Introduction
Prostate cancer is the second most frequently
diagnosed cancer and the sixth leading cause of cancer-associated
mortality in males worldwide, accounting for 14% (903,500) of total
new cancer cases and 6% (258,400) of total cancer mortalities in
males in 2008 (1). In the USA,
prostate cancer alone accounted for 27% (233,000) of new cancer
cases in men in 2014, and the estimated number of prostate cancer
mortalities was ~10% (29,480) (2).
Conventional therapy to eradicate tumor cells, including surgery,
chemotherapy, radiotherapy and hormonal treatments, has resulted in
prolonged survival and successful treatment in certain patients
(3). However, relapse and metastasis
occur frequently, and are generally unresponsive to conventional
therapy. Therefore, the development of novel agents for prostate
cancer therapy is clinically important.
Natural products are a promising source for the
development of novel cancer therapies, due to their potential
effectiveness and low toxicity profiles (4). Flavonoids, a major class of natural
products commonly found in fruits and vegetables, are demonstrably
useful for the biologically active properties that inhibit tumor
promotion and metastasis, such as apoptosis induction and cell
cycle arrest in cancer cells (5–8).
One of the main components in Herba oxytropis
is 2′,4′-dihydroxychalcone (TFC) (9).
Experimental studies have demonstrated the anti-tumor activity of
TFC in cancer cells (10,11). Previously, studies have demonstrated
that TFC may induce the apoptosis of MGC-803 cells via
downregulation of survivin mRNA expression (9,12).
However, the exact anti-tumor mechanism of TFC remains unclear.
In the present study, the authors investigated the
anti-tumor effect of TFC on human prostate cancer cells. TFC was
revealed to potently inhibit the proliferation of PC-3 human
prostate cancer cells by induction of apoptosis. The results of the
present study may provide a scientific explanation for the
antitumor mechanism of TFC.
Materials and methods
Reagents
The reagent 2′,4′-dihydroxychalcone (>95% purity)
was isolated from O. falcata herb, as described by Lu et
al (13). The structure of TFC is
exhibited in Fig. 1. The compound was
dissolved in dimethyl sulfoxide (DMSO). The final concentration of
DMSO was <1% (v/v). Ham's −12 Nutrient Mixture (F-12) was
purchased from Life Technologies (Grand Island, NY, USA).
Invitrogen fetal bovine serum was purchased from Thermo Fisher
Scientific (Waltham, MA, USA). Cell counting kit (CCK-8) was
purchased from Beyotime Institute of Biotechnology (Haimen,
Jiangsu, China). Fluorescein isothiocyanate (FITC) Annexin V
Apoptosis Detection kit and Cycletest plus DNA Reagent kit were
obtained from BD Pharmingen (San Diego, CA, USA). Other chemicals
were of analytical grade from commercial suppliers (Sinopharm
Chemical Reagent Co., Ltd., Nanjing, China).
Cell culture
The human prostate cancer PC-3 cell line was
purchased from the Cell Bank of China Academy of Sciences
(Shanghai, China) and maintained in F-12 culture medium containing
10% fetal bovine serum, 100 U/ml penicillin and 100 U/ml
streptomycin, at 37°C and supplied with 95% room air and 5%
CO2.
Cell viability assay
PC-3 cells in the exponential phase of growth were
placed in a 96-well plate (5,000 cells/well). Following a 4-h
incubation period, the cells were treated with compounds or with
the vehicle (vehicle control, 1% DMSO) for 24 h. Then 10 µl of
CCK-8 was added and the plates were incubated for another 4 h. The
absorbance at 450 nm was recorded by a Benchmark™ Plus microplate
reader (Bio-Rad Laboratories, Hercules, CA, USA).
Cell morphology
The PC-3 cells were incubated with or without TFC
for 24 h in a 5% CO2 incubator at 37°C. Then, the cells
were observed for morphological changes using an Eclipse TE2000-U
inverted microscope (Nikon Instruments, Melville, NY, USA) and
images were captured.
Flow cytometry analysis to determine
cell cycle distribution and apoptosis
Flow cytometry was used to analyze the cell cycle
distribution and apoptosis subsequent to treatment with TFC.
Briefly, for the apoptosis analysis assay, the PC-3 cells were
placed in a 6-well plate (1×106 cells/well) and treated
with various doses of TFC for 24 h. The cells were then collected,
washed with phosphate-buffered saline (PBS) and re-suspended in 1X
binding buffer. FITC Annexin V and propidium iodide (PI) reagent
were added and staining occurred for 15 min prior to flow cytometry
analysis. For the cell cycle analysis assay performed subsequent to
TFC treatment, the cells were collected and fixed with 70% ethanol
overnight. The cells were then handled according to the
manufacturer's instructions with Cycletest plus DNA Reagent kit,
prior to analysis by flow cytometry. The cells in the G0/G1, S and
G2/M phases were gated out as appropriate.
