Introduction
Epidermal growth factor receptor (EGFR) is a
transmembrane glycoprotein, with an intracellular component that
acts as a tyrosine kinase (1). As the
EGFR/K-ras pathway is commonly activated in metastatic colorectal
cancer (mCRC), it is an attractive target for molecular therapy
(1).
CRC is one of the most common malignancies in men
and women (2). Cetuximab, a
monoclonal antibody (mAb) directed against EGFR, has shown activity
in monotherapy and in combination with chemotherapy
(chemoimmunotherapy) in various lines of mCRC treatment (3–6). An
analysis of Cetuximab Combined with Irinotecan in First-Line
Therapy for Metastatic Colorectal Cancer (CRYSTAL) phase III and
Oxaliplatin and Cetuximab in First-Line Treatment of mCRC (OPUS)
phase II randomized clinical trials showed statistically
significant improvement in overall survival (OS), progression-free
survival (PFS) and overall response rate (ORR) in patients without
K-ras mutation receiving cetuximab with first-line chemotherapy
(6). The CO.17 trial proved that
cetuximab monotherapy administered following progression on
chemotherapy lines (oxaliplatin and irinotecan with 5-fluorouracil)
also improves OS, PFS and ORR (5).
Inhibition of the EGFR/K-ras pathway by cetuximab is connected with
numerous side-effects, such as skin toxicity, diarrhea,
hypomagnesemia and other dyselectrolytemias or infusion reactions
(1,7).
A previous study by our group (7)
analyzed skin toxicity associated with cetuximab-based therapy;
acne-like rash was observed at a frequency of 80% and paronychia at
20%.
Hypomagnesemia may be a result of insufficient
magnesium (Mg) supplementation in the diet, hormonal imbalance,
antibiotic usage or alcoholism (8,9). The
National Cancer Institute Common Terminology Criteria for Adverse
