Introduction
The myelodysplastic syndromes (MDS) are a
heterogeneous group of clonal hematopoietic stem-cell disorders
characterized by abnormal differentiation, morphology, and
maturation of hematopoietic cells in bone marrow (1). Patients with higher-risk MDS, which
include intermediate-2 and high risk of the International
Prognostic Scoring System (IPSS), have a peculiar propensity to
evolve into acute myeloid leukemia (AML), and often have a median
survival time of <12 months (2,3).
Currently, hypomethylating agents (such as azacitidine and
decitabine), lenalidomide, intensive chemotherapy, and
allo-hematopoietic stem cell transplantation may be therapeutic
options for patients with higher-risk MDS (4). However, high-intensity chemotherapy is
generally reserved for higher-risk patients, particularly young
patients eligible for intensive therapy that lack a suitable stem
cell donor (2,5). Unfortunately, treatment of MDS patients
with chemotherapy only results in a few long-term survivors
(2,5,6).
Therefore, innovative approaches for the treatment of higher-risk
MDS should be developed.
Homoharringtonine (HHT), a plant alkaloid isolated
from the Chinese evergreen Cephalotaxus harringtonia, was
first investigated in China and has been used for the treatment of
chronic myeloid leukemia, AML, and MDS (7–12). It was
reported that HHT functions as a protein synthesis inhibitor at the
initiation and elongation phases of translation, and that it is a
potent apoptosis inducer for hematopoietic malignancies such as AML
(13–15). In vitro experiments have
demonstrated that HHT inhibited a variety of antiapoptotic proteins
including Mcl-1, xIAP and Bcl-2, downregulated Akt pathway and
sensitized AML cells to TRAIL-induced apoptosis via increase of
death receptor 4 (DR4) and DR5 (13,15–20). Our
previous pilot study revealed that HHT combined with cytarabine
(AraC) and aclarubicin (HAA regimen) resulted in a complete
remission (CR) rate of 83% in the patients with de novo AML
and an estimated 3-year overall survival rate of 53%, which is more
effective than any other induction remission regimens currently
available (11). Therefore, in the
present study, the same regimen was used to treat patients with
MDS-refractory anemia with excess blasts (RAEB). The current study
presents a retrospective analysis of the outcome of the HAA regimen
as induction chemotherapy in the patients with MDS-RAEB.
Patients and methods
Patients
Between January 2005 and June 2012, 24 patients with
MDS-RAEB diagnosed according to the World Health Organization (WHO)
classification (21) were enrolled
into the presentstudy. The study was approved by the Ethics
Committee of the First Affiliated Hospital, College of Medicine,
Zhejiang University (Hangzhou, China). All the patients signed
informed consent prior to the enrollment. Other eligibility
criteria were an Eastern Cooperative Oncology Group performance
status of 0–2 and normal cardiac function (23). The clinical parameters assessed at
presentation included age, sex, hemoglobin (Hb), white blood cell
count (WBC), platelet count and percentage of bone marrow (BM)
blasts. A cytogenetic study at diagnosis on BM was performed.
Treatment
All the enrolled patients received induction
therapy: This consisted of a combination of HHT (2 mg/m2
intramuscularly twice daily, days 1–3) with aclarubicin (12
mg/m2, days 1–7) and AraC (75 mg/m2 injected
subcutaneously twice daily, days 1–7). The response evaluation
criteria for the patients were as follows (24): i) CR was defined as normalization of
blood counts with ≥1.0×109/l, Hb ≥110 g/dl, platelets
≥100×109/l, and marrow blasts ≤5% without evidence of
dysplasia. ii) Partial remission (PR) was defined as CR except that
marrow blasts should reduce by ≥50% compared with the pretreatment
levels. iii) Bone marrow CR was defined as ≤5% marrow blasts
without evidence of dysplasia, but complete recovery of blood
counts was not achieved. Toxicity of induction therapy was graded
according to the National Cancer Institute common toxicity criteria
(10). For the patients with CR or
PR, a second course was repeated using the same drugs and doses,
whereas patients failing to response were offered palliative care.
