Introduction
Breast cancer (BC) is a common malignancy that is a
serious threat to the health of women. It has been reported that
~1.5 million women are diagnosed with BC annually in the world and
that nearly 0.5 million succumb to this disease (1). With accumulating studies on BC, the
therapeutic schemes for BC have become much more mature, evolving
from the initial local excision to current surgery-based
comprehensive treatments, including radiotherapy, chemotherapy,
endocrine therapy, biological immune therapy and molecular-targeted
therapy.
The estrogen receptor (ER) is often found in BC and
this cancer is consequently labeled as ER-positive (ERP).
Furthermore, as the occurrence and development of BC is so closely
associated with the expression of the ER (2), endocrine therapy has been widely used as
an effective therapeutic method. In the past few decades, endocrine
therapeutic drugs have significantly improved the clinical outcomes
of BC patients, as well as their quality of life (3,4). Recently,
two multi-center, large-scale, prospective clinical trials further
established the positive effect of endocrine therapy in BC
treatment (5,6).
However, with the extension of endocrine treatment,
certain studies found that a few BC patients showed resistance to
endocrine therapy. The clinical data indicated that although there
were BC-ERP patients who were suitable for endocrine therapy, ~30%
of BC-ERP patients exhibited resistance to endocrine drugs in the
early stages of treatment (primary resistance), and ~40% BC-ERP of
patients showed the effectiveness of endocrine therapy prior to
exhibiting gradually reduced sensitivity or resistance with the
extension of treatment time (7,8). This ERP
status greatly affected the clinical efficacy, and even lead to the
failure of clinical BC treatment. Recently, certain studies
reported that ERP may be associated with the following factors:
Certain receptors, such as human epidermal growth factor-2,
insulin-like growth factor receptor and fibroblast growth factor
receptor, the phosphoinositide 3-kinase-Akt signal pathway and the
abnormal expression of associated microRNAs (9–15).
However, these studies did not take the clinical factors into
consideration. Therefore the present study analyzed 45 patients who
experienced the relapse and metastasis of BC between November 2007
and March 2013, and attempted to identify the clinical factors that
were involved in the resistance to endocrine therapy.
Materials and methods
Subjects
BC patients who were treated in the Department of
General Surgery, Jinling Hospital, Medical School of Nanjing
University (Nanjing, Jiangsu, China) between November 2007 and
March 2013 were enrolled in the study. The inclusion criteria were
as follows: i) No metastasis when initially treated; ii) positive
ER immunohistochemical results; iii) receipt of endocrine therapy;
iv) metastasis or recurrence occurring following endocrine therapy;
and v) complete clinical and retrospective follow-up data. This
study was conducted in accordance with the Declaration of Helsinki.
This study was conducted with approval from the Ethics Committee of
Jinling Hospital, Medical School of Nanjing University. Written
informed consent was obtained from all participants.
Research methods
A retrospective survey was performed in the 45 BC
patients that met the inclusion criteria. The basic information,
relevant test results and survival information were collected,
including the age of onset, menstrual status at onset, pathological
and lymph node status, immunohistochemistry, radiotherapy,
endocrine therapy drugs and disease-free survival time. The cut-off
for PR positivity was immunohistochemical staining in ≥10% of tumor
cells. The scoring of CerbB2 by immunohistochemical was: -, no
membrane staining; +, weak and incomplete membrane staining, ++,
strong, complete membrane staining in ≤30% of tumor cells or
weak/moderate heterogeneous complete membrane staining in ≥10% of
tumor cells; or +++, strong, complete, homogeneous membrane
staining in >30% of tumor cells. Outcome indices included the
recurrence or metastasis of BC, and the follow-up time was 7–120
months. The local recurrence was confirmed by pathological
diagnosis, and the sites of distant metastasis were determined by
examinations such as ultrasound, X-ray, bone scan, computed
tomography, magnetic resonance imagining or positron emission
tomography. The disease-free survival time was calculated from the
date of diagnosis to the date of recurrence.