Events (CTCAE) version 4.0 are used to grade levels of
hypomagnesemia (Table I) (10). The most common symptom of
hypomagnesemia is weakness. There are also other problems,
including irritability, arrhythmias or metabolic and neuromuscular
disorders, which may be revealed in the case of higher grades of
this dyselectrolytemia (8,9). However, the incidence and severity of
hypomagnesemia were not assessed in the aforementioned clinical
trials.
 | Table I.Grades of hypomagnesemia according to
common terminology criteria for adverse events v.4.0. |
Table I.
Grades of hypomagnesemia according to
common terminology criteria for adverse events v.4.0.
| Grade | Hypomagnesemia |
|---|
| 1 | <LLN-1.2 mg/dl,
<LLN-0.5 mmol/l |
| 2 | <1.2–0.9 mg/dl,
<0.5–0.4 mmol/l |
| 3 | <0.9-0.7 mg/dl,
<0.4-0.3 mmol/l |
| 4 | <0.7 mg/dl,
<0.3 mmol/l, life-threatening consequences |
| 5 | Mortality |
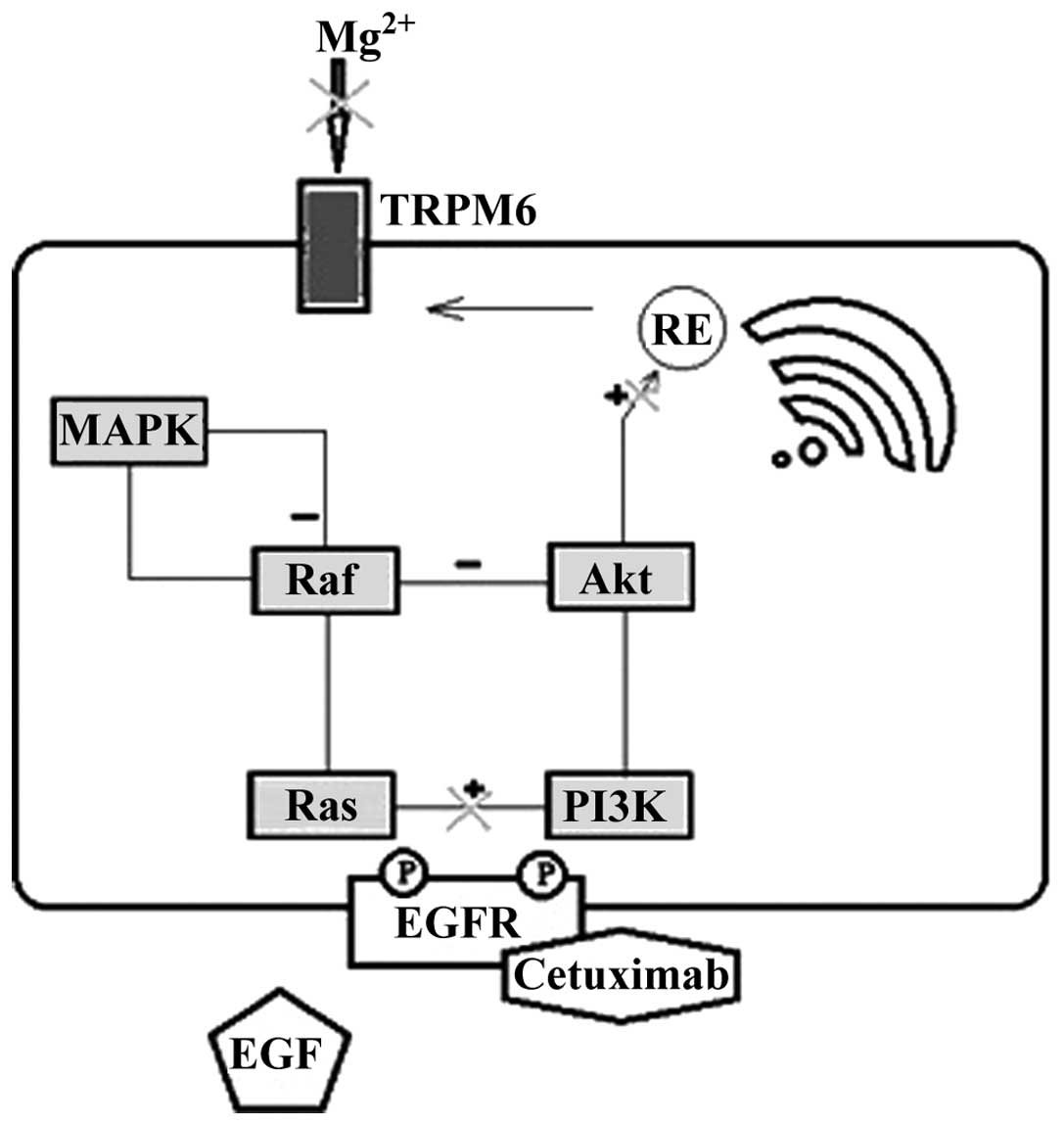
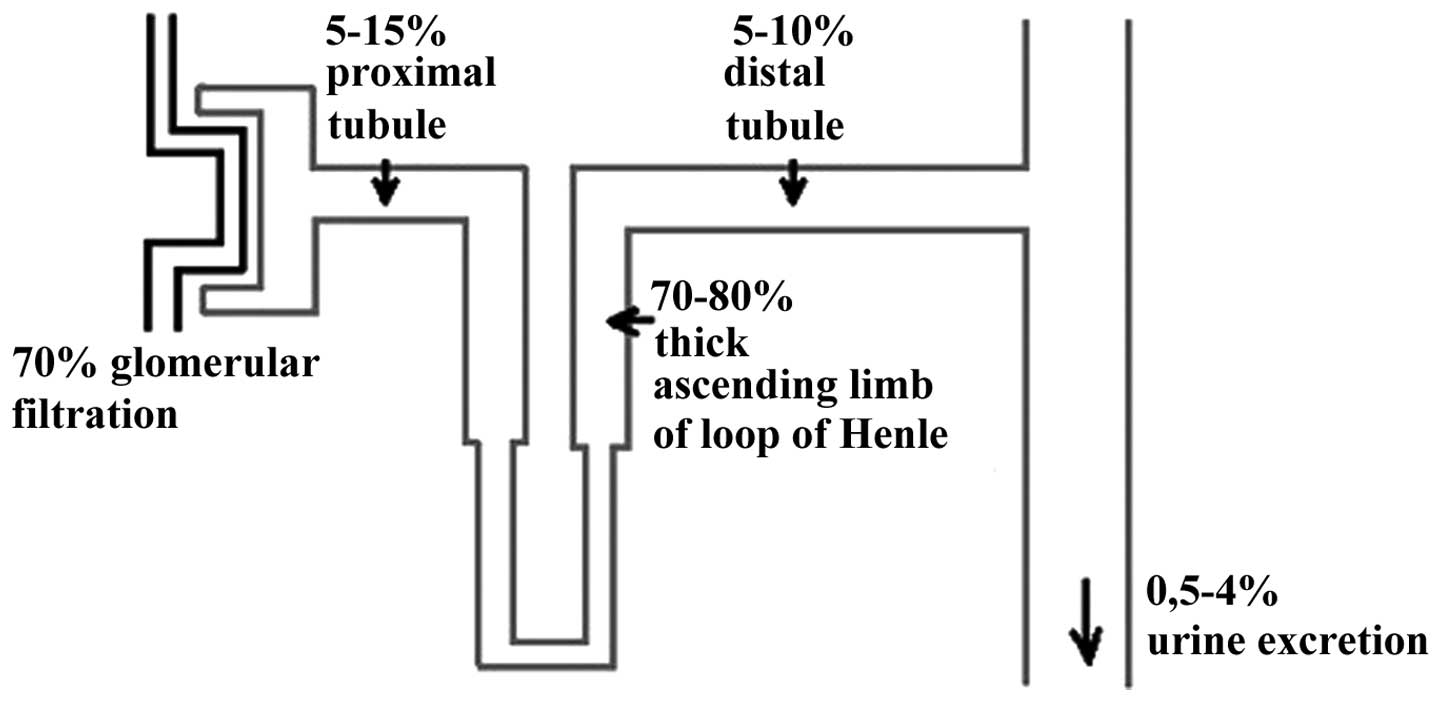
Cells building the renal tubule are characterized by
a high level of EGFR expression. The mechanism of Mg wasting during
treatment with cetuximab is associated with the blockage of
EGFR-dependent transient receptor potential channel 6 (TRPM6) in
the nephron (Fig. 1) (11,12). The
blockage of this pathway results in insufficient activation of the
TRPM6 epithelial ion channel and Mg wasting (11,12). The
process takes place mainly in the distal convoluted tube of the
nephron, where the expression of TRPM6 is the greatest (Fig. 2). The other suggested mechanism is via
indirect tubular nephrotoxicity (13). The fact that after cessation of
therapy with cetuximab the Mg concentration in the plasma returns
to normal suggests the reversibility of this process (12,14).