Postremission therapy was offered in rotation after the achievement
of CR as following: HAA regimen, AraC (150 mg/m2, days
1–7) in combination with a second drug, which including
daunorubicin (45 mg/m2, days 1–3), idarubicin (10
mg/m2, days 1–3), etoposide (75 mg/m2, days
1–5), mitoxantrone (10 mg/m2, days 1–3), or aclarubicin
(12 mg/m2, days 1–5). During chemotherapy, the patients
received subcutaneous injections of granulocyte colony-stimulating
factor at 5 mg/kg, from the day neutrophil count was
<0.5×109/l until the neutrophil count was
>1.0×109/l on 3 successive days. Maintenance therapy
was administered to all CR patients and continued until the patient
was 3 years in remission.
Statistical analysis
Fisher's exact test was used in order to detect the
factors that influenced the CR rate. Time-to-event analysis was
performed according to the Kaplan-Meier method, and the log-rank
test was applied to assess differences between subgroups. All
statistical analysis was performed using SPSS 10.0 statistical
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a stastically significant difference.
Results
Patient characteristics
The baseline characteristics for 24 patients
enrolled in the present study are summarized in Table I. The median age of this population
was 41 years (range 18–66 years); 6 patients were >50 years and
18 patients were <50 years. According to the WHO classification,
9 patients had a diagnosis of MDS-RAEB I and 15 had RAEB II. Of the
