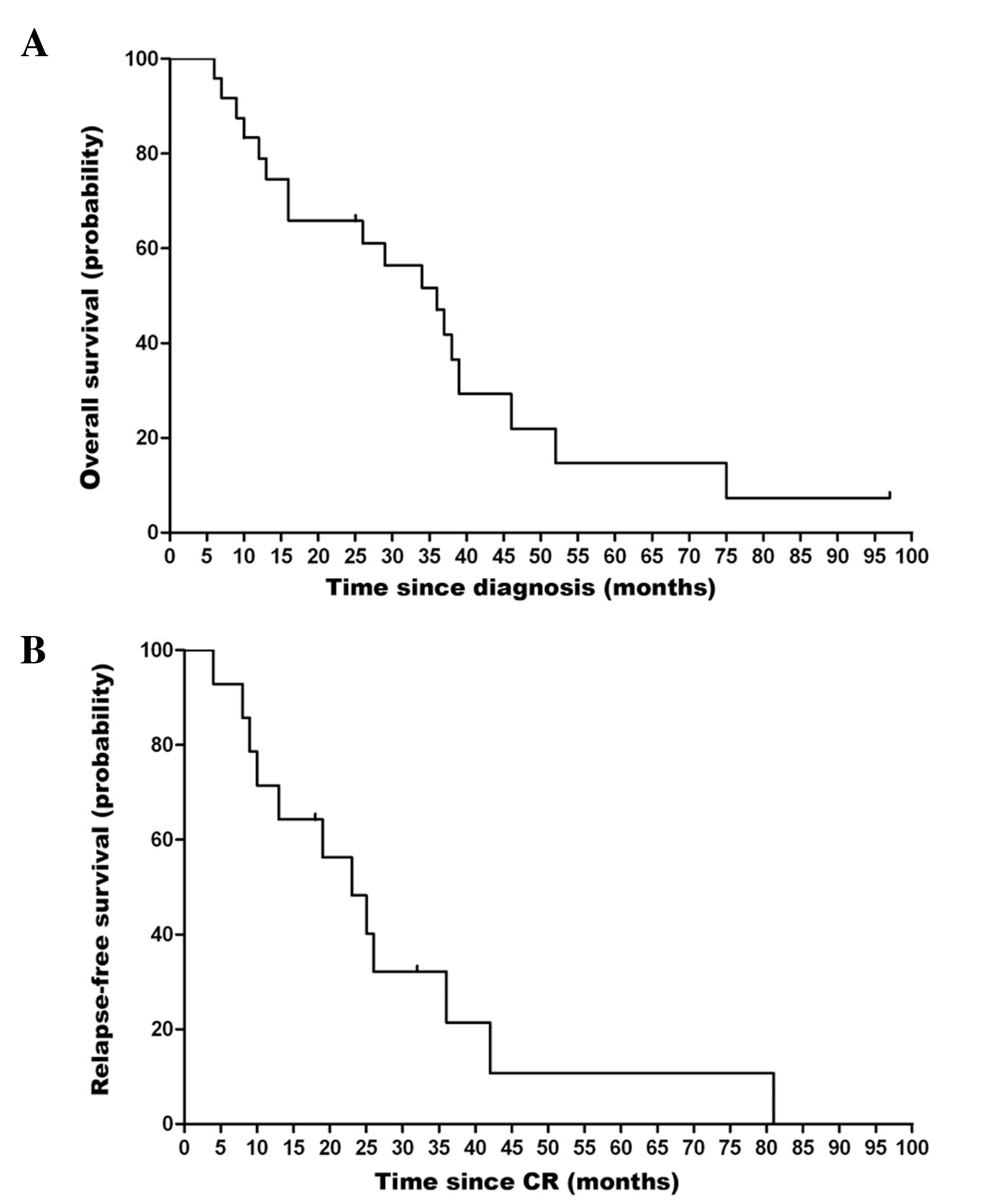
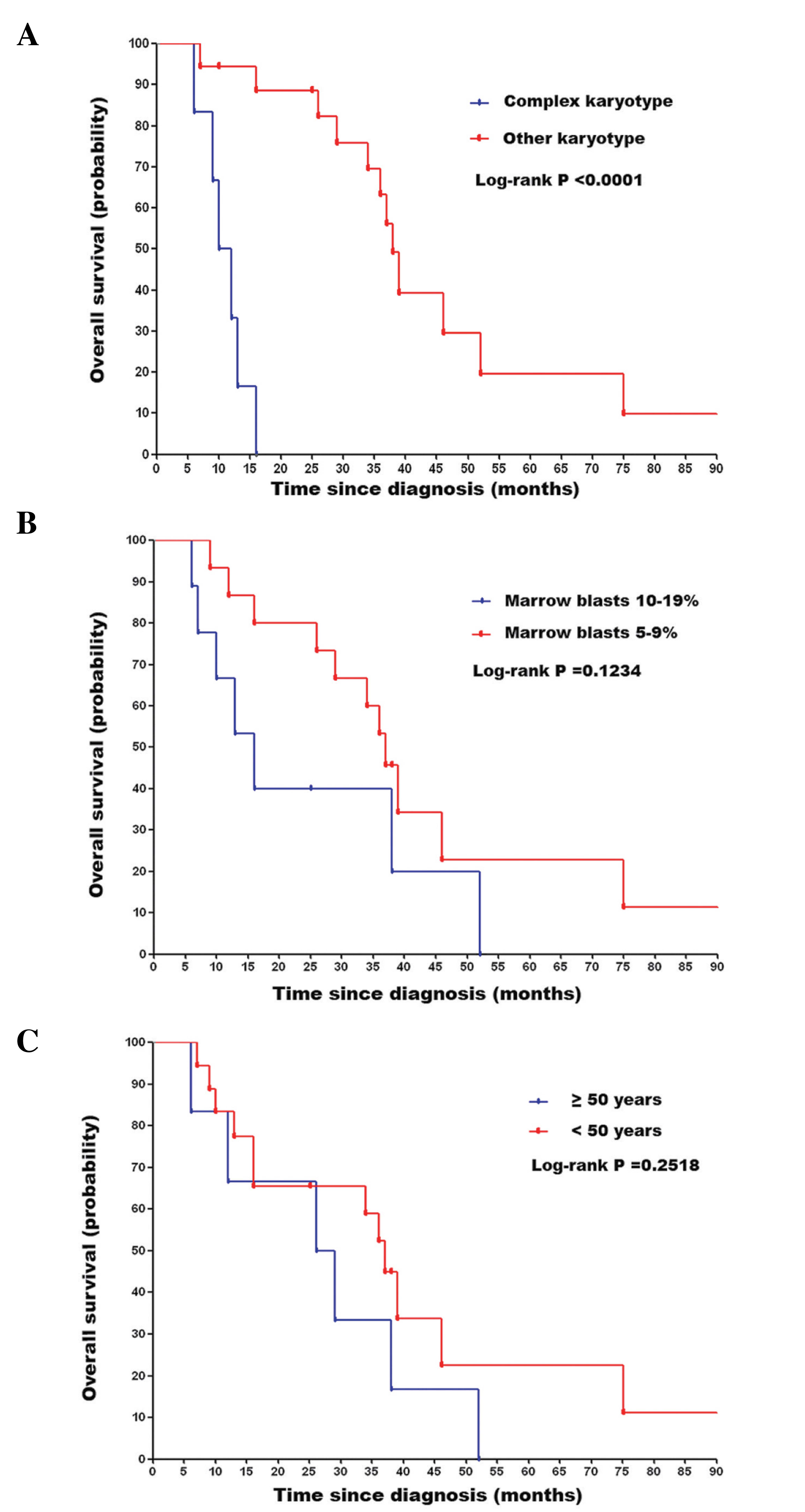
|
1
|
Nimer SD: Myelodysplastic syndromes.