The reason for the present study was to estimate the
frequency and severity of hypomagnesemia among patients with mCRC
treated with cetuximab. The study also aimed to measure the extent
of serum Mg assessment in this group of patients.
Patients and methods
Patients
Between October 2009 and June 2013, a retrospective
analysis of the records of 52 patients from the Department of
Clinical Oncology, University Hospital, Jagiellonian University
Medical College (Kraków, Poland) was performed. The inclusion
criteria for the study were as follows: An age of ≥18 years, a
diagnosis of CRC confirmed by an available histopathological
report, no K-ras mutation, presence of metastases on diagnostic
imaging (magnetic resonance imaging, computed tomography, positron
emission tomography or bone scintigraphy), and receipt of at least
2 doses of cetuximab. The exclusion criteria included: Concurrent
malignancies (also in the past), malabsorption or genetic Mg
wasting syndromes, a history of hypomagnesemia prior to the
treatment, alcoholism, diarrhea (grade 3 or greater according to
CTCAE v.4.0) during the 2 months prior to the start of treatment
and while on the treatment with cetuximab, concurrent
administration of diuretics (thiazide, loop diuretics), and a lack
of consent to participate in the study.
The analyzed factors included: Sociodemographic
data, localization of the primary tumor and metastases, clinical
staging according to the 7th edition of Tumor-Node-Metastasis
system (15), type of treatment
received (including line of systemic treatment), reason for therapy
ending, the presence of hypomagnesemia associated with cetuximab
therapy and its intensity. Hypomagnesemia was classified according
to the CTCAE v.4.0 (Table I)
(10).
Statistical analysis
Statistical evaluation was conducted using computer
software Statistica 11.0 PL (StatSoft Poland, Krakow, Poland).
Descriptive statistics are used in the form of percentage
distribution, range or mean ± standard deviation. When comparing
the quantitative variables, Student's t-test was applied; if there
was absence of a normal distribution of factors, the Mann-Whitney U
test was used. To check the association between quantitative
variables, Spearman' test was conducted and the χ2 test
was applied when comparing qualitative variables. Factors
potentially associated with the risk of developing hypomagnesemia
were also assessed using logistic regression analysis. P<0.05
was used to indicate a statistically significant difference.
Ethical approval
The present study was approved by the Jagiellonian
University Medical College Ethical Committee (registry number,
KB/254/B/2011). The data was collected and analyzed in accordance
with the ethical standards laid down in the 1964 Declaration of
Helsinki with its amendments.
Literature search
A literature search of the MEDLINE database (between
January 2005 and May 2014; http://www.ncbi.nlm.nih.gov/pubmedhealth/; accessed
1st June 2014) and UpToDate (http://www.uptodate.com/; accessed 1st June 2014) was
performed to find an association between treatment with cetuximab
and hypomagnesemia. The key words ‘anti-EGFR’, ‘cetuximab’,
‘hypomagnesemia’, ‘magnesium’, ‘metastases’, ‘colorectal cancer’,
‘colon cancer’, ‘monoclonal antibody’ and ‘TRPM6’ were used in
various combinations.
Results
Of the 52 patients analyzed, 27 patients who
fulfilled all the inclusion criteria and none of the exclusion
criteria were enrolled into the study. In the excluded cases, 21
lacked a serum Mg level assessment, and the remainder developed
grade 3 diarrhea or an anaphylactic reaction.
Table II shows the
baseline characteristics of the study population, which was
composed of 7 females and 20 males, with a median (± standard
deviation) age of 55.0±11.6 years. Cetuximab was administered as a
palliative regiment at a standard dose of 400 mg/m2 as a
first dose and at 250 mg/m2 in each subsequent dose
regardless of whether it was administered as monotherapy or in
combination with standard chemotherapy. Chemotherapy regimens were
based on irinotecan, oxaliplatin or capecytabine alone. The main
reason for treatment termination in the patients treated with
monotherapy or immunochemotherapy was progression of the disease
(92.6%). The median duration of treatment with cetuximab was 98
days (range, 15–546).
 | Table II.Baseline characteristics of the
studied population. |
Table II.