Western blot analysis
The PC-3 cells were treated with TFC for 24 h and
the treated cells were collected, washed three times with PBS and
lysed in cell lysis buffer. The cell lysates were separated on a
15% polyacrylamide gel and transferred to a polyvinyl difluoride
membrane (EMD Millipore, Billerica, MA, USA). Subsequent to
blocking with Block Ace (AppliChem GmbH, Darmstadt, Germany) for 4
h at room temperature, the membrane was incubated overnight with
primary antibodies, and then for 2 h with secondary antibodies. All
antibodies were obtained from Cell Signaling Technology (Danvers,
MA, USA). The following rabbit anti-human primary antibodies were
used for western blot analysis: Anti-p27Kip1
(monoclonal; clone, D69C12; cat no. 3686); anti-PTEN (monoclonal;
clone, D4.3; cat no. 9188); anti-caspase-7 (polyclonal; cat no.
9492); anti-caspase-3 (monoclonal; clone, 8G10; cat no. 9665); and
anti-β-actin (monoclonal; clone, 13E5; cat no. 4970). Anti-rabbit
IgG conjugated to horseradish peroxidase (cat no. 7074) was used as
the secondary antibody. The primary antibodies were used at a
dilution of 1:1,000. The secondary antibodies were used at a
dilution of 1:3,000 and visualized with an enhanced
chemiluminescence system (Amersham Imager 600; GE Healthcare Life
Sciences, Little Chalfont, UK).
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA). The results
were expressed as the mean ± standard deviation. P<0.05 or
P<0.01 was considered to indicate a statistically significant
difference.
Results
TFC inhibits PC-3 cell growth in a
dose-dependent manner
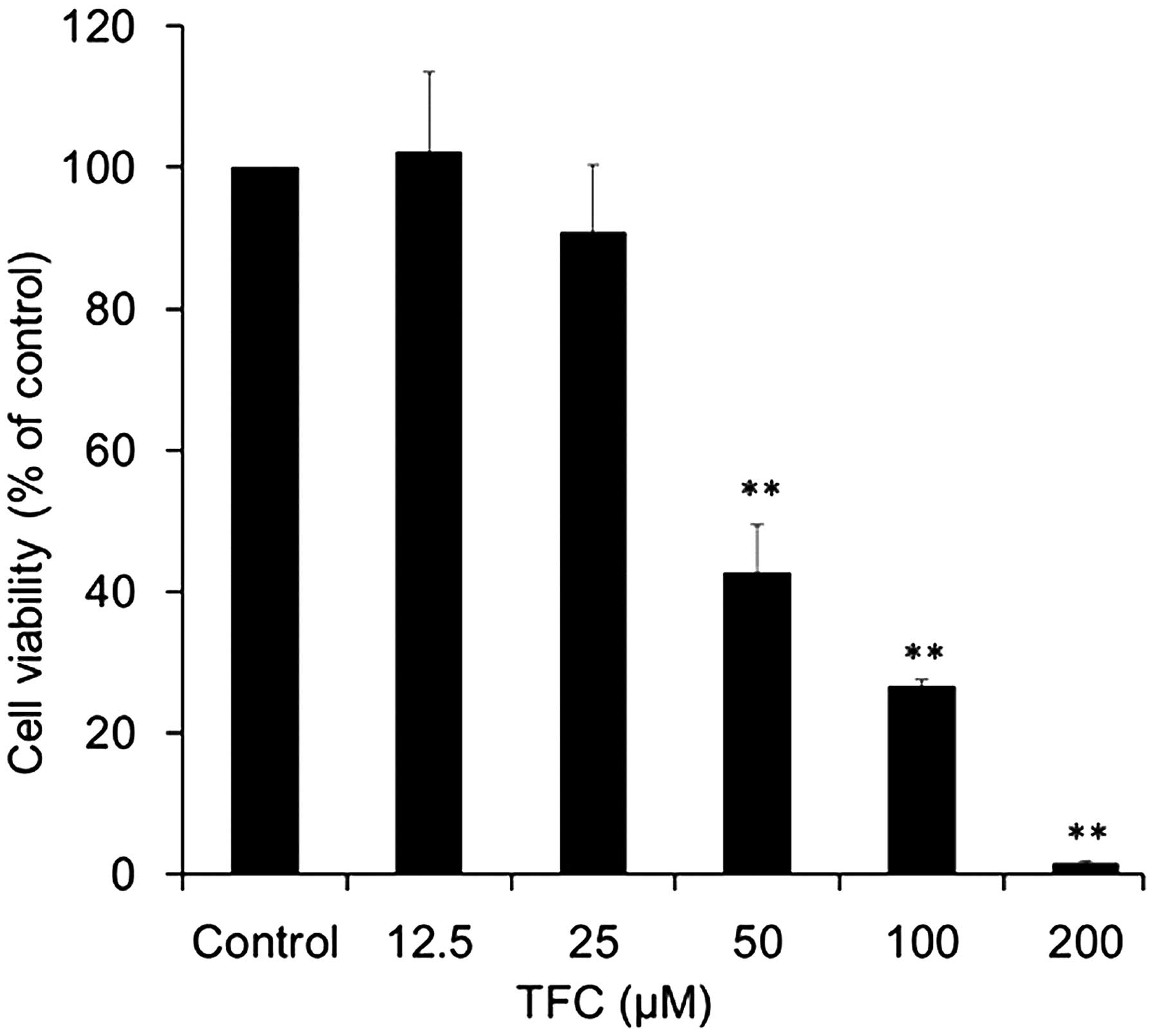
The CCK-8 assay was used as an indirect measure to
determine the viability of PC-3 cells exposed to TFC. TFC
significantly suppressed the cell viability of PC-3 cells in a
dose-dependent manner (Fig. 2). The
cell viability was reduced to 90.81, 42.51, 26.52 and 1.51% when
cells were treated with 25, 50, 100 and 200 µM TFC, respectively.