Statistical analysis
The measurement data in this study were expressed as
the mean ± standard deviation, and the counting data were expressed
as rates. The Kaplan-Meier method was used to create the survival
curve, the log-rank test was used to compare the disease-free
survival rate and the Cox regression analysis was used to
investigate the associated factors that affected the survival time.
SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA) was
used for the statistical analysis. All statistical tests were
two-sided, and statistical significance was defined as
P<0.05.
Results
Basic data
A total of 45 BC cases were enrolled into this
study, with a minimum age of 27 years old, a maximum age of 87
years old and an average age of 46.76±11.89 years old. The median
disease-free survival time was 31 months. The remaining basic data
are shown in Table I.
 | Table I.Baseline patient demographics and
clinical characteristics. |
Table I.
Baseline patient demographics and
clinical characteristics.
| Index | Frequency, n | Ratio, % | Effective ratio,
% | Accumulated ratio,
% |
|---|
| Age, years |
|
|
|
|
| ≤50 | 27 | 60.0 | 60.0 | 60.0 |
|
>50 | 18 | 40.0 | 40.0 | 100.0 |
| Menstrual status at
onset |
|
|
|
|
|
Menostasis at onset | 32 | 71.1 | 71.1 | 71.1 |
| No
menostasis at onset | 13 | 28.9 | 28.9 | 100.0 |
| Staging |
|
|
|
|
| II | 22 | 48.9 | 48.9 | 48.9 |
| III | 21 | 46.7 | 46.7 | 95.6 |
|
Unclear | 2 | 4.4 | 4.4 | 100.0 |
| Radiotherapy |
|
|
|
|
| No | 25 | 55.6 | 55.6 | 55.6 |
| Yes | 20 | 44.4 | 44.4 | 100.0 |
| PR |
|
|
|
|
| − | 12 | 26.7 | 26.7 | 26.7 |
| + | 33 | 73.3 | 73.3 | 100.0 |
| CerbB2 |
|
|
|
|
| − and
+ | 31 | 68.9 | 68.9 | 68.9 |
| ++ and
+++ | 14 | 31.1 | 31.1 | 100.0 |
| Endocrine
therapy |
|
|
|
|
|
Tamoxifen | 34 | 75.6 | 75.6 | 75.6 |
|
Aromatizing enzyme
inhibitor | 11 | 24.4 | 24.4 | 100.0 |
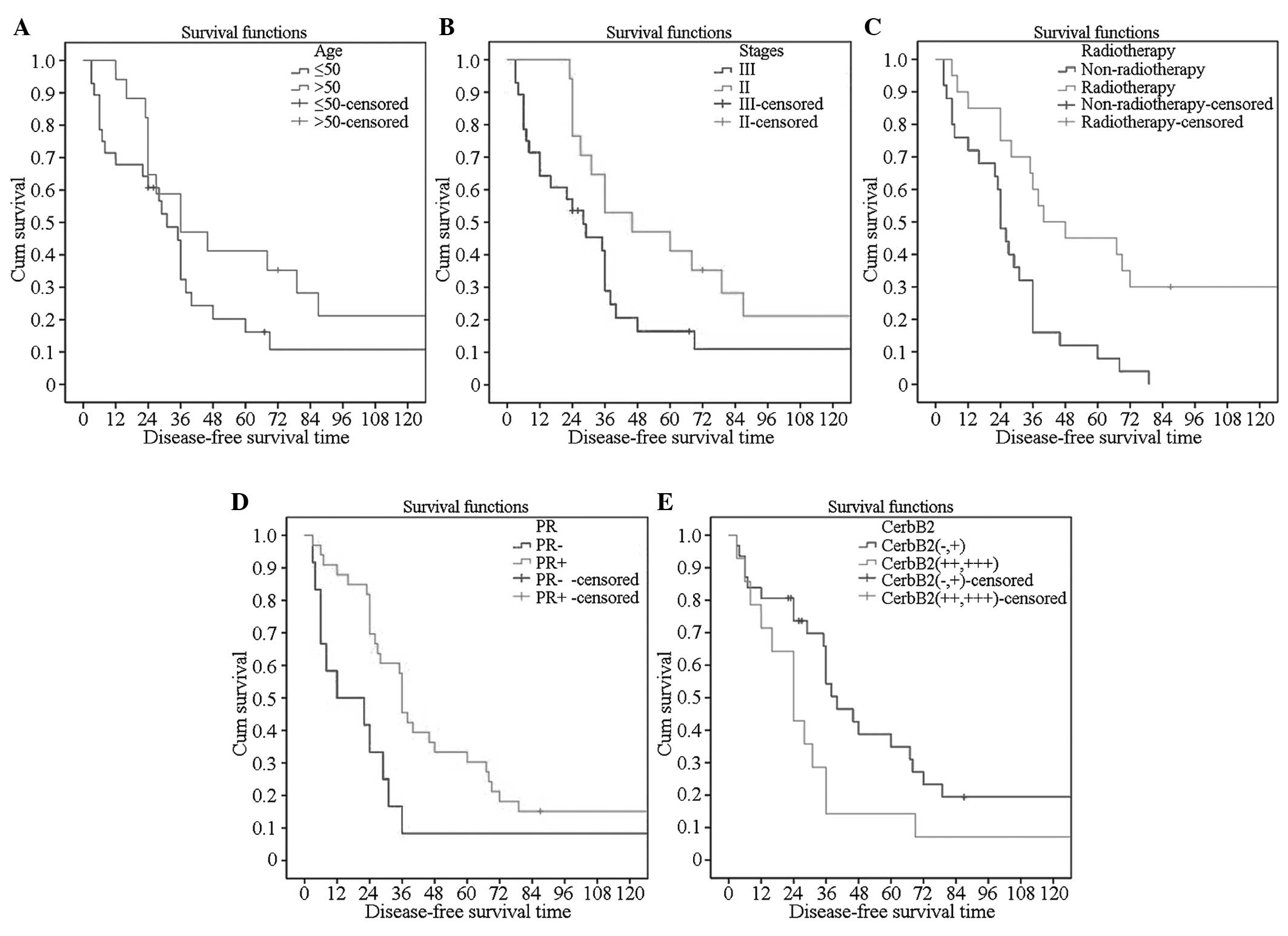
Single factor analysis of effects of
different clinical indicators on endocrine resistance
The disease-free survival times were observed with
regard to the age of onset, menstrual status at onset, BC staging,
chemotherapy status, endocrine therapy and different levels of PR
and CerbB2, and then survival curves were created. It was