Baseline characteristics of the
studied population.
| Parameter | Value |
|---|
| Age, years |
|
|
Median | 55.0 |
|
Range | 27–72 |
| Gender, n (%) |
|
|
Women | 7
(25.9) |
|
Men | 20 (74.1) |
| Primary tumor
localization, n (%) |
|
|
Rectum | 13 (48.1) |
|
Colon | 14 (51.9) |
| No. of organs
involved with metastases, n (%) |
|
| 1 | 21 (77.8) |
|
>1 | 6
(22.2) |
| Location of
metastasesa, n (%) |
|
|
Liver | 18 (66.7) |
|
Other | 14 (51.9) |
| Cetuximab treatment
line, n (%) |
|
| 1 | 9
(33.3) |
|
>1 | 18 (66.7) |
| Type of therapy, n
(%) |
|
|
Monotherapy | 4
(14.8) |
|
Chemoimmunotherapy | 23 (85.2) |
| Reason for
treatment ending, n (%) |
|
| Cancer
progression | 25
(92.6) |
|
Side-effects | 0
(0.0) |
|
Decision of a physician | 1
(3.7) |
| Lack of
data | 1
(3.7) |
In 29.6% of all patients randomly assessed (more
than once), the Mg level indicated hypomagnesemia. The majority of
cases (22.2%) were grade 1 according to CTCAE v.4.0, while 1
patient of grade 2 and 1 patient of grade 3 was revealed.
There was no statistically significant correlation
between the presence of hypomagnesemia (none vs. any) or the grade
of hypomagnesemia and patient age (≥55 vs. <55 years; P=0.1 and
P=0.1, respectively), duration of treatment (P=0.9 and P=0.3,
respectively), type of treatment (monotherapy vs. in combination
with chemotherapy; P=0.3 and P=0.6, respectively), line of systemic
treatment (P=0.3 and P=0.2, respectively), localization of primary
tumor (rectum vs. colon; P=0.6 and P=0.6, respectively) or
metastases (liver vs. other localizations; P=0.3 and P=0.3,
respectively), and number of metastases (1 vs. >1; P=0.6 and
P=0.9, respectively). There was an upward trend in a logistic
regression model showing that the risk of developing hypomagnesemia
increases with age (odds ratio, 1.10; 95% confidence interval,
0.97–1.25). However, the trend did not reach statistical
significance (P=0.1).
None of the patients had the treatment discontinued
due to the hypomagnesemia.
Discussion
Targeted therapy with mAbs has become a widely used
treatment option for cancer patients. In comparison with standard
chemotherapy, targeted drugs show lower risk of severe systemic
adverse effects. First suggestions with regard to the requirement
for Mg measurement and supplementation appeared in 2005 (16). The summary of product characteristics
produced for Erbitux (cetuximab) estimates the frequency of
hypomagnesemia in >10% of patients treated with the drug
(17). Table III (17–29) shows
the results of other studies regarding hypomagnesemia as a
side-effect of cetuximab. In the available results of retrospective
studies, the percentage of patients with any grade of
hypomagnesemia varied from 6.3–93.3% with grade 3/4 in 0 to 27%
(16,25–30). One
of the first studies of this dyselectrolytemia associated with
cetuximab by Fakih et al (14)
showed a high incidence of grade 3 and 4 compared with later
studies (27%). This may be due to the fact that only patients with
both baseline Mg level and level assessed during the treatment were
included. As checking the serum Mg concentration was not mandatory
at the time of this study, it may be hypothesized that patients
with baseline levels were those more prone to suffer from
hypomagnesemia due to other causes or had dyselectrolytemia found
in past laboratory tests. The same inclusion criteria were
introduced in a study by do Pazo-Oubiña et al, however, in
this study the incidence was higher for grade 1 and 3, with no
patient suffering from grade 4 (25).
To omit this potential bias, in the present study, the enrollment
of patients without baseline Mg level (but without hypomagnesemia
in the history) was also decided upon. Taking into consideration
only a sub-group with baseline assessment, the incidence in the
present study was slightly higher, but did not reach the levels
shown in the two aforementioned studies (data not shown).
 | Table III.Literature data on hypomagnesemia as
a side-effect of cetuximab compared with the results of the present
study. |
Table III.