The half maximal inhibitory concentration (IC50) was
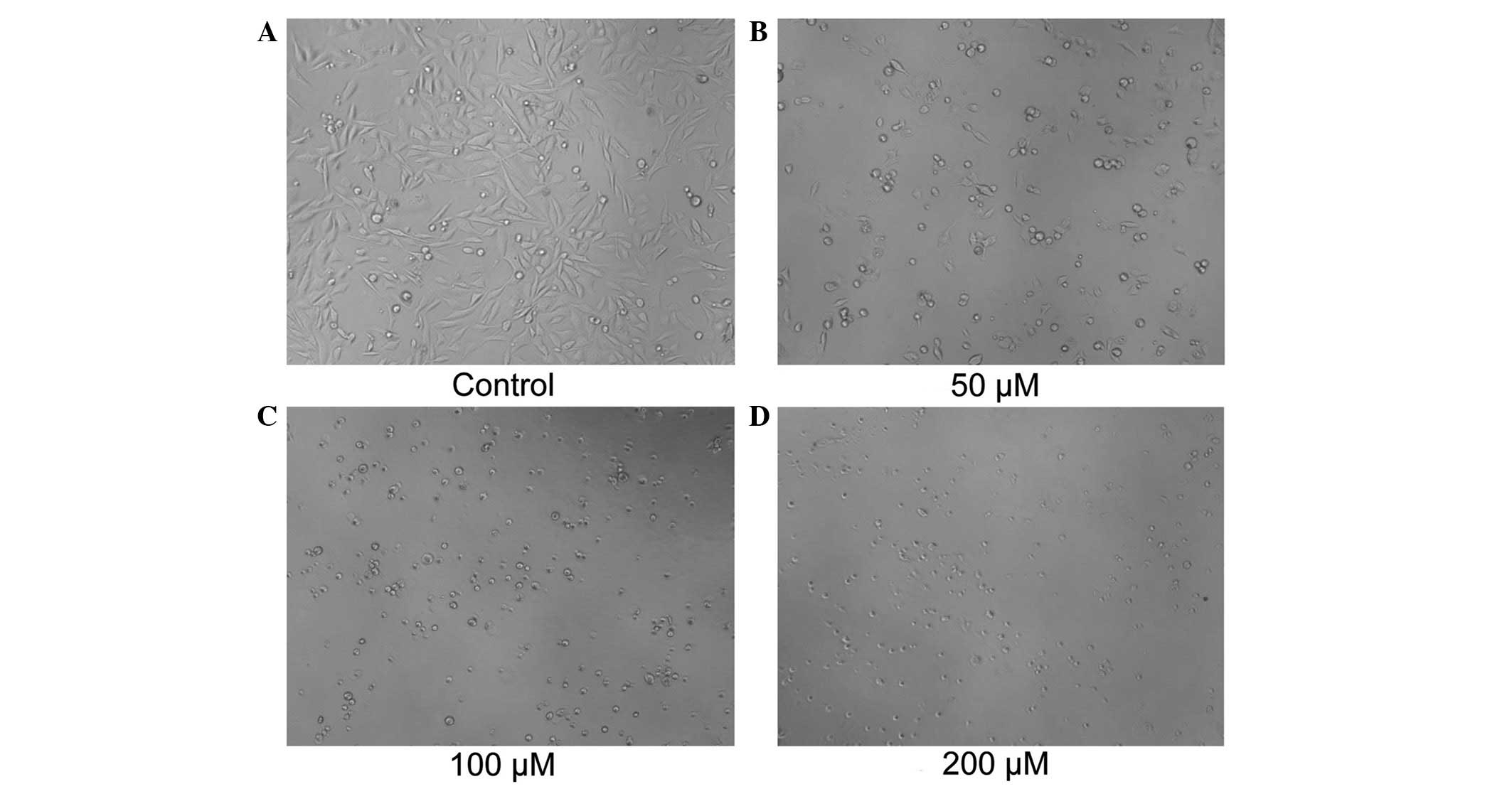
53.82 µM. Morphological changes were observed using microscopy
(Fig. 3). The PC-3 cells treated with
TFC for 24 h exhibited marked morphological changes. TFC caused
individual cells to shrink and separate from neighboring cells. The
cells appeared refringent and finally detached from the monolayer
by TFC administration.