22 patients who had cytogenetic studies performed, 5 patients
(22.7%) had intermediate-1, 13 patients (59.1%) had intermediate-2,
and 4 patients (18.2%) had high risk MDS, according to the IPSS
(2,13).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | n (range) |
|---|
| Patients | 24 |
| Male/female | 9/15 |
| Median age | 41 years (18–66) |
| FAB classification
subtype |
|
| RAEB
I | 9 |
| RAEB
II | 15 |
| Median WBC count | 3.15×109/l
(1.0–13.3) |
| Median platelet
count | 53.5×109/l
(7–198) |
| Median
hemoglobin | 7.4 g/dl
(3.5–11.0) |
| Median marrow blast
% | 10.75% (6–19) |
| Cytogenetics (%) |
|
|
Intermediate risk | 18 (81.8) |
| High
risk | 4 (18.2) |
| Not
available | 2 |
Response and treatment outcome
A total of 14/24 patients (58.3%) achieved CR
(including bone marrow CR) after the first course of induction
treatment, and PR was observed in 5 patients (20.7%) for an overall
response rate of 79%. Univariate analysis of factors influencing CR
demonstrated that the age, gender, marrow blast count, and
cytogenetic abnormalities of patient had no significant influence
on CR rate (Table II).
 | Table II.Complete remission analyses. |
Table II.
Complete remission analyses.
|
Characteristics | CR/total | CR rate (%) | P-value |
|---|
| Age |
|
| 0.665 |
|
<50 | 11/18 | 61 |
|
|
≤50 | 3/6 | 50 |
|
| Gender |
|
| 0.403 |
|
Male | 4/9 | 44 |
|
|
Female | 10/15 | 66.6 |
|
| Cytogenetic |
|
| 0.1 |