Literature data on hypomagnesemia as
a side-effect of cetuximab compared with the results of the present
study.
|
|
|
| Hypomagnesemia,
% |
|
|
|---|
|
|
|
|
|
|
|
|---|
| First author/s
(ref.) | Year | Type of cancer | G1 |
| G2 | G3 |
| G4 | Any grade | No. of patients
enrolled | Type of study |
|---|
| Present study | 2014 | Colorectal | 22.2 |
| 3.7 | 3.7 |
| 0.0 | 29.6 |
27 | Retrospective |
| Chen et al
(18) | 2013 | Colorectal |
| No data |
|
| 2.9 |
| 25.8a | 2769 | Meta-analysis |
| Cao et al
(19) | 2010 | Colorectal, head
and neck, non small-cell lung, liver, ovarian, esophageal,
gastric |
| No data |
|
| 5.6 |
| 36.7b | 3006 | Meta-analysis |
| Price et al
(20) | 2014 | Colorectal |
| 15.1 |
| 2.0 |
| 0.6 | 17.7 |
503 | Prospective |
| Lordick (21) | 2013 | Gastric |
| 19 |
| 7 |
| 3 | 29 |
446 | Prospective |
| Vickers (22) | 2013 | Colorectal | 20.6 |
| 2.8 | 0.9 |
| 0.5 | 24.8 |
218 | Prospective |
| Weickhardt et
al (23) | 2012 | Colorectal |
| 20 |
|
| 18 |
| 38 |
50 | Prospective |
| Vincenzi et
al (24) | 2008 | Colorectal | 4.4 |
| 0.0 | 0.0 |
| 0.0 | 4.4 |
68 | Prospective |
| Tejpar et al
(12)c | 2007 | Colorectal | 35 |
| 13 | 3 |
| 3 | 54 |
98 | Prospective |
| Do Pazo-Oubiña
et al (25)d | 2013 | Colorectal | 31.3 |
| 0.0 | 12.5 |
| 0.0 | 43.8 |
16 | Retrospective |
| Demizu et al
(26)e | 2013 | No data | 73.3 |
| 13.3 | 6.7 |
| 0.0 | 93.3 |
15 | Retrospective |
| Melichar et
al (27) | 2012 | Colorectal |
| No data |
| 6 |
| 4 | 56 |
51 | Retrospective |
| Vincenzi et
al (28) | 2011 | Colorectal | 5.6 |
| 0.7 | 0.0 |
| 0.0 | 6.3 |
143 | Retrospective |
| Maliaka and Ledford
(29) | 2010 | Head and neck,
colorectal | 48 |
| 5 |
| 2 |
| 55 |
58 | Retrospective |
| Fakih et al
(30) | 2006 | Colorectal | No data |
| 8 | 8 |
| 19 | No data |
48 | Retrospective |
| Schrag et al
(15) | 2005 | Colorectal |
| No data |
| 11.8 |
| 2.9 | No data |
34 | Retrospective |
Notably, prospective studies also showed significant
discrepancies in the assessment of hypomagnesemia incidence, with
any grade found in 4.4 to 54% of patients (12,20–24). Even
when not taking into consideration the study by Tejpar et
al, which checked the effects of cetuximab, matuzumab and
panitumumab, the differences are significant (4.4 to 38%). The
present data are the closest to those obtained by Vickers et
al (22). Building on their
research and conclusions, the huge differences in results [for
example when compared with the studies by Vincenzi et al
(24,28)] may be associated with different
baseline serum Mg concentrations or applied types of concomitant
and past systemic treatments.
Vickers et al (22) performed a sub-group analysis, which
found hypomagnesemia to be more commonly presented in patients
without K-ras mutation (19 vs. 27%; the difference was not
statistically assessed for the significance).
Looking at the two meta-analyses found in the
literature search, any grade of hypomagnesemia was observed in 25.8
and 36.7% of patients, and grade 3/4 in 2.9 and 5.6% of patients,
respectively (18,19). These results are consistent with the
present study observations. Grades 1 and 2 were estimated in none
of these meta-analyses (18,19). According to Chen et al
(18), patients with mCRC have a
higher incidence of hypomagnesemia grade 3/4 than patients with
other malignancies. Comparing their results obtained for patients
with mCRC with an earlier meta-analysis performed by Cao et
al (19) on a group of patients
with various malignancies it can be noted that this incidence was
actually higher in the latter study. Additionally, a retrospective
study by Maliakal and Ledford that also enrolled patients with head
and neck cancer had one of the highest percentages of patients with
hypomagnesemia. Table III presents
only a sub-group of patients from the study by do Pazo-Oubiña et
al (mCRC treated with cetuximab) (25). In this study, patients with head and
neck carcinoma were also enrolled, and it was concluded that
overall hypomagnesemia was less common in mCRC patients than head
and neck cancer patients (43.8 vs. 72.2%).
In the present study, no grade 4 hypomagnesemia was
observed, which is generally consistent with the majority of other
studies where this metabolic complication was a rare event at grade
4. Only one study estimated the level of grade 3/4 hypomagnesemia
at 27% (30). Thus, it may be assumed
that Mg depletion is a common, but not life-threatening
complication in the population of patients treated with
EGFR-targeting mAbs. Also, certain other studies indicated that
there was no requirement for therapy termination (or reduction) due
to hypomagnesemia caused by cetuximab (12). However, it has also been indicated
that this metabolic side-effect may influence treatment in severely
affected individuals (12).