TFC induces apoptosis in PC-3
cells
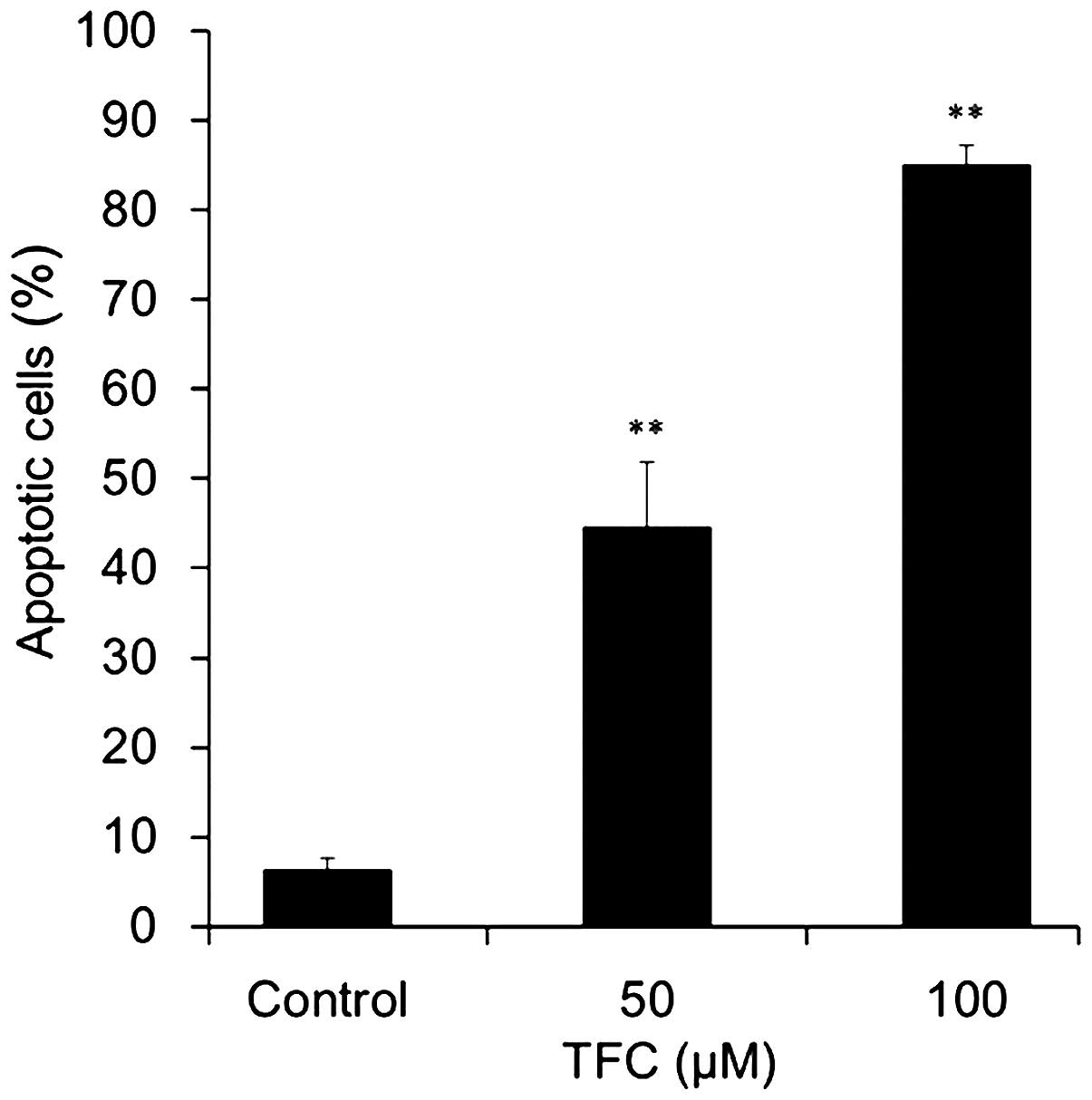
To determine whether the reduction of cell viability
in PC-3 cells was associated with the induction of apoptosis, flow
cytometry analysis was used to assess the number of apoptotic cells
following TFC treatment. Apoptotic cells were significantly
increased in a dose-dependent manner when compared with the control
group (Fig. 4). The percentage of
apoptotic cells reached 44.58±7.19 and 85.05±2.14% when treated
with 50 and 100 µM TFC, respectively. These results demonstrated
that TFC inhibited the proliferation of PC-3 cells by inducing
apoptosis.