|
Intermediate risk | 10/16 | 63.6 |
|
| High
risk | 3/6 | 50 |
|
| Marrow blast
count |
|
| 0.678 |
|
5–9% | 6/9 | 60 |
|
|
10–19% | 8/15 | 57.1 |
|
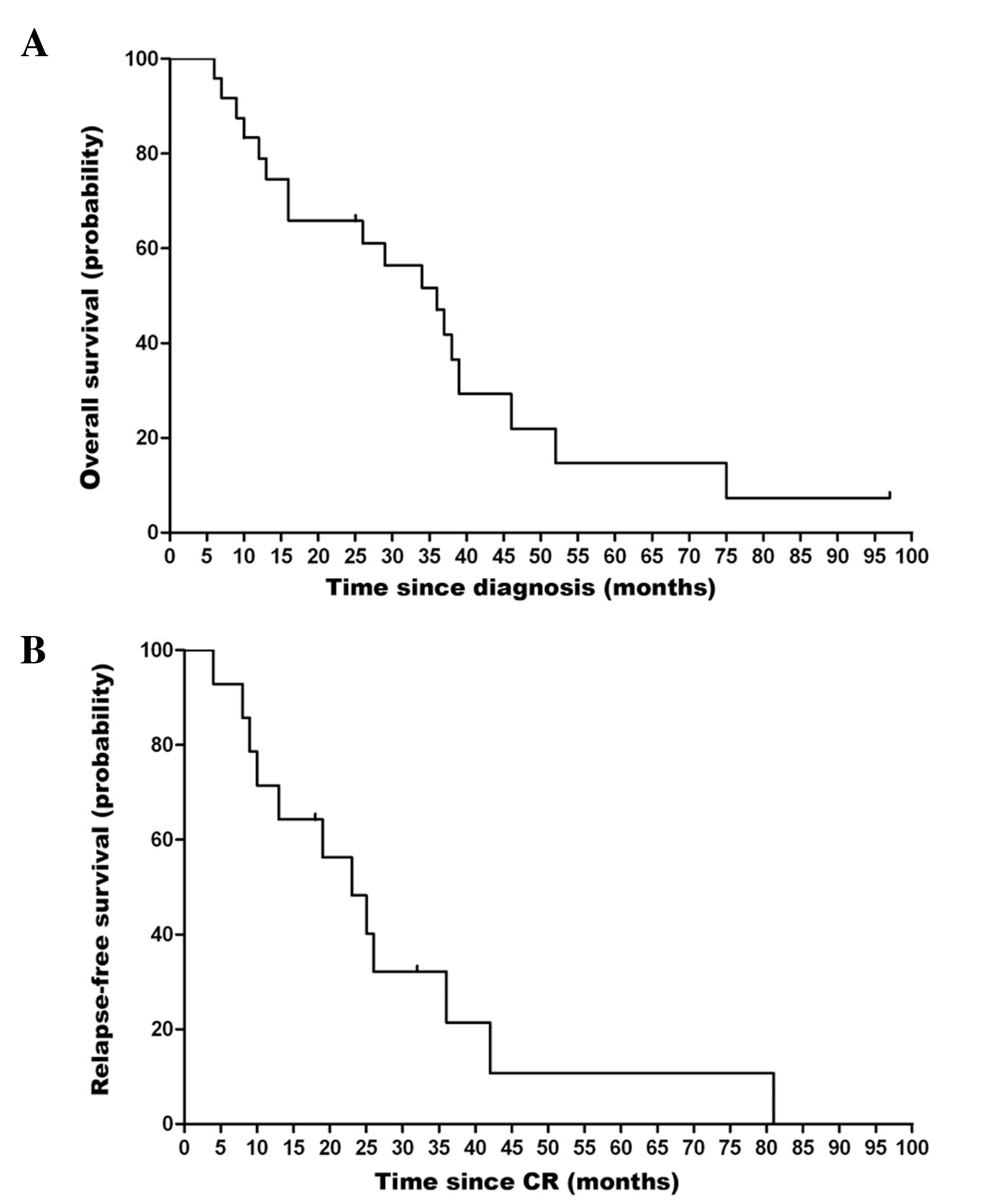
Fig. 1A shows the
overall survival (OS) curve for the 24 patients. The median OS time
was 36.2 months [95% confidence interval (CI), 24.6–47.4 months],
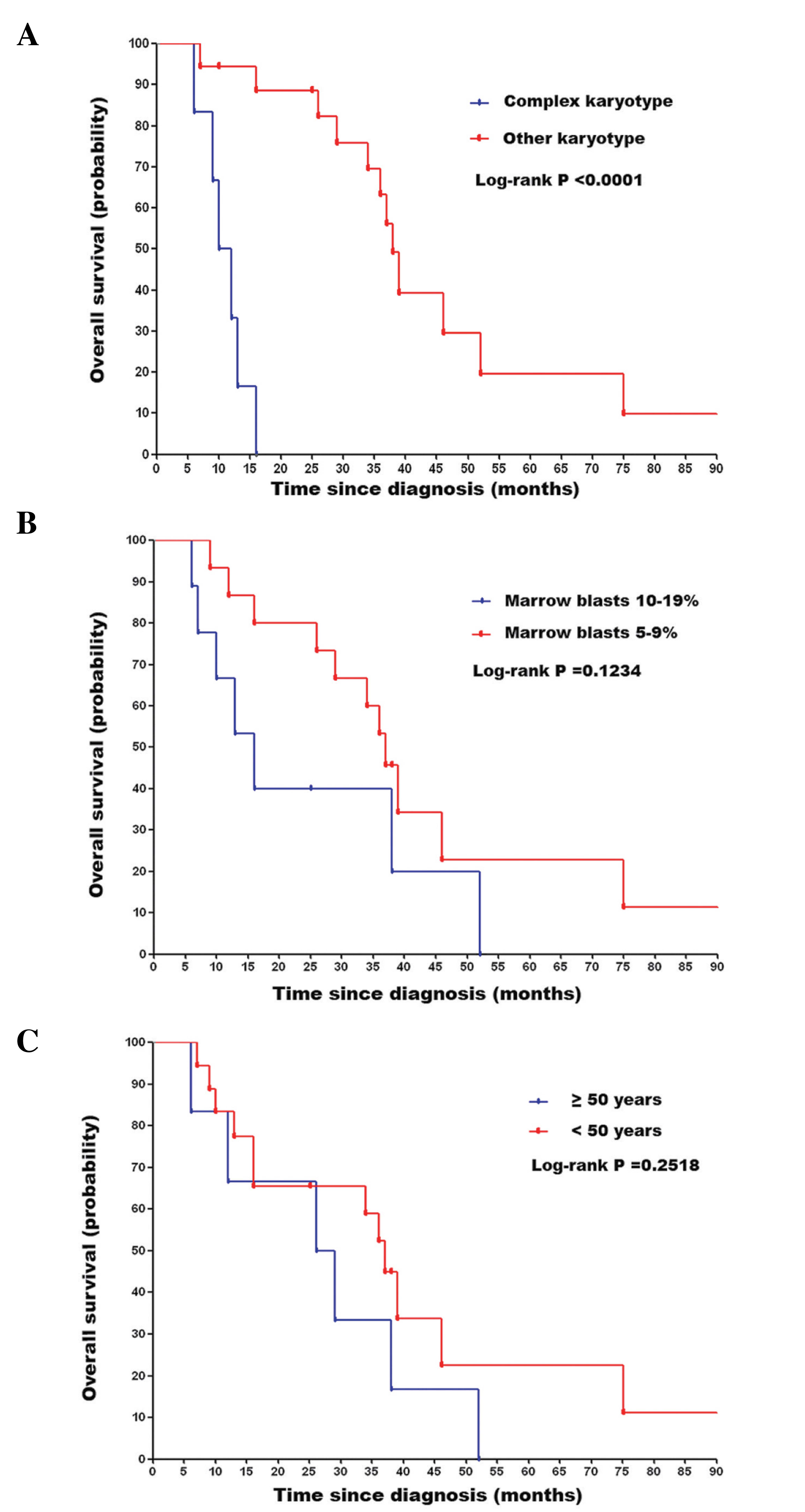
and the estimated 3 year OS rate was 45.8%. Univariate analysis of
the factors that influenced OS showed a negative impact of complex
karyotype (P<0.0001; Fig. 2A).
Whereas, marrow blast count and age had no significant impacts on
survival (Fig. 2B and C). Fig. 1B shows the relapse-free survival (RFS)
curve for the 14 patients who achieved CR. The median RFS for these
patients was 27.7 months (95% CI, 12.9–33.1 months).
Toxicity
Mortality was not associated with treatment with the
HAA regimen as an induction therapy. The most common toxicities for
cycle 1 were myelosuppression, which occurred in all patients. The
nadirs of the neutrophil count and platelet were
0.2×109/l (range 0.1–0.35×109/l) and
4×109/l (range 2–13×109/l), respectively. The
median times to recovery of neutrophil (≥1.0×109/l) and
recovery of the platelets (≥20×109/l) were 24.5 days
(95% CI, 19.9–29.2 days) and 27.2 days (95% CI, 18.5–35.9 days),
respectively. The most common nonhematological toxicities were
nausea, emesis, diarrhea and constipation, which were mild with
grades I–II.