The main reason for Mg wasting is the blockage of
EGFR-dependent TRPM6 in the nephron resulting in impaired renal
reabsorption (12,22). There are also suggestions that
blocking EGFR by cetuximab may affect the absorption of Mg in the
gut (12,16), or that the tubular damage in the
kidneys is caused by mAb precipitation (14). Few factors that may predispose to the
development and severity of hypomagnesemia during the treatment
with cetuximab are taken into consideration. Certain studies
propose that concurrent chemotherapy with platinum agents is
indicated, as these affect Mg level most significantly (31). Also, the time factor appears to play
an important role (30,31). Results of one study showed an increase
in hypomagnesemia incidence proportional to the duration of the
treatment (30). No such association
was observed in the present study.
Tejpar et al found an association between an
older patient age and Mg wasting (12). This trend was also observed in the
present study, although it was not statistically significant. The
connection appears to be logical, as ageing is also connected with
other conditions leading to Mg loss, such as glomerulosclerosis or
deterioration in renal function (32). Notably, higher baseline level may be
connected with more prompt Mg reduction (12,22).
Hypomagnesemia resulting from EGFR blockage may be a
class effect for all mAbs directed against this receptor. Exact
differences between mAbs have not yet been assessed (12).
There are no reliable and precise recommendations
concerning Mg measurement and supplementation in patients with mCRC
receiving anti-EGFR mAbs. The Erbitux summary of product
characteristics claims only that the assessment of serum Mg level
(and that of other electrolytes) prior to and periodically during
the treatment with cetuximab and, as appropriate, supplementation
of electrolytes is recommended (17).
Additionally, studies have made suggestions that regular Mg
screening should be performed, particularly in patients treated
simultaneously with platinum-based agents (25,31). The
suggested interval for serum Mg measurement is 4–8 weeks plus the
baseline level (25).
As there are no particular recommendations for Mg
replacement in this particular group of patients, it appears
reasonable to follow general guidelines for Mg supplementation
(9). Certain cancer centers have
created their own treatment guidelines (26,30). Fakih
et al (30) administered
intravenous Mg sulfate daily or 3 times/week, at 6–10 g per dose,
in patients with grade 3 and 4 hypomagnesemia. Also Tejpar et
al (12) performed daily
intravenous Mg supplementation in severely affected individuals. It
is notable that oral Mg supplementation in cancer patients may be
ineffective due to diarrhea or malabsorption (12,30).
Results of the study comparing oral low- and high-dose Mg
supplementation in this group of patients are expected to be
published (12).
Due to its retrospective character and small
population size, the present study is characterized by certain
limitations. Data regarding sociodemographic status, as well as
information on the treatment and disease were gathered from medical
records. Sporadically, the information was incomplete. Only half
the patients (27/52; 51.9%) entered the study. The reason for this
was mainly as there was no information on Mg level due to the lack
of recommendations suggesting regular Mg measurement. However,
previously described studies on cetuximab efficacy have also not
carefully assessed the frequency of hypomagnesemia (3–6). In a
study performed by Schrag et al, only 22.1% of patients (34
patients) entered the retrospective studies assessing Mg level
(16,25), while in a recently published study by
do Pazo-Oubiña et al, 33.8% of patients (68 patients)
received mAb anti-EGFR. Currently, all patients in the Department
of Clinical Oncology, University Hospital of Krakow, undergo
regular Mg level assessment once every 4 weeks, and in the case of
any abnormalities, every 2 weeks (prior to every cetuximab
infusion), or more often if required.
It would also be interesting to observe the
frequency and intensity of Mg decrease from its baseline level
prior to the treatment; however, these data were not present in all
patient records. One study on 98 patients with mCRC revealed a
decrease in serum Mg concentration in 97% of patients during the
treatment with mAbs directed against EGFR (12).
Published data on the effect of Mg level on tumor
growth are inconclusive (33). There
are studies claiming that early hypomagnesemia may work as an
inexpensive positive predictive factor for the treatment with
cetuximab (24,28). This connection has already been
described for the skin-related toxicity caused by this mAb
(7). One study has suggested that
hypomagnesemia may function in decreasing the proliferation of
cells (33). However, other recently
published studies suggest an opposite association and a decrease in
OS time in patients with hypomagnesemia (22). Vickers et al (22) hypothesized that the predictive meaning
of hypomagnesemia may be associated with the severity of this
side-effect, with lower levels being associated with better
treatment outcome and higher levels with worse treatment outcome.
However, due to the limited number of patients and a variety of
treatment lines, it was not possible to perform statistical
analysis of the possible correlations between protocol
type/response to the treatment and hypomagnesemia occurrence or
grading.