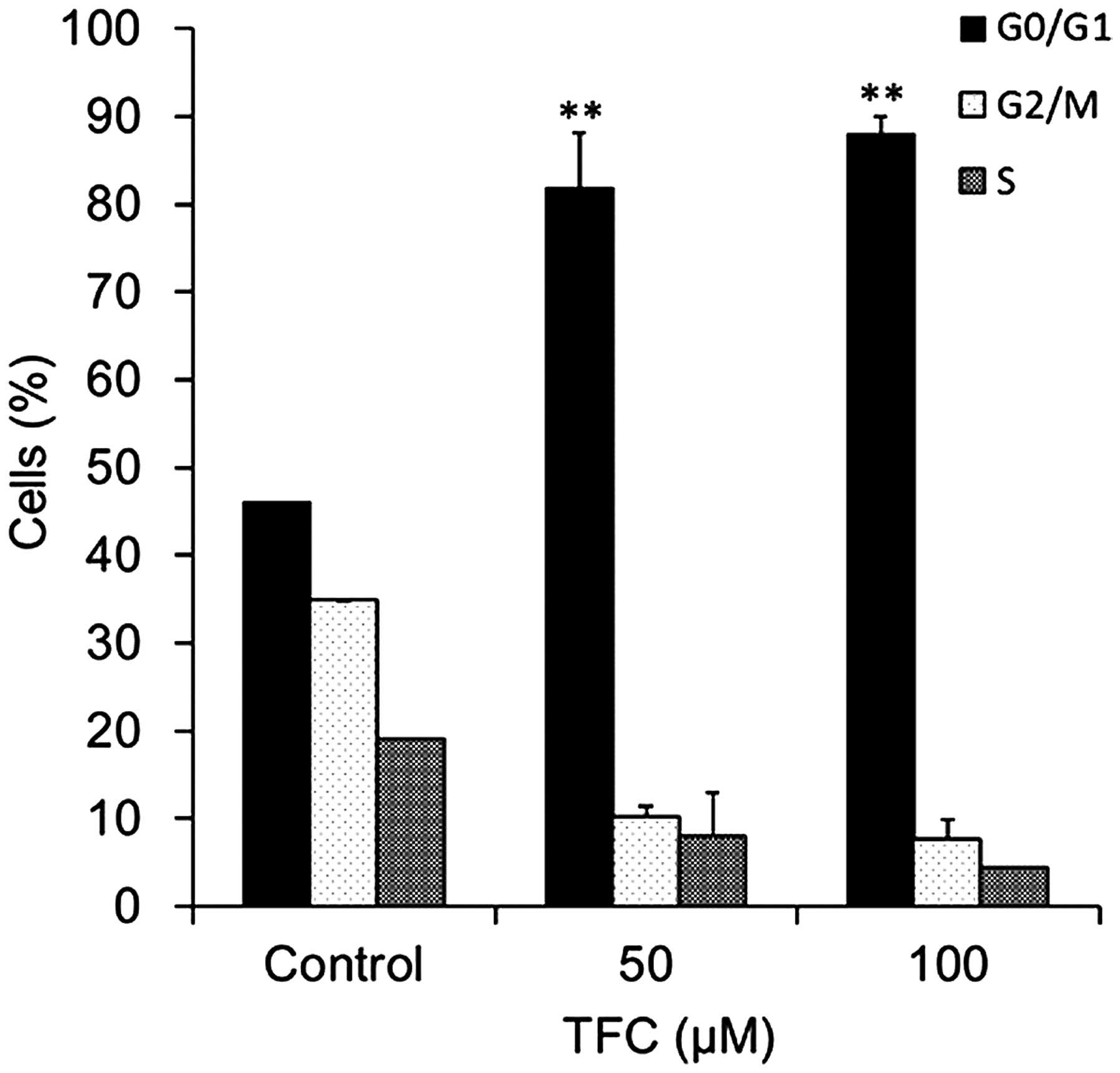
TFC arrests the cell cycle of PC-3
cells in the G0/G1 phase
Cell cycle distribution was assessed by flow
cytometry. The results demonstrated that TFC treatment caused G0/G1
phase cell cycle arrest in PC-3 cells (Fig. 5). The percentage of cells in the G0/G1
phase significantly increased between 46.06% in the cells not
treated with TFC and 89.87% in cells treated with 100 µM TFC. These
results indicated that TFC induced apoptosis in PC-3 cells by
arresting the cell cycle in the G0/G1 phase.