Blood. 111:4841–4851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukumoto JS and Greenberg PL: Management
of patients with higher risk myelodysplastic syndromes. Crit Rev
Oncol Hematol. 56:179–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Witte T, Suciu S, Peetermans M, et al:
Intensive chemotherapy for poor prognosis myelodysplasia (MDS) and
secondary acute myeloid leukemia (sAML) following MDS of more than
6 months duration. A pilot study by the Leukemia Cooperative Group
of the European Organisation for Research and Treatment in Cancer
(EORTC-LCG). Leukemia. 9:1805–1811. 1995.PubMed/NCBI
|
|
4
|
Garcia-Manero G and Fenaux P:
Hypomethylating agents and other novel strategies in
myelodysplastic syndromes. J Clin Oncol. 29:516–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Estey EH, Kantarjian HM, O'Brien S, et al:
High remission rate, short remission duration in patients with
refractory anemia with excess blasts (RAEB) in transformation
(RAEB-t) given acute myelogenous leukemia (AML)-type chemotherapy
in combination with granulocyte-CSF (G-CSF). Cytokines Mol Ther.
1:21–28. 1995.PubMed/NCBI
|
|
6
|
Estey E, Thall P, Beran M, Kantarjian H,
Pierce S and Keating M: Effect of diagnosis (refractory anemia with
excess blasts, refractory anemia with excess blasts in
transformation, or acute myeloid leukemia [AML]) on outcome of
AML-type chemotherapy. Blood. 90:2969–2977. 1997.PubMed/NCBI
|
|
7
|
Feldman EJ, Seiter KP, Ahmed T, Baskind P
and Arlin ZA: Homoharringtonine in patients with myelodysplastic
syndrome (MDS) and MDS evolving to acute myeloid leukemia.
Leukemia. 10:40–42. 1996.PubMed/NCBI
|
|
8
|
Stone RM, Donohue KA, Stock W, et al: A
phase II study of continuous infusion homoharringtonine and
cytarabine in newly diagnosed patients with chronic myeloid
leukemia: CALGB study 19804. Cancer Chemother Pharmacol.
63:859–864. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quintás-Cardama A, Kantarjian H,
Garcia-Manero G, et al: Phase I/II study of subcutaneous
homoharringtonine in patients with chronic myeloid leukemia who
have failed prior therapy. Cancer. 109:248–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Lü S, Yang J, et al: A
homoharringtonine-based induction regimen for the treatment of
elderly patients with acute myeloid leukemia: a single center
experience from China. J Hematol Oncol. 2:322009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin J, Jiang DZ, Mai WY, et al:
Homoharringtonine in combination with cytarabine and aclarubicin
resulted in high complete remission rate after the first induction
therapy in patients with de novo acute myeloid leukemia. Leukemia.
20:1361–1367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu WL, Jin J and Qian WB:
Homoharringtonine in combination with cytarabine and aclarubicin as
induction therapy improves remission and survival of patients with
higher risk myelodysplastic syndromes. Chin Med J (Engl).
123:108–110. 2010.PubMed/NCBI
|
|
13
|
Yin S, Wang R, Zhou F, Zhang H and Jing Y:
Bcl-xL is a dominant antiapoptotic protein that inhibits
homoharringtonine-induced apoptosis in leukemia cells. Mol
Pharmacol. 79:1072–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tujebajeva RM, Graifer DM, Karpova GG and
Ajtkhozhina NA: Alkaloid homoharringtonine inhibits polypeptide
chain elongation on human ribosomes on the step of peptide bond
formation. FEBS Lett. 257:254–256. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen R, Guo L, Chen Y, et al:
Homoharringtonine reduced Mcl-1 expression and induced apoptosis in
chronic lymphocytic leukemia. Blood. 117:156–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jie H, Donghua H, Xingkui X, et al:
Homoharringtonine-induced apoptosis of MDS cell line MUTZ-1 cells
is mediated by the endoplasmic reticulum stress pathway. Leuk
Lymphoma. 48:964–977. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lindqvist LM, Vikström I, Chambers JM, et
al: Translation inhibitors induce cell death by multiple mechanisms
and Mcl-1 reduction is only a minor contributor. Cell Death Dis.
3:e4092012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meng HT, Li L, Liu H, Wang Y, Li GC and
Qian WB: Homoharringtonine acts synergistically with SG235-TRAIL, a
conditionally replicating adenovirus, in human leukemia cell lines.