Finally, it should be noted that the prevalence of
hypomagnesemia in the healthy population has been estimated as
between 2.5 and 15%. A review by Saif suggested an even higher
prevalence among cancer patients due to higher urinary and
gastrointestinal loss (e.g., diarrhea), malnutrition and poor
dietary intake (9). Patients with
neoplastic diseases also commonly present with weakness/fatigue,
which is the most common symptom of mild hypomagnesemia. It would
be extremely difficult to estimate the frequency of this
side-effect of cetuximab in a retrospective study, and of other
symptoms, such as irritability. For this reason, similar to certain
other studies (12), it was decided
against collecting data on hypomagnesemia symptoms in the present
study.
The results shown in this study and previously
published records regarding skin-related toxicity (7) demonstrate that cetuximab-related
side-effects present their specific characteristics regardless of
whether the drug is used as a monotherapy or in combination with
standard chemotherapy. It is essential to know the main symptoms of
hypomagnesemia in order to ensure the safety of treatment with
cetuximab. Physicians should focus on actively searching for
hypomagnesemia and other typical adverse effects in this group of
patients. The data regarding this side-effect remain limited, with
the hypomagnesemia incidence being assessed at between 4.4 to
93.3%, depending on the study. These metabolic complications are
not usually life-threatening, nor do they lead to treatment
termination, but require monitoring and treatment. As the extent of
Mg monitoring in patients treated with mAbs directed against EGFR
is insufficient, it is reasonable to introduce recommendations
concerning Mg measurement and supplementation in this population.
Physicians should remember that hypomagnesemia may be revealed as a
side-effect of cancer treatment, not only as a result of diarrhea
or malabsorption.
Acknowledgements
The authors would like to thank Dr Agnieszka Pacek
and Dr Magdalena Kozioł for their invaluable support in data
collection, and Ms. Joanna Gołąb for editing the original
manuscript.
References
|
1
|
Fakih M and Vincent M: Adverse events
associated with anti-EGFR therapies for the treatment of metastatic
colorectal cancer. Curr Oncol. 17(Suppl 1): S18–S30.
2010.PubMed/NCBI
|
|
2
|
Boyle P and Langman JS: ABC of colorectal
cancer: Epidemiology. BMJ. 321:805–808. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saltz LB, Meropol NJ, Loehrer PJ Sr,
Needle MN, Kopit J and Mayer RJ: Phase II trial of cetuximab in
patients with refractory colorectal cancer that expresses the
epidermal growth factor receptor. J Clin Oncol. 22:1201–1208. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jonker DJ, O'Callaghan CJ, Karapetis CS,
Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et
al: Cetuximab for the treatment of colorectal cancer. N Engl J Med.
357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bokemeyer C, Van Cutsem E, Rougier P,
Ciardiello F, Heeger S, Schlichting M, Celik I and Köhne CH:
Addition of cetuximab to chemotherapy as first-line treatment for
KRAS wild-type metastatic colorectal cancer: Pooled analysis of the
CRYSTAL and OPUS randomized clinical trials. Eur J Cancer.
48:1466–1475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pacek A, Kozioł M, Püsküllüoğlu M,
Tomaszewski KA, Ochenduszko S, Zygulska AL and Krzemieniecki K:
Assessment of skin-related toxicity in patients with metastatic
colorectal cancer treated with cetuximab. Acta Dermatovenerol
Croat. 22:137–144. 2014.PubMed/NCBI
|
|
8
|
Martin KJ, González EA and Slatopolsky E:
Clinical consequences and management of hypomagnesemia. J Am Soc
Nephrol. 20:2291–2295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saif MW: Management of hypomagnesemia in
cancer patients receiving chemotherapy. J Support Oncol. 6:243–248.
2008.PubMed/NCBI
|
|
10
|
National Cancer Institute Common Toxicity
Criteria. Version 4.0. May 28–2009.http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmAccessed.
May 2–2014
|
|
11
|
Groenestege WM, Thébault S, van der Wijst
J, van den Berg D, Janssen R, Tejpar S, van den Heuvel LP, van
Cutsem E, Hoenderop JG, Knoers NV, et al: Impaired basolateral
sorting of pro-EGF causes isolated recessive renal hypomagnesemia.
J Clin Invest. 117:2260–2267. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tejpar S, Piessevaux H, Claes K, Piront P,
Hoenderop JG, Verslype C and Van Cutsem E: Magnesium wasting
associated with epidermal-growth-factor receptor-targeting
antibodies in colorectal cancer: A prospective study. Lancet Oncol.
8:387–394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang XD, Jia C, Corvalan JR, Wang P and
Davis CG: Development of ABX-EGF, a fully human anti-EGF receptor
monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol.
38:17–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fakih M: Anty-EGFR monoclonal
antibody-induced hypomagnesaemia. Lancet Oncol. 8:366–367. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. (7th). (New
York, NY). Springer. 1432010.
|
|
16
|
Schrag D, Chung KY, Flombaum C and Saltz
L: Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer
Inst. 97:1221–1224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Erbitux-Summary of product
characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000558/WC500029119.pdfAccessed.