demonstrated that patients with an age of onset of >50 years,
stage II disease, radiotherapy, PR(+) and CerbB2 (− and +) showed a
higher incidence of resistance to endocrine therapy (Fig. 1).
Cox univariate regression
analysis
The disease-free survival time, recurrence and
metastasis were set as the dependent variables. By contrast, the
age of onset, menopausal status at onset, lymph node status,
clinical staging, radiotherapy, endocrine therapy, PR expression
and CerbB2 expression were set as the independent variables for the
Cox univariate regression analysis. The results revealed that the
age of onset, radiotherapy, endocrine therapy, PR expression and
CerbB2 expression exhibited an impact on the disease-free survival
time (Table II).
 | Table II.Univariate Cox regression
analysis. |
Table II.
Univariate Cox regression
analysis.
|
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Index | B | SE | Index | P-value | RR | Lower limit | Upper limit |
|---|
| Age of onset | −0.860 | 0.369 | 5.430 | 0.020a | 0.423 | 0.205 | 0.872 |
| Menstrual status at
onset | −0.036 | 0.332 | 0.012 | 0.913 | 0.965 | 0.503 | 1.849 |
| Staging | −0.265 | 0.327 | 0.658 | 0.417 | 0.767 | 0.404 | 1.456 |
| Radiotherapy | −1.085 | 0.350 | 9.629 | 0.002a | 0.338 | 0.170 | 0.671 |
| Endocrine
therapy |
0.990 | 0.368 | 7.220 | 0.007b | 2.692 | 1.307 | 5.542 |
| PR expression | −0.832 | 0.363 | 5.234 | 0.022a | 0.435 | 0.214 | 0.888 |
| CerbB2
expression | −0.502 | 0.140 | 2.124 | 0.017a | 0.605 | 0.460 | 0.796 |
Cox multivariate regression
analysis
The disease-free survival time, recurrence and
metastasis were set as the dependent variables. Those clinical
indicators that had statistical significance in the Cox univariate
regression analysis, namely the age of onset, radiotherapy,
endocrine therapy, PR expression and CerbB2 expression, were set as
the independent variables for the Cox regression with the Enter
method. The model testing results indicated that the model had
statistical significance (Table
III). The Cox regression analysis showed that the different
ages of onset exhibited a statistical significant effect on the
endocrine therapy (P=0.019), with a standardized odds ratio (OR)
value of 3.658 and a 95% confidence interval (CI) of 1.235–10.836.