Discussion
Higher-risk MDS represents a significant therapeutic
treatment challenge for any current chemotherapeutics because of
poor responses to combination chemotherapy and shorter survival
(25). Previous large studies using
various induction regimens containing AraC in various combinations
with idarubicin, fludarabine, topotecan, and cyclophosphamide
demonstrated CR rates of ~40–60% for higher-risk MDS, median OS
rate 10.4–13 months, and treatment-related early death 10–20%
(2,3,26,27). In the present study, an HAA regimen
was implemented for MDS-RAEB in adults who were aged 18–66 years. A
total of 17/22 patients (77.3%) were scored as higher-risk by IPSS
(28). The HAA regimen produced a CR
rate of 58.3%, and a median OS of 36.2 months. The median RFS time
of the patients who achieved CR was 27.7 months. This compares
favorably with the outcomes of traditional intensive chemotherapies
reported previously (26,27,29). The
patient selection appears to be likely reason for this, since a
high proportion of younger patients was included. Previously,
Fenaux et al (30) reported
that RAEB and predominantly RAEB in transformation (RAEBt) patients
treated with an anthracycline-AraC regimen whose CR exceeded 2
years were young with normal karyotypes. Consistent with this
result, in the present study, patients with other karyotypes
[normal, −7, −8, -Y and del(20q)], had an improved OS compared with
those with complex karyotypes. However, it is worth noting that no
statistical difference in OS was observed in comparison of high age
(≥50 years) vs. low age (<50 years), in the present study.
For patients with higher-risk MDS, HAA regimen was
found to be effective in inducing durable remissions although the
number of patients in the current study was too small to make any
definitive conclusions. HHT has also been administered to adults
with MDS (7,12,31–33). Daver
et al (31) reported that the
CR rate was 11% in patients with intermediate and high risk MDS,
who received HHT alone. In addition, combination therapy of HHT and
low-dose AraC has been demonstrated to be an effective strategy for
advanced MDS and RAEBt and CR was observed in 46.9% (32); however, the median OS was shorter
(18.2 months). Encouraging results from an open-label, randomized,
controlled phase III study using an HAA regimen as an induction
therapy in de novo AML patients aged 14–59 years have been
reported (34). In that study,
150/206 patients (73%) received HAA regimen achieved CR versus
125/205 (61%) in the patients who were treated with daunorubicin
and AraC (DA regimen); 3-year event-free survival was 35.4% versus
23.1% (P=0.0023) (34). These
differences appeared to be mainly a consequence of improved outcome
in the patients with favorable and intermediate cytogenetics.
Moreover, our recent study (35)
showed in vitro synergy between HHT and aclarubicin. This
combination therapy could synergistically induce the apoptosis of
CD34+/CD38− primary AML cells. The probable
mechanism of synergy arises from the inhibition of PI3K/Akt and
WNT/β-catnin signaling pathways, which may be the reason for the
clinical benefit of HAA regimen in treating AML and higher-risk
MDS.
Despite the presence of myelosuppresion in all of
the patients in the present study, which resulted in accompanying
risks of infectious complications, the induction mortality was
notably low (0 by day 30) compared with previous reports in
patients with AML who received an HAA regimen (4% to 5.8% by day
30) (11,34). One of the major issues concerned with
HHT is cardiovascular complications. A previous study showed that a
high dose HHT (5–6 mg/m2/day) was associated with severe
hypotension and cardiovascular collapse (36). The studies using HAA regimen in AML
demonstrated that only 2% of patients had toxic cardiac effects
(11,34). Collectively, these results support the
safety of HAA regimen.
Altogether, the data from the present study
indicates that the HAA regimen may improve the outcome of de
novo higher-risk MDS patients, particularly of those with
favorable and intermediate cytogenetics. However, this conclusion
was obtained from the comparison with historical controls. Thus, a
prospective controlled study is needed to confirm these
results.
References
|
1
|
Nimer SD: Myelodysplastic syndromes.