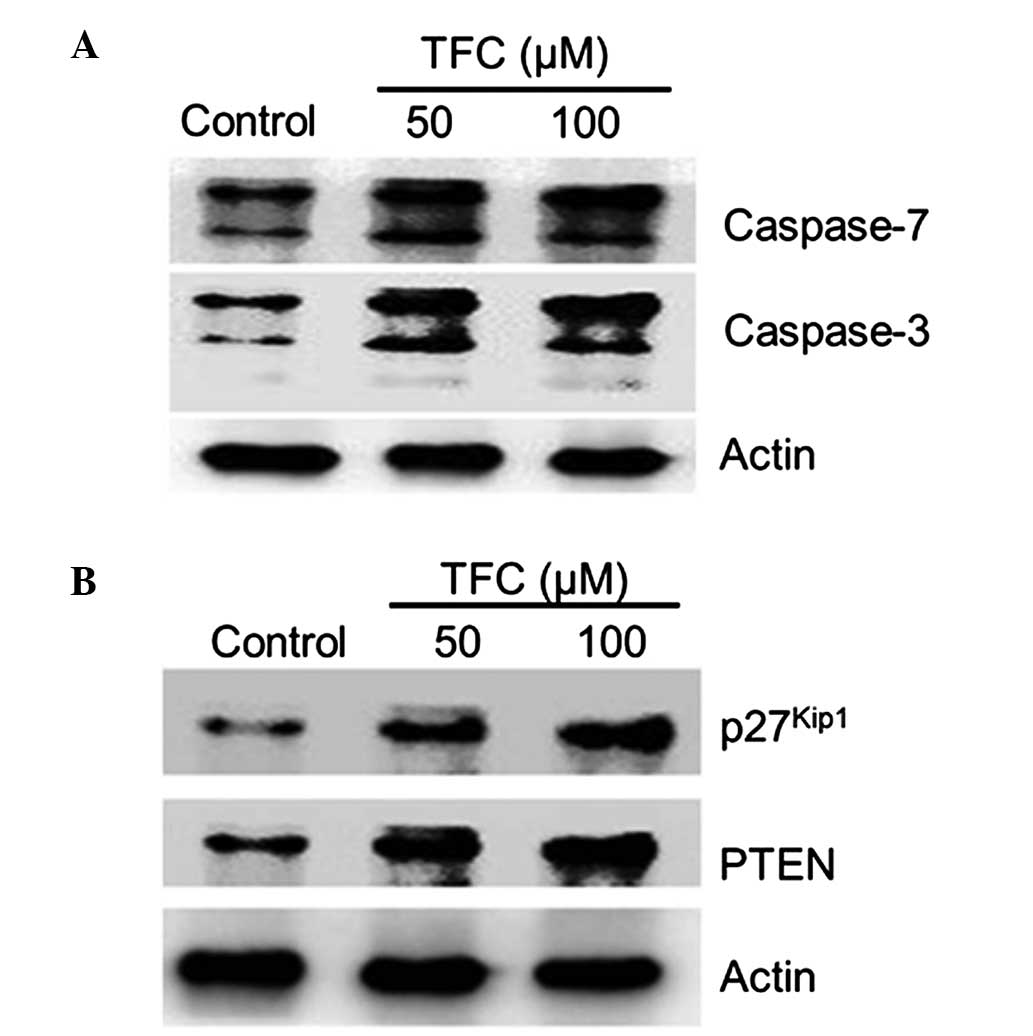
TFC increases caspase-3/7 activation
in PC-3 cells
Caspases are crucial mediators of programmed cell
death, termed apoptosis (14). Out of
these mediators, caspase-3 and caspase-7 are responsible for the
proteolytic cleavage of numerous key proteins, including adenosine
diphosphate (ADP)-ribose polymerase and poly(ADP-ribose)
polymerase, that induce cell apoptosis (14). In the present study, TFC treatment
significantly increased the activation of caspase-3 and −7
(Fig. 6A), demonstrating the
importance of caspases in the induction of apoptosis.
TFC upregulates PTEN and
p27Kip1 expression in PC-3 cells
The expression levels of the cyclin-dependent kinase
inhibitor p27Kip1 have been revealed to be a reliable
prognostic marker in prostate cancer, as low or absent expression
of p27Kip1 correlates with poor prognosis (15). In addition, PTEN, a tumor-suppressor
gene and one of the most promising biomarkers for prostate cancer,
also acts as a valid and accurate tool for the prediction of
prognosis and support of clinical decisions (16,17). To
determine the effect of TFC on these biomarkers in prostate cancer,
western blot analysis was performed to assess the effect of TFC on
the expression of p27Kip1 and PTEN in PC-3 cells.
Notably, the expression of PTEN and p27Kip1 was
significantly upregulated subsequent to treatment with TFC
(Fig. 6B).
Discussion
Apoptosis, an ordered and orchestrated cellular
process that occurs in physiological and pathological conditions,
plays an important role in the treatment of cancer and is a popular
target of numerous treatment strategies (18). In general, drug-induced apoptosis is a
major advancement for the treatment of cancer (19). In the present study, it was
demonstrated that TFC may effectively inhibit the growth of PC-3
cells, induce apoptosis, activate caspase-3/-7 and block the cell
cycle in the G0/G1 phase. TFC was revealed to be a potential
compound for prostate cancer therapy.