Acta Pharmacol Sin. 30:1529–1536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng H, Yang C, Jin J, Zhou Y and Qian W:
Homoharringtonine inhibits the AKT pathway and induces in vitro and
in vivo cytotoxicity in human multiple myeloma cells. Leuk
Lymphoma. 49:1954–1962. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuroda J, Kamitsuji Y, Kimura S, et al:
Anti-myeloma effect of homoharringtonine with concomitant targeting
of the myeloma-promoting molecules, Mcl-1, XIAP, and beta-catenin.
Int J Hematol. 87:507–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vardiman JW, Harris NL and Brunning RD:
The World Health Organization (WHO) classification of the myeloid
neoplasms. Blood. 100:2292–2302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clark TG, Stewart ME, Altman DG, Gabra H
and Smyth JF: A prognostic model for ovarian cancer. Br J Cancer.
85:944–952. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheson BD, Greenberg PL, Bennett JM, et
al: Clinical application and proposal for modification of the
International Working Group (IWG) response criteria in
myelodysplasia. Blood. 108:419–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeidan AM, Linhares Y and Gore SD: Current
therapy of myelodysplastic syndromes. Blood Rev. 27:243–259. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wattel E, De Botton S, Luc Laï J, et al:
Long-term follow-up of de novo myelodysplastic syndromes treated
with intensive chemotherapy: incidence of long-term survivors and
outcome of partial responders. Br J Haematol. 98:983–991. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oosterveld M, Muus P, Suciu S, et al:
EORTC, EBMT, SAKK, GIMEMA Leukemia Groups and the MD Anderson
Cancer Center: Chemotherapy only compared to chemotherapy followed
by transplantation in high risk myelodysplastic syndrome and
secondary acute myeloid leukemia; two parallel studies adjusted for
various prognostic factors. Leukemia. 16:1615–1621. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Greenberg P, Cox C, LeBeau MM, et al:
International scoring system for evaluating prognosis in
myelodysplasticsyndromes. Blood. 89:2079–2088. 1997.PubMed/NCBI
|
|
29
|
Beran M, Shen Y, Kantarjian H, et al:
High-dose chemotherapy in high-risk myelodysplastic syndrome:
covariate-adjusted comparison of five regimens. Cancer.
92:1999–2015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fenaux P, Morel P, Rose C, Lai JL, Jouet
JP and Bauters F: Prognostic factors in adult de novo
myelodysplastic syndromes treated by intensive chemotherapy. Br J
Haematol. 77:497–501. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Daver N, Vega-Ruiz A, Kantarjian HM, et
al: A phase II open-label study of the intravenous administration
of homoharringtonine in the treatment of myelodysplastic syndrome.
Eur J Cancer Care (Engl). 22:605–611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu L, Li X, Su J, He Q, Zhang X, Chang C
and Pu Q: Efficacy and safety of CHG regimen (low-dose cytarabine,
homoharringtonine with G-CSF priming) as induction chemotherapy for
elderly patients with high-risk MDS or AML transformed from MDS.
Cancer Res Clin Oncol. 137:1563–1569. 2011. View Article : Google Scholar
|
|
33
|
Wu L, Li X, Su J, et al: Effect of
low-dose cytarabine, homoharringtonine and granulocyte
colony-stimulating factor priming regimen on patients with advanced
myelodysplastic syndrome or acute myeloid leukemia transformed from
myelodysplastic syndrome. Leuk Lymphoma. 50:1461–1467. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin J, Wang JX, Chen FF, et al:
Homoharringtonine-based induction regimens for patients with
de-novo acute myeloid leukaemia: a multicentre, open-label,
randomised, controlled phase 3 trial. Lancet Oncol. 14:599–608.
2008. View Article : Google Scholar
|
|
35
|
Wang L, You LS, Ni WM, et al: β-Catenin
and AKT are promising targets for combination therapy in acute
myeloid leukemia. Leuk Res. 37:1329–1340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Legha SS, Keating M, Picket S, Ajani JA,
Ewer M and Bodey GP: Phase I clinical investigation of
homoharringtonine. Cancer Treat Rep. 68:1085–1091. 1984.PubMed/NCBI
|