Different radiotherapies also exhibited statistical significance
(P=0.006), with a standardized OR value of 2.838 and a 95% CI of
1.342–6.000. Different expression levels of PR also exhibited
statistical significance (P=0.002), with a standardized OR value of
2.631 and a 95% CI of 1.416–4.889. Furthermore, different
expression levels of CerbB2 exhibited statistical significance
(P=0.043), with a standardized OR value of 2.631 and a 95% CI of
1.416–4.889. The Cox multivariate regression analysis showed that
the different ages of onset, the use of pre-operative radiotherapy,
and the different expression levels of PR and CerbB2 exhibited
statistical significance with regard to the post-endocrine-therapy
disease-free survival time, which indicated that these factors may
affect the endocrine therapy resistance of BC (Table IV).
 | Table III.Model test of Cox regression. |
Table III.
Model test of Cox regression.
| Parameter | Value |
|---|
| 2-fold logarithm
likelihood value | 203.737 |
| χ2 | 30.42 |
| Degrees of
freedom | 9 |
| P-value | <0.0001 |
 | Table IV.Multivariate Cox regression
analysis. |
Table IV.
Multivariate Cox regression
analysis.
|
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Index | Estimated
value | SD | Wald | P-value | Standardized
estimated value | Lower limit | Upper limit |
|---|
| Age of onset |
1.297 | 0.554 | 5.488 | 0.019a | 3.658 | 1.235 | 10.836 |
| Staging |
0.090 | 0.484 | 0.035 | 0.853 | 1.094 | 0.424 | 2.822 |
| Radiotherapy |
1.043 | 0.382 | 7.439 | 0.006b | 2.838 | 1.342 | 6.000 |
| Endocrine
therapy |
0.762 | 0.457 | 2.785 | 0.095 | 2.142 | 0.875 | 5.243 |
| PR expression |
0.967 | 0.316 | 9.371 | 0.002b | 2.631 | 1.416 | 4.889 |
| CerbB2
expression | −0.961 | 0.476 | 4.077 | 0.043a | 0.382 | 0.150 | 0.972 |
Discussion
Currently, endocrine therapy is an important part of
comprehensive BC treatment (16).
Although molecular typing and screening in recent years have
provided an effective method for choosing the most sensitive
candidates for endocrine therapy, a considerable number of patients
exist that are not sensitive to endocrine therapy (17,18).
Therefore, further investigation of the specific indicators is
necessary for improving the efficacy of endocrine treatment. The
present study aimed to search for novel indicators for screening
the sensitive populations and predicting the efficacy of the
treatment.