Expression levels of p27Kip1 have been
revealed as reliable prognostic markers in prostate cancer
(15). Studies have linked loss of
p27Kip1 expression with the development and progression
of prostate carcinoma, and animal models have implicated this gene
in the development of prostate carcinoma (20–23). In
addition, p27Kip1 is also a cell-cycle regulatory
protein that interacts with cyclin-dependent kinase (CDK)2 and
CDK4, inhibiting cell cycle progression at the G1 phase (24). An increase in p27Kipl
protein expression causes proliferating cells to exit from the cell
cycle, whereas a decrease in p27Kip1 protein expression
promotes quiescent cells to resume cell proliferation (25). By contrast, PTEN, a tumor-suppressor
gene, is one of the most promising biomarkers for prostate cancer
(16,17). PTEN is hypothesized to operate via the
action of its phosphatase protein product. This phosphatase is
involved in the regulation of the cell cycle and prevents cells
from growing and dividing rapidly (26). The expression of PTEN can inhibit cell
cycle progression, induce G0/G1 cell cycle arrest, inhibit cell
migration and induce apoptosis (27–30).
Consistent with these previous findings, the expression of
p27Kip1 and PTEN was significantly upregulated with TFC
treatment in PC-3 cells in the present study (Fig. 6B). The upregulation of
p27Kip1 and PTEN may contribute to the cell cycle arrest
in the G0/G1 phase and the induction of apoptosis. Furthermore,
previous studies demonstrated that PTEN regulates the
ubiquitin-dependent degradation of the CDK inhibitor
p27Kip1 through the Skp, Cullin, F-box containing
ubiquitin E3 ligase complex (31). It
is possible that the activation of p27Kip1 may be due to
the upregulation of PTEN in PC-3 cells. However, numerous studies
are required to test this hypothesis.
In conclusion, the present study demonstrated that
TFC treatment in PC-3 cells can upregulate the expression of
p27Kip1 and PTEN, block the cell cycle in the G0/G1
phase and induce apoptosis. However, additional studies are
required to determine the exact mechanisms of action and to explore
genetic and signal transduction pathways. Finally, the present
results revealed that TFC may be a potential compound for prostate
cancer therapy.
Acknowledgements
This study was kindly supported by the Zhenjiang
Science and Technology Project (grant no. SH2012040).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brawley OW, Barnes S and Parnes H: The
future of prostate cancer prevention. Ann Ny Acad Sci. 952:145–152.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crowell JA: The chemopreventive agent
development research program in the Division of Cancer Prevention
of the US National Cancer Institute: An overview. Eur J Cancer.
41:1889–1910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yue R, Li B, Shen Y, Zeng H, Li B, Yuan H,
He Y, Shan L and Zhang W: 6-C-methyl flavonoids isolated from Pinus
densata inhibit the proliferation and promote the apoptosis of the
HL-60 human promyelocytic leukaemia cell line. Planta Med.
79:1024–1030. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Costa SS, Couceiro JN, Silva IC, Ddo
Malvar C, Coutinho MA, Camargo LM, Muzitano MF and Vanderlinde FA:
Flavonoids in the therapy and prophylaxis of flu: A patent review.
Expert Opin Ther Pat. 22:1111–1121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Chen Y, Wang J, Chen J, Aggarwal
BB, Pang X and Liu M: Xanthohumol, a prenylated chalcone derived
from hops, suppresses cancer cell invasion through inhibiting the
expression of CXCR4 chemokine receptor. Curr Mol Med. 12:153–162.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Priyadarsini Vidya R, Murugan Senthil R,
Maitreyi S, Ramalingam K, Karunagaran D and Nagini S: The flavonoid
quercetin induces cell cycle arrest and mitochondria-mediated
apoptosis in human cervical cancer (HeLa) cells through p53
induction and NF-κB inhibition. Eur J Pharmacol. 649:84–91. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lou CH, Yang GM, Cai H, Zou M, Xu Z, Li Y,
Zhao F, Li W, Tong L, Wang M and Cai B:
2′,4′-Dihydroxychalcone-induced apoptosis of human gastric cancer
MGC-803 cells via down-regulation of survivin mRNA. Toxicol In
Vitro. 24:1333–1337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen JJ, Lee HH, Duh CY and Chen IS:
Cytotoxic chalcones of flavonoids from the leaves of Muntingia
calabura. Planta Med. 71:970–973. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pouget C, Lauthier F, Simon A, Fagnere C,
Basly JP, Delage C and Chulia AJ: Flavonoids: Structural
requirements for antiproliferative activity on breast cancer cells.
