoelch ML, Regezi JA, Dekker NP, Ng IO,
McMillan A, Ziober BL, Le QT, Silverman S and Fu KK: Cell cycle
proteins and the development of oral squamous cell carcinoma. Oral
Oncol. 35:333–342. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saito T, Nakajima T and Mogi K:
Immunohistochemical analysis of cell cycle-associated proteins p16,
pRb, p53, p27 and Ki-67 in oral cancer and precancer with special
reference to verrucous carcinomas. J Oral Pathol Med. 28:226–232.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Venkatesan TK, Kuropkat C, Caldarelli DD,
Panje WR, Hutchinson JC Jr, Chen S and Coon JS: Prognostic
significance of p27 expression in carcinoma of the oral cavity and
oropharynx. Laryngoscope. 109:1329–1333. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kudo Y, Takata T, Ogawa I, Zhao M, Sato S,
Takekoshi T, Miyauchi M and Nikai H: Reduced expression of p27
(Kip1) correlates with an early stage of cancer invasion in oral
squamous cell carcinoma. Cancer Lett. 151:217–222. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kapranos N, Stathopoulos GP, Manolopoulos
L, Kokka E, Papadimitriou C, Bibas A, Yiotakis J and Adamopoulos G:
P53, p21 and p27 protein expression in head and neck cancer and
their prognostic value. Anticancer Res. 21:521–528. 2001.PubMed/NCBI
|
|
27
|
Kudo Y, Kitajima S, Sato S, Miyauchi M,
Ogawa I and Takata T: High expression of S-phase kinase-interacting
protein 2, human F-box protein, correlates with poor prognosis in
oral squamous cell carcinomas. Cancer Res. 61:7044–7047.
2001.PubMed/NCBI
|
|
28
|
Harada K, Supriatno, Yoshida H and Sato M:
Low p27Kip1 expression is associated with poor prognosis in oral
squamous cell carcinoma. Anticancer Res. 22:2985–2989.
2002.PubMed/NCBI
|
|
29
|
Kuo MY, Hsu HY, Kok SH, Kuo RC, Yang H,
Hahn LJ and Chiang CP: Prognostic role of p27 (Kip1) expression in
oral squamous cell carcinoma in Taiwan. Oral Oncol. 38:172–178.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shintani S, Mihara M, Nakahara Y, Kiyota
A, Ueyama Y, Matsumura T and Wong DT: Expression of cell cycle
control proteins in normal epithelium, premalignant and malignant
lesions of oral cavity. Oral Oncol. 38:235–243. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuropkat C, Venkatesan TK, Caldarelli DD,
Panje WR, Hutchinson J, Preisler HD, Coon JS and Werner JA:
Abnormalities of molecular regulators of proliferation and
apoptosis in carcinoma of the oral cavity and oropharynx. Auris
Nasus Larynx. 29:165–174. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi HR, Tucker SA, Huang Z, Gillenwater
AM, Luna MA, Batsakis JG and El-Naggar AK: Differential expressions
of cyclin-dependent kinase inhibitors (p27 and p21) and their
relation to p53 and Ki-67 in oral squamous tumorigenesis. Int J
Oncol. 22:409–414. 2003.PubMed/NCBI
|
|
33
|
Shintani S, Li C, Mihara M, Hino S,
Nakashiro K and Hamakawa H: Skp2 and Jab1 expression are associated
with inverse expression of p27 (KIP1) and poor prognosis in oral
squamous cell carcinomas. Oncology. 65:355–362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harada K, Supriatno, Yamamoto S, Kawaguchi
S, Yoshida H and Sato M: Cepharanthine exerts antitumor activity on
oral squamous cell carcinoma cell lines by induction of p27Kip1.
Anticancer Res. 23:1441–1448. 2003.PubMed/NCBI
|
|
35
|
Kitajima S, Kudo Y, Ogawa I, Bashir T,
Kitagawa M, Miyauchi M, Pagano M and Takata T: Role of Cks1
overexpression in oral squamous cell carcinomas: Cooperation with
Skp2 in promoting p27 degradation. Am J Pathol. 165:2147–2155.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rodolico V, Barresi E, Di Lorenzo R,
Leonardi V, Napoli P, Rappa F and Di Bernardo C: Lymph node
metastasis in lower lip squamous cell carcinoma in relation to
tumour size, histologic variables and p27Kip1 protein expression.