Considering the close association between ER and BC,
the sensitivity to endocrine therapy in patients with different
levels of ER was first analyzed. The ATAC trial compared the
efficacy of tamoxifen (TAM) and anastrozole, from which one result
showed that the recurrence rate of ER+/PR−
patients was significantly higher than that of
ER+/PR+ patients. Due to the different PR
status, this cancer could not simply be referred to as
receptor-positive BC. In 2007, experts in the St. Gallen conference
came to the consensus that ER+/PR− was
included in the endocrine incomplete reaction type (19). Arpino et al (20) reported that 70% of BC patients with
double-positive ER and PR were sensitive to endocrine therapy,
while only 34% of ER+/PR− BC patients were
sensitive to endocrine therapy. This data confirmed that besides
ER, PR also played an important role in forecasting the efficacy of
endocrine therapy. In ER+ BC patients, PR−
patients were more prone to generating TAM resistance than the
PR+ patients, therefore leading to treatment failure
(21). In the present study, the Cox
multivariate regression analysis showed that the treatment efficacy
of ER+/PR+ BC patients was significantly
better than those who were ER+/PR−, which was
consistent with other studies. Nicholson et al (22) first reported that the efficacy of TAM
towards metastatic BC patients with CerbB2 overexpression was
decreased from 38 to 7% compared with those with no CerbB2; Wright
et al (23)also showed that
high CerbB2 expression made the response of ER+ BC
patients towards TAM decrease from 48 to 20%. Meng et al
(24) considered that the higher the
expression level of CerbB2 the quicker the progress of BC
metastasis or recurrence, and suggested that the overall
disease-free survival time would also be short, all represented as
the different levels of endocrine therapy resistance. In the
present study, the Cox multivariate regression analysis showed that
the risk of endocrine resistance in the patients with high CerbB2
expression (++ and +++) was higher than that in those with low or
no expression of CerbB2 (− and +). This was also basically
consistent with the results of the study by Gregory et al
(25).
The present results also indicated that certain
clinical parameters may predict the sensitivity of endocrine
therapy. According to the Cox multivariate regression analysis,
patients >50 years old at onset was less sensitive to endocrine
therapy than those ≤50 years old. This may be as the degree of
malignancy in the young BC patients was higher, with a more
aggressive nature, which would be more prone to relapse and
metastasis; while the elder BC patients exhibited slow progression,
with a prognosis that was relatively improved. Although BC is a
hormone-dependent tumor, the present study found that menopause
exhibited no significant effect on endocrine therapy resistance.
This conclusion was inconsistent with some previous studies
(26). A small sample size, selection
criteria and different age-division ranges may also contribute to
this conclusion. The application of BC chemotherapeutic drugs
reduced the risk of BC recurrence and metastasis, and prolonged the
survival time. No studies exist to confirm the impact of
radiotherapy on endocrine therapy resistance, however, in the
present study, radiotherapy had a positive impact, which probably
resulted from the small sample size and requires future large-scale
clinical trials for further verification.
The aromatase inhibitors (AIs) were effective
towards the TAM-resistant BC, and have been approved as a
second-line drug against postmenopausal metastatic BC (27). The 91-month follow-up data for IES031
showed that compared with TAM, exemestane significantly improved
disease-free survival, and reduced the risks of local and distant
recurrence, while significantly increasing the overall survival
rate of ER+ patients for unknown reasons. The results of
a 2.75-year follow-up by TEAM also showed that, compared with TAM,
exemestane significantly reduced the risks of recurrence and
distant metastasis (28). A clinical
study (29) showed that with regard
to the postmenopausal BC patients, when TAM treatment generated
resistance, the application of second-line drugs (AIs) would still
be effective. A total of 30% of the BC patients who were resistant
to the AI therapy could obtain a clinical benefit from fulvestrant
treatment, which also indicated that selective estrogen receptor
modulators would play a role against the AI-resistant cells.
Therefore, the sequential or combined application of endocrine
therapy drugs could avoid endocrine therapy resistance to a certain
extent. In the present study, univariate Cox regression analysis
demonstrated that the patients receiving tamoxifen exhibited a
significantly improved disease-free survival rate compared with
those receiving AIs, however, the difference was not observed in
the multivariate Cox model. Upon review of the clinical data, aside
from the small sample size, the results were also impacted by the
fact that among the 45 BC patients, 6 patients did not experience
relapse or metastasis within 10 years. Of these patients, 5 were
administrated TAM, and among these 5, 1 patient underwent a
modified radical mastectomy combined with bilateral oophorectomy
and 1 patient underwent a uterine adnexectomy for other
gynecological disease prior to BC diagnosis. Therefore, the
gynecological surgeries affected the endocrine status, which may
have had a greater impact on the results.
Taken together, the present study demonstrated that
certain clinicopathological parameters, including younger age of
onset, not receiving radiotherapy, a low expression level of PR and
a high expression level of CerbB2, may be risk factors that
contribute to tamoxifen or AIs resistance. Hence, patients with
these characteristics should be cautiously supervised during
endocrine therapy.