Blood. 111:4841–4851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukumoto JS and Greenberg PL: Management
of patients with higher risk myelodysplastic syndromes. Crit Rev
Oncol Hematol. 56:179–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Witte T, Suciu S, Peetermans M, et al:
Intensive chemotherapy for poor prognosis myelodysplasia (MDS) and
secondary acute myeloid leukemia (sAML) following MDS of more than
6 months duration. A pilot study by the Leukemia Cooperative Group
of the European Organisation for Research and Treatment in Cancer
(EORTC-LCG). Leukemia. 9:1805–1811. 1995.PubMed/NCBI
|
|
4
|
Garcia-Manero G and Fenaux P:
Hypomethylating agents and other novel strategies in
myelodysplastic syndromes. J Clin Oncol. 29:516–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Estey EH, Kantarjian HM, O'Brien S, et al:
High remission rate, short remission duration in patients with
refractory anemia with excess blasts (RAEB) in transformation
(RAEB-t) given acute myelogenous leukemia (AML)-type chemotherapy
in combination with granulocyte-CSF (G-CSF). Cytokines Mol Ther.
1:21–28. 1995.PubMed/NCBI
|
|
6
|
Estey E, Thall P, Beran M, Kantarjian H,
Pierce S and Keating M: Effect of diagnosis (refractory anemia with
excess blasts, refractory anemia with excess blasts in
transformation, or acute myeloid leukemia [AML]) on outcome of
AML-type chemotherapy. Blood. 90:2969–2977. 1997.PubMed/NCBI
|
|
7
|
Feldman EJ, Seiter KP, Ahmed T, Baskind P
and Arlin ZA: Homoharringtonine in patients with myelodysplastic
syndrome (MDS) and MDS evolving to acute myeloid leukemia.
Leukemia. 10:40–42. 1996.PubMed/NCBI
|
|
8
|
Stone RM, Donohue KA, Stock W, et al: A
phase II study of continuous infusion homoharringtonine and
cytarabine in newly diagnosed patients with chronic myeloid
leukemia: CALGB study 19804. Cancer Chemother Pharmacol.
63:859–864. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quintás-Cardama A, Kantarjian H,
Garcia-Manero G, et al: Phase I/II study of subcutaneous
homoharringtonine in patients with chronic myeloid leukemia who
have failed prior therapy. Cancer. 109:248–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Lü S, Yang J, et al: A
homoharringtonine-based induction regimen for the treatment of
elderly patients with acute myeloid leukemia: a single center
experience from China. J Hematol Oncol. 2:322009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin J, Jiang DZ, Mai WY, et al:
Homoharringtonine in combination with cytarabine and aclarubicin
resulted in high complete remission rate after the first induction
therapy in patients with de novo acute myeloid leukemia. Leukemia.
20:1361–1367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu WL, Jin J and Qian WB:
Homoharringtonine in combination with cytarabine and aclarubicin as
induction therapy improves remission and survival of patients with
higher risk myelodysplastic syndromes. Chin Med J (Engl).
123:108–110. 2010.PubMed/NCBI
|
|
13
|
Yin S, Wang R, Zhou F, Zhang H and Jing Y:
Bcl-xL is a dominant antiapoptotic protein that inhibits
homoharringtonine-induced apoptosis in leukemia cells. Mol
Pharmacol. 79:1072–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tujebajeva RM, Graifer DM, Karpova GG and
Ajtkhozhina NA: Alkaloid homoharringtonine inhibits polypeptide
chain elongation on human ribosomes on the step of peptide bond
formation. FEBS Lett. 257:254–256. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen R, Guo L, Chen Y, et al:
Homoharringtonine reduced Mcl-1 expression and induced apoptosis in
chronic lymphocytic leukemia. Blood. 117:156–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jie H, Donghua H, Xingkui X, et al:
Homoharringtonine-induced apoptosis of MDS cell line MUTZ-1 cells
is mediated by the endoplasmic reticulum stress pathway. Leuk
Lymphoma. 48:964–977. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lindqvist LM, Vikström I, Chambers JM, et
al: Translation inhibitors induce cell death by multiple mechanisms
and Mcl-1 reduction is only a minor contributor. Cell Death Dis.
3:e4092012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meng HT, Li L, Liu H, Wang Y, Li GC and
Qian WB: Homoharringtonine acts synergistically with SG235-TRAIL, a
conditionally replicating adenovirus, in human leukemia cell lines.