Oral Oncol. 40:92–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rodolico V, Aragona F, Cabibi D, Di
Bernardo C, Di Lorenzo R, Gebbia N, Gulotta G, Leonardi V and
Ajello F: Overexpression of cyclin D1 and interaction between
p27Kip1 and tumour thickness predict lymph node metastases
occurrence in lower lip squamous cell carcinoma. Oral Oncol.
41:268–275. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fillies T, Woltering M, Brandt B, Van
Diest JP, Werkmeister R, Joos Uz and Buerger H: Cell cycle
regulating proteins p21 and p27 in prognosis of oral squamous cell
carcinomas. Oncol Rep. 17:355–359. 2007.PubMed/NCBI
|
|
39
|
Queiroz AB, Focchi G, Dobo C, Gomes TS,
Ribeiro DA and Oshima CT: Expression of p27, p21 (WAF/Cip1) and p16
(INK4a) in normal oral epithelium, oral squamous papilloma and oral
squamous cell carcinoma. Anticancer Res. 30:2799–2803.
2010.PubMed/NCBI
|
|
40
|
Martín-Ezquerra G, Salgado R, Toll A, Baró
T, Mojal S, Yébenes M, Garcia-Muret MP, Solé F, Quitllet FA,
Espinet B and Pujol RM: CDC28 protein kinase regulatory subunit 1B
(CKS1B) expression and genetic status analysis in oral squamous
cell carcinoma. Histol Histopathol. 26:71–77. 2011.PubMed/NCBI
|
|
41
|
Gao L, Huang S, Ren W, Zhao L, Li J, Zhi
K, Zhang Y, Qi H and Huang C: Jun activation domain-binding protein
1 expression in oral squamous cell carcinomas inversely correlates
with the cell cycle inhibitor p27. Med Oncol. 29:2499–2504. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Monteiro LS, Diniz-Freitas M,
Garcia-Caballero T, Warnakulasuriya S, Forteza J and Fraga M:
Combined cytoplasmic and membranous EGFR and p53 overexpression is
a poor prognostic marker in early stage oral squamous cell
carcinoma. J Oral Pathol Med. 41:559–567. 2012.PubMed/NCBI
|
|
43
|
Perisanidis C, Perisanidis B, Wrba F,
Brandstetter A, El Gazzar S, Papadogeorgakis N, Seemann R, Ewers R,
Kyzas PA and Filipits M: Evaluation of immunohistochemical
expression of p53, p21, p27, cyclin D1 and Ki67 in oral and
oropharyngeal squamous cell carcinoma. J Oral Pathol Med. 41:40–46.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Canzonieri V, Barzan L, Franchin G,
Vaccher E, Talamini R, Sulfaro S and Baldassarre G: Alteration of
G1/S transition regulators influences recurrences in head and neck
squamous carcinomas. J Cell Physiol. 227:233–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang M, Li J, Wang L, Tian Z, Zhang P, Xu
Q, Zhang C, Wei F and Chen W: Prognostic significance of p21, p27
and survivin protein expression in patients with oral squamous cell
carcinoma. Oncol Lett. 6:381–386. 2013.PubMed/NCBI
|
|
46
|
Shintani S, Li C, Mihara M, Hino S,
Nakashiro K and Hamakawa H: Skp2 and Jab1 expression are associated
with inverse expression of p27 (KIP1) and poor prognosis in oral
squamous cell carcinomas. Oncology. 65:355–362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fan GK, Chen J, Ping F and Geng Y:
Immunohistochemical analysis of P57 (kip2), p53 and hsp60
expressions in premalignant and malignant oral tissues. Oral Oncol.
42:147–153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pignataro L, Sambataro G, Pagani D and
Pruneri G: Clinico-prognostic value of D-type cyclins and p27 in
laryngeal cancer patients: A review. Acta Otorhinolaryngol Ital.
25:75–85. 2005.PubMed/NCBI
|