References
|
1
|
de Martel C, Ferlay J, Franceschi S,
Vignat J, Bray F, Forman D and Plummer M: Global burden of cancers
attributable to infections in 2008: A review and synthetic
analysis. Lancet Oncol. 13:607–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oliveras-Ferraros C, Vazquez-Martin A,
Cufí S, Torres-Garcia VZ, Sauri-Nadal T, Barco SD, Lopez-Bonet E,
Brunet J, Martin-Castillo B and Menendez JA: Inhibitor of Apoptosis
(IAP) survivin is indispensable for survival of HER2 gene-amplified
breast cancer cells with primary resistance to HER1/2-targeted
therapies. Biochem Biophys Res Commun. 407:412–419. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Regan MM, Neven P, Giobbie-Hurder A,
Goldhirsch A, Ejlertsen B, Mauriac L, Forbes JF, Smith I, Láng I,
Wardley A, et al: Assessment of letrozole and tamoxifen alone and
in sequence for postmenopausal women with steroid hormone
receptor-positive breast cancer: The BIG 1–98 randomised clinical
trial at 8.1 years median follow-up. Lancet Oncol. 12:1101–1108.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bliss JM, Kilburn LS, Coleman RE, Forbes
JF, Coates AS, Jones SE, Jassem J, Delozier T, Andersen J,
Paridaens R, et al: Disease-related outcomes with long-term
follow-up: An updated analysis of the intergroup exemestane study.
J Clin Oncol. 30:709–717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davies C, Pan H, Godwin J, Gray R,
Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A,
Bonfill X, et al: Long-term effects of continuing adjuvant
tamoxifen to 10 years versus stopping at 5 years after diagnosis of
oestrogen receptor-positive Breast cancer: ATLAS, a randomised
trial. Lancet. 381:805–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burstein HJ, Temin S, Anderson H, Buchholz
TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Rowden D, Solky
AJ, et al: Adjuvant endocrine therapy for women with hormone
receptor-positive breast cancer: American society of clinical
oncology clinical practice guideline focused update. J Clin Oncol.
32:2255–2269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Traub F, Feist H, Kreipe HH and Pich A:
SELDI-MS-based expression profiling of ductal invasive and lobular
invasive human Breast carcinomas. Patholo Res Pract. 201:763–770.
2005. View Article : Google Scholar
|
|
8
|
Caldon CE, Sergio CM, Kang J,
Muthukaruppan A, Boersma MN, Stone A, Barraclough J, Lee CS, Black
MA, Miller LD, et al: Cyclin E2 overexpression is associated with
endocrine resistance but not insensitivity to CDK2 inhibition in
human Breast cancer cells. Mol Cancer Ther. 11:1488–1499. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Osborne CK and Schiff R: Mechanisms of
endocrine resistance in breast cancer. Annu Rev Med. 62:233–247.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garcia-Becerra R, Santos N, Diaz L and
Camacho J: Mechanisms of resistance to endocrine therapy in breast
cancer: Focus on signaling pathways, miRNAs and genetically based
resistance. Int J Mol Sci. 14:108–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Hara J, Vareslija D, McBryan J, Bane F,
Tibbitts P, Byrne C, Conroy RM, Hao Y, Gaora PÓ, Hill AD, et al:
AIB1: ERα transcriptional activity is selectively enhanced in
aromatase inhibitor-resistant breast cancer cells. Clin Cancer Res.
18:3305–3315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hawsawi Y, El-Gendy R, Twelves C, Speirs V
and Beattie J: Insulin-like growth factor-oestradiol crosstalk and
mammary gland tumourigenesis. Biochim Biophys Acta. 1836:345–353.