May 2–2014
|
|
18
|
Chen P, Wang L, Li H, Liu B and Zou Z:
Incidence and risk of hypomagnesemia in advanced cancer patients
treated with cetuximab: A meta-analysis. Oncol Lett. 5:1915–1920.
2013.PubMed/NCBI
|
|
19
|
Cao Y, Liao C, Tan A, Liu L and Gao F:
Meta-analysis of incidence and risk of hypomagnesemia with
cetuximab for advanced cancer. Chemotherapy. 56:459–465. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Price TJ, Peeters M, Kim TW, Li J, Cascinu
S, Ruff P, Suresh AS, Thomas A, Tjulandin S, Zhang K, et al:
Panitumumab versus cetuximab in patients with
chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal
cancer (ASPECCT): A randomised, multicentre, open-label,
non-inferiority phase 3 study. Lancet Oncol. 15:569–579. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lordick F, Kang YK, Chung HC, Salman P, Oh
SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et
al: Capecitabine and cisplatin with or without cetuximab for
patients with previously untreated advanced gastric cancer
(EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol.
14:490–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vickers MM, Karapetis CS, Tu D,
O'Callaghan CJ, Price TJ, Tebbutt NC, Van Hazel G, Shapiro JD,
Pavlakis N, Gibbs P, et al: Association of hypomagnesemia with
inferior survival in a phase III, randomized study of cetuximab
plus best supportive care versus best supportive care alone: NCIC
CTG/AGITG CO.17. Ann Oncol. 24:953–960. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weickhardt AJ, Price TJ, Chong G, Gebski
V, Pavlakis N, Johns TG, Azad A, Skrinos E, Fluck K, Dobrovic A, et
al: Dual targeting of the epidermal growth factor receptor using
the combination of cetuximab and erlotinib: Preclinical evaluation
and results of the phase II DUX study in chemotherapy-refractory,
advanced colorectal cancer. J Clin Oncol. 30:1505–1512. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vincenzi B, Santini D, Galluzzo S, Russo
A, Fulfaro F, Silletta M, Battistoni F, Rocci L, Zobel BB, Adamo V,
et al: Early magnesium reduction in advanced colorectal cancer
patients treated with cetuximab plus irinotecan as predictive
factor of efficacy and outcome. Clin Cancer Res. 14:4219–4224.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pazo-Oubiña Do F, Estefanell-Tejero A,
Riu-Viladoms G, Anglada-Martínez H, Molas-Ferrer G and Creus-Baró
N: Magnesium monitoring practice in monoclonal anti-epidermal
growth factor receptor antibodies therapy. J Clin Pharm Ther.
38:101–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Demizu M, Ueda H, Osawa M, Chihara S,
Igarashi T, Yano K, Kimura F, Tanaka N and Hiratsuka M: Effect of
magnesium supplementation on early-stage hypomagnesemia in patients
treated with cetuximab. Gan To Kagaku Ryoho. 40:897–900.
2013.PubMed/NCBI
|
|
27
|
Melichar B, Králíčková P, Hyšpler R,
Kalábová H, Cerman J Jr, Holečková P, Studentová H and Malířová E:
Hypomagnesaemia in patients with metastatic colorectal carcinoma
treated with cetuximab. Hepatogastroenterology. 59:366–371.
2012.PubMed/NCBI
|
|
28
|
Vincenzi B, Galluzzo S, Santini D, Rocci
L, Loupakis F, Correale P, Addeo R, Zoccoli A, Napolitano A,
Graziano F, et al: Early magnesium modifications as a surrogate
marker of efficacy of cetuximab-based anticancer treatment in KRAS
wild-type advanced colorectal cancer patients. Ann Oncol.
22:1141–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maliakal P and Ledford A: Electrolyte and
protein imbalance following anti-EGFR therapy in cancer patients: A
comparative study. Exp Ther Med. 1:307–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fakih MG, Wilding G and Lombardo J:
Cetuximab-induced hypomagnesemia in patients with colorectal
cancer. Clin Colorectal Cancer. 6:152–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stintzing S, Fischhaber D, Mook C, Modest
DP, Giessen C, Schulz C, Haas M, Boeck S, Michl M, Stemmler J, et
al: Clinical relevance and utility of cetuximab-related changes in
magnesium and calcium serum levels. Anticancer Drugs. 24:969–974.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thomas SE, Anderson S, Gordon KL, Oyama
TT, Shankland SJ and Johnson RJ: Tubulointerstitial disease in
aging: Evidence for underlying peritubular capillary damage, a
potential role for renal ischemia. J Am Soc Nephrol. 9:231–242.
1998.PubMed/NCBI
|
|
33
|
Wolf FI, Cittadini AR and Maier JA:
Magnesium and tumors: Ally or foe? Cancer Treat Rev. 35:378–382.
2009. View Article : Google Scholar : PubMed/NCBI
|