Acta Pharmacol Sin. 30:1529–1536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng H, Yang C, Jin J, Zhou Y and Qian W:
Homoharringtonine inhibits the AKT pathway and induces in vitro and
in vivo cytotoxicity in human multiple myeloma cells. Leuk
Lymphoma. 49:1954–1962. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuroda J, Kamitsuji Y, Kimura S, et al:
Anti-myeloma effect of homoharringtonine with concomitant targeting
of the myeloma-promoting molecules, Mcl-1, XIAP, and beta-catenin.
Int J Hematol. 87:507–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vardiman JW, Harris NL and Brunning RD:
The World Health Organization (WHO) classification of the myeloid
neoplasms. Blood. 100:2292–2302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clark TG, Stewart ME, Altman DG, Gabra H
and Smyth JF: A prognostic model for ovarian cancer. Br J Cancer.
85:944–952. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheson BD, Greenberg PL, Bennett JM, et
al: Clinical application and proposal for modification of the
International Working Group (IWG) response criteria in
myelodysplasia. Blood. 108:419–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeidan AM, Linhares Y and Gore SD: Current
therapy of myelodysplastic syndromes. Blood Rev. 27:243–259. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wattel E, De Botton S, Luc Laï J, et al:
Long-term follow-up of de novo myelodysplastic syndromes treated
with intensive chemotherapy: incidence of long-term survivors and
outcome of partial responders. Br J Haematol. 98:983–991. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oosterveld M, Muus P, Suciu S, et al:
EORTC, EBMT, SAKK, GIMEMA Leukemia Groups and the MD Anderson
Cancer Center: Chemotherapy only compared to chemotherapy followed
by transplantation in high risk myelodysplastic syndrome and
secondary acute myeloid leukemia; two parallel studies adjusted for
various prognostic factors. Leukemia. 16:1615–1621. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Greenberg P, Cox C, LeBeau MM, et al:
International scoring system for evaluating prognosis in
myelodysplasticsyndromes. Blood. 89:2079–2088. 1997.PubMed/NCBI
|
|
29
|
Beran M, Shen Y, Kantarjian H, et al:
High-dose chemotherapy in high-risk myelodysplastic syndrome:
covariate-adjusted comparison of five regimens. Cancer.
92:1999–2015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fenaux P, Morel P, Rose C, Lai JL, Jouet
JP and Bauters F: Prognostic factors in adult de novo
myelodysplastic syndromes treated by intensive chemotherapy. Br J
Haematol. 77:497–501. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Daver N, Vega-Ruiz A, Kantarjian HM, et
al: A phase II open-label study of the intravenous administration
of homoharringtonine in the treatment of myelodysplastic syndrome.
Eur J Cancer Care (Engl). 22:605–611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu L, Li X, Su J, He Q, Zhang X, Chang C
and Pu Q: Efficacy and safety of CHG regimen (low-dose cytarabine,
homoharringtonine with G-CSF priming) as induction chemotherapy for
elderly patients with high-risk MDS or AML transformed from MDS.
Cancer Res Clin Oncol. 137:1563–1569. 2011. View Article : Google Scholar
|
|
33
|
Wu L, Li X, Su J, et al: Effect of
low-dose cytarabine, homoharringtonine and granulocyte
colony-stimulating factor priming regimen on patients with advanced
myelodysplastic syndrome or acute myeloid leukemia transformed from
myelodysplastic syndrome. Leuk Lymphoma. 50:1461–1467. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin J, Wang JX, Chen FF, et al:
Homoharringtonine-based induction regimens for patients with
de-novo acute myeloid leukaemia: a multicentre, open-label,
randomised, controlled phase 3 trial. Lancet Oncol. 14:599–608.
2008. View Article : Google Scholar
|
|
35
|
Wang L, You LS, Ni WM, et al: β-Catenin
and AKT are promising targets for combination therapy in acute
myeloid leukemia. Leuk Res. 37:1329–1340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Legha SS, Keating M, Picket S, Ajani JA,
Ewer M and Bodey GP: Phase I clinical investigation of
homoharringtonine. Cancer Treat Rep. 68:1085–1091. 1984.PubMed/NCBI
|