2013.PubMed/NCBI
|
|
13
|
Hasson SP, Rubinek T, Ryvo L and Wolf I:
Endocrine resistance in breast cancer: Focus on the
phosphatidylinositol 3-kinase/akt/mammalian target of rapamycin
signaling pathway. Breast Care (Basel). 8:248–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao X, Di Leva G, Li M, Fang F, Devlin C,
Hartman-Frey C, Burow ME, Ivan M, Croce CM and Nephew KP:
MicroRNA-221/222 confers breast cancer fulvestrant resistance by
regulating multiple signaling pathways. Oncogene. 30:1082–1097.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ward A, Shukla K, Balwierz A, König R,
Sahin O and Wiemann S: MicroRNA-519a is a novel oncomir conferring
tamoxifen resistance by targeting a network of tumour-suppressor
genes in ER+ breast cancer. J Pathol. 233:368–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zelnak AB and O'Regan RM: Optimizing
endocrine therapy for breast cancer. J Natl Compr Canc Netw.
13:e56–64. 2015.PubMed/NCBI
|
|
17
|
Regan MM: Predicting benefit of endocrine
therapy for early breast cancer. Breast S0960–9776. 00167–00168.
2015.
|
|
18
|
Feng Q, Zhang Z, Shea MJ, Creighton CJ,
Coarfa C, Hilsenbeck SG, Lanz R, He B, Wang L, Fu X, et al: An
epigenomic approach to therapy for tamoxifen-resistant breast
cancer. Cell Res. 24:809–819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goss PE, Ingle JN, Martino S, Robert NJ,
Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard
KI, et al: National Cancer Institute of Canada Clinical Trials
Group MA.17: Efficacy of letrozole extended adjuvant therapy
according to estrogen receptor and progesterone receptor status of
the primary tumor: National Cancer Institute of Canada Clinical
Trials Group MA.17. J Clin Oncol. 25:2006–2011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arpino G, Weiss H, Lee AV, Schiff R, De
Placido S, Osborne CK and Elledge RM: Estrogen receptor-positive,
progesterone receptor-negative breast cancer: Association with
growth factor receptor expression and tamoxifen resistance. J Natl
Cancer Inst. 97:1254–1261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Richer JK, Jacobsen BM, Manning NG, Abel
MG, Wolf DM and Horwitz KB: Differential gene regulation by the two
progesterone receptor isoforms in human breast cancer cells. J Biol
Chem. 277:5209–5218. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nicholson RI and Johnston SR: Endocrine
therapy-current benefits and limitations. Breast Cancer Res Treat.
93:S3–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wright C, Nicholson S, Angus B, et al:
Relationship between c-erbB-2 protein product expression and
response to endocrine therapy in advanced breast cancer. Br J
Cancer. 65:118–121. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng S, Tripathy D, Shete S, Ashfaq R,
Haley B, Perkins S, Beitsch P, Khan A, Euhus D, Osborne C, et al:
HER-2 gene amplification can be acquired as breast cancer
progresses. Proc Natl Acad Sci USA. 101:9393–9398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gregory RK, Powles TJ, Salter J, Chang JC,
Ashley S and Dowsett M: Prognostic relevance of cerbB2 expression
following neoadjuvant chemotherapy in patients in a randomised
trial of neoadjuvant versus adjuvant chemoendocrine therapy. Breast
Cancer Res Treat. 59:171–175. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan P, Yue W, Wang JP, Aiyar S, Li Y, Kim
TH and Santen RJ: Mechanisms of resistance to structurally diverse
antiestrogens differ under premenopausal and postmenopausal
conditions: Evidence from in vitro breast cancer cell models.
Endocrinology. 150:2036–2045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bliss JMI, Kilburn LS, Coleman RE, Forbes
JF, Coates AS, Jones SE, Jassem J, Delozier T, Andersen J,
Paridaens R, et al: Disease-related outcomes with long-term
follow-up: An updated analysis of the intergroup exemestane study.
J Clin Oncol. 30:709–717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van de Velde CJ, Rea D, Seynaeve C, Putter
H, Hasenburg A, Vannetzel JM, Paridaens R, Markopoulos C, Hozumi Y,
Hille ET, et al: Adjuvant tamoxifen and exemestane in early breast
cancer (TEAM): a randomised phase 3 trial. Lancet. 377:321–331.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jha K, Shukla M and Pandey M: Survivin
expression and targeting in breast cancer. Surg Oncol. 21:125–131.
2012. View Article : Google Scholar : PubMed/NCBI
|