Bioorg Med Chem Lett. 11:3095–3097. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lou C, Wang M, Yang G, Cai H, Li Y, Zhao
F, Yang H, Tong L and Cai B: Preliminary studies on anti-tumor
activity of 2′,4′-dihydroxychalcone isolated from Herba oxytropis
in human gastric cancer MGC-803 cells. Toxicol In Vitro.
23:906–910. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu F and Xu XJ: Studies on flavonoids of
Oxytropis falcata. Zhongguo Zhong Yao Zazhi. 32:318–320. 2007.(In
Chinese).
|
|
14
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Macri E and Loda M: Role of p27 in
prostate carcinogenesis. Cancer Metastasis Rev. 17:337–344. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pourmand G, Ziaee AA, Abedi AR, Mehrsai A,
Alavi HA, Ahmadi A and Saadati HR: Role of PTEN gene in progression
of prostate cancer. Urol J. 4:95–100. 2007.PubMed/NCBI
|
|
17
|
Liu Y, Hegde P, Zhang F, Hampton G and Jia
S: Prostate cancer - a biomarker perspective. Front Endocrinol
(Lausanne). 3:722012.PubMed/NCBI
|
|
18
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Canc Res. 30:872011.
View Article : Google Scholar
|
|
19
|
Saddoughi SA, Song P and Ogretmen B: Roles
of bioactive sphingolipids in cancer biology and therapeutics.
Subcell Biochem. 49:413–440. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo YP, Sklar GN, Borkowski A and
Kyprianou N: Loss of the cyclin-dependent kinase inhibitor
p27(Kip1) protein in human prostate cancer correlates with tumor
grade. Clin Cancer Res. 3:2269–2274. 1997.PubMed/NCBI
|
|
21
|
Yang RM, Naitoh J, Murphy M, Wang HJ,
Phillipson J, de Kernion JB, Loda M and Reiter RE: Low p27
expression predicts poor disease-free survival in patients with
prostate cancer. J Urol. 159:941–945. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsihlias J, Kapusta LR, DeBoer G,
Morava-Protzner I, Zbieranowski I, Bhattacharya N, Catzavelos GC,
Klotz LH and Slingerland JM: Loss of cyclin-dependent kinase
inhibitor p27Kip1 is a novel prognostic factor in localized human
prostate adenocarcinoma. Cancer Res. 58:542–548. 1998.PubMed/NCBI
|
|
23
|
Cordon-Cardo C, Koff A, Drobnjak M,
Capodieci P, Osman I, Millard SS, Gaudin PB, Fazzari M, Zhang ZF,
Massague J and Scher HI: Distinct altered patterns of p27Kip1 gene
expression in benign prostatic hyperplasia and prostatic carcinoma.
J Natl Cancer Inst. 90:1284–1291. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kato JY, Matsuoka M, Polyak K, Massague J
and Sherr CJ: Cyclic AMP-induced G1 phase arrest mediated by an
inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell.
79:487–496. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Le XF, Claret FX, Lammayot A, Tian L,
Deshpande D, LaPushin R, Tari AM and Bast RC Jr: The role of
cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2
antibody-induced G1 cell cycle arrest and tumor growth inhibition.
J Biol Chem. 278:23441–23450. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chu EC and Tarnawski AS: PTEN regulatory
functions in tumor suppression and cell biology. Med Sci Monit.
10:RA235–RA241. 2004.PubMed/NCBI
|
|
27
|
Furnari FB, Huang HJ and Cavenee WK: The
phosphoinositol phosphatase activity of PTEN mediates a
serum-sensitive G1 growth arrest in glioma cells. Cancer Res.
58:5002–5008. 1998.PubMed/NCBI
|
|
28
|
Persad S, Attwell S, Gray V, Delcommenne
M, Troussard A, Sanghera J and Dedhar S: Inhibition of
integrin-linked kinase (ILK) suppresses activation of protein
kinase B/Akt and induces cell cycle arrest and apoptosis of
PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA.
97:3207–3212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Backman SA, Ghazarian D, So K, Sanchez O,
Wagner KU, Hennighausen L, Suzuki A, Tsao MS, Chapman WB, Stambolic
V and Mak TW: Early onset of neoplasia in the prostate and skin of
mice with tissue-specific deletion of Pten. Proc Natl Acad Sci USA.
101:1725–1730. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie F, Su M, Qiu W, Zhang M, Guo Z, Su B,
Liu J, Li X and Zhou L: Kaempferol promotes apoptosis in human
bladder cancer cells by inducing the tumor suppressor, PTEN. Int J
Mol Sci. 14:21215–21226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mamillapalli R, Gavrilova N, Mihaylova VT,
Tsvetkov LM, Wu H, Zhang H and Sun H: PTEN regulates the
ubiquitin-dependent degradation of the CDK inhibitor p27(Kip1)
through the ubiquitin E3 ligase SCF(SKP2). Curr Biol. 11:263–267.
2001. View Article : Google Scholar : PubMed/NCBI
|