Introduction
Renal medullary carcinoma (RMC) is a rare subtype of
renal cell carcinoma that most commonly occurs in adolescents and
young adults with sickle cell (SC) hemoglobinopathies (1). According to the World Health
Organization (WHO) classification of renal tumors, RMC is a
distinct entity with unique biological behavior and distinctive
pathological and morphogenetic characteristics (2,3). The most
common symptoms of RMC are hematuria and flank or abdominal pain,
which may lead to a misdiagnosis of urinary tract infection or
renal abscess in certain patients, prior to a neoplasm being
suspected (1).
A number of cases of RMC in non-African Americans
without sickle cell anemia or sickle cell trait were reported
within a series of 33 highly aggressive RMC first described in 1995
(1). The histopathological features
of RMC include epithelial cells with reticular, adenoid cystic
plasia, and prominent inflammation (4).
At present, the prognosis for patients with RMC
remains very poor, since~95% of cases present at an advanced stage
at the time of diagnosis and the tumor is resistant to chemotherapy
in addition to biological therapy (4–6). A mean
survival of 19 weeks from the time of initial diagnosis of RMC was
reported by Simpson et al (7).
Surgery of radical nephrectomy without metastatic disease appears
to prolong survival of the patients (8,9).
Pathologically, RMC arises from the renal medulla,
grows rapidly in an infiltrative pattern, and invades the renal
sinuses (10). Previous studies on
RMC have documented the pathological and clinical features of this
rare form of renal carcinoma (11).
However, there are limited studies on RMC focusing on computed
tomography (CT) imaging findings (10,12).
Patients with RMC present a poor prognosis, and nearly all patients
succumb to the disease within several months following diagnosis.
Therefore, an accurate diagnosis of RMC is important, since an
early diagnosis may improve the prognosis of these patients.
Therefore, the aim of the present study was to investigate the CT
imaging findings in 6 cases of RMC.
Patients and methods
Patients
An institutional review board exemption and a waiver
of the requirement for written informed consent from the patients
to perform the present retrospective study were obtained from the
First Affiliated Hospital of Fujian Medical University (Fuzhou,
China). A search in the pathology records and the picture archiving
and communication system of the hospital identified 6 patients with
RMC, who were hospitalized at the First Affiliated Hospital of
Fujian Medical University between 2003 and 2014. Details of the
patients, including age, gender, ethnicity and clinical symptoms,
were recorded, in addition to characteristics of the tumor,
including size, location (right or left), surgery or biopsy
confirmation, and presence of metastasis, necrosis and/or
hemorrhage, pyelocaliectasis, vascular invasion and SC trait.
Multi-slice CT examinations
All examinations were performed on multi-slice CT
(MSCT) scanners (Aquilion 16 and Aquilion ONE; Toshiba Medical
Systems Corporation, Otawara-shi, Japan), using the following
abdominal scanning parameters: i) Detector collimation, 16.0×0.5 mm
(n=4) or 320.0×0.5 mm (n=2); ii) gantry rotation time, 0.35–0.50
sec; iii) pitch, 1.0–1.4; iv) tube voltage, 120 kV; and v)
abdominal reference tube current, 60–120 mA. All images were
reconstructed from the contrast-enhanced MSCT scans with a slice
thickness of 0.75–1.00 mm and reconstruction increments of 0.5
mm.
For contrast-enhanced CT scanning, an 80–100-ml
bolus of iopromide (300 mg/ml; Bayer HealthCare Pharmaceuticals,
Berlin, Germany) was administered at a rate of 4–6 ml/sec via
injection into an antecubital vein, followed by injection of 40 ml
saline solution. The enhanced CT scans were initiated at 20–25 sec
following injection for the arterial (cortical) phase; after 65–75
sec for the cortico-medullary (medullary) phase; and after 270–300
sec for the excretory (delayed) phase. In all cases where an
initial non-contrast CT scan was available, the degree and pattern
of enhancement of the tumor were determined in the nephrographic
phase.
Pathological examination
Evaluation of gross specimens was conducted to
assess their shape; presence of necrotic components; formation of
fibrous capsule; and invasion into the renal pelvis, calyx, ureter,
renal vein or inferior vena cava. Light microscopy was used to
evaluate pathological specimens, using an XP-201 polarizing
microscope (Nanjing Jiangnan Novel Optics Co., Ltd., Nanjing,
China), and an immunohistochemical analysis was also conducted. The
tissue was obtained from the surgical resection or biopsy specimens
in six cases. All tumors were fixed in neutral buffered formalin
and all were paraffin embedded. Four-micron-thick sections of
paraffin-embedded materials were cut, deparaffinized in xylene and
rehydrated in descending dilution of ethanol. The sections were
subjected to heat-induced epitope antigen retrieval using an
electric pressue cooker set at 120°C for 5 min. For CAM5.2, enzyme
treatment (IP enzyme [ventana,Tucson,AZ,USA]) was used in addition
to the heat-induced epitope antigen retrieval. The tissue was
pretreated with 3% hydrogen peroxidase for 10 min to block
endogenous peroxidase activity. The sections were incubated in 10%
normal goat serum in PBS for 10 min to block non-specific binding.
The sections were washed three times for 5 min with TBS between
incubation steps. The sections were incubated with the primary
antibodies listed in Table II for 60
min. The sections were then washed as before and incubated with the
secondary antibody, RealTM EnVision (K5007; Dako Denmark) for 30
min. Real TM DAB+Chromogen (Dako Denmark) was used as a chromogen
for antigen localization. Slides were exposed to diaminobenzidine
for 5 min. After immunostaining, the sections were counterstained
with hematoxylin, coverslipped and sealed. PBS was used as a
negative control for the primary antibody for each group. All renal
tumors were confirmed to be RMC based upon the histological
examination and immunohistochemical findings (1,13).
 | Table II.Antibodies used for
immunohistochemical analysis. |
Table II.
Antibodies used for
immunohistochemical analysis.
| Antibody | Clone | Dilution | Manufacturer | Catalog no. | Host Target
species |
|---|
| CAM5.2 | CAM5.2 | prediluted | Thermo Fisher
Scientific, Fremont, CA, USA | ZM-0316 | Mouse/Human |
| EMA | E29 | 1:200 | Thermo Fisher
Scientific, Fremont, CA, USA | Kit-0011-2 | Mouse/Human |
| CK(H) | 34BE-12 | 1:400 | Thermo Fisher
Scientific, Fremont, CA, USA | MAB-0052 | Mouse/Human |
| P504S | 13H4 | 1:100 | Zeta, Sierra Madre,
CA, USA | ZA0227 | Rabbit/Human |
| Cytokeratin | AE1/AE3 | 1:200 | Dako, Glostrup,
Denmark | Kit-0009-2 | Mouse/Human |
Image analysis
The imaging characteristics of the tumors were
retrospectively evaluated by two genitourinary radiologists in
consensus. The following parameters were evaluated in the tumors:
Location; size; presence of calcification, cystic or necrotic
components; and attenuation on unenhanced CT scan. The degree of
enhancement of the tumors on enhanced CT was assessed during the
aforementioned 3 phases, and the results were expressed in
Hounsfield units (HUs). The presence of a capsule, hydronephrosis,
perinephric stranding, vascular and renal tissue invasion, and
metastases to retroperitoneal lymph node or other locations, was
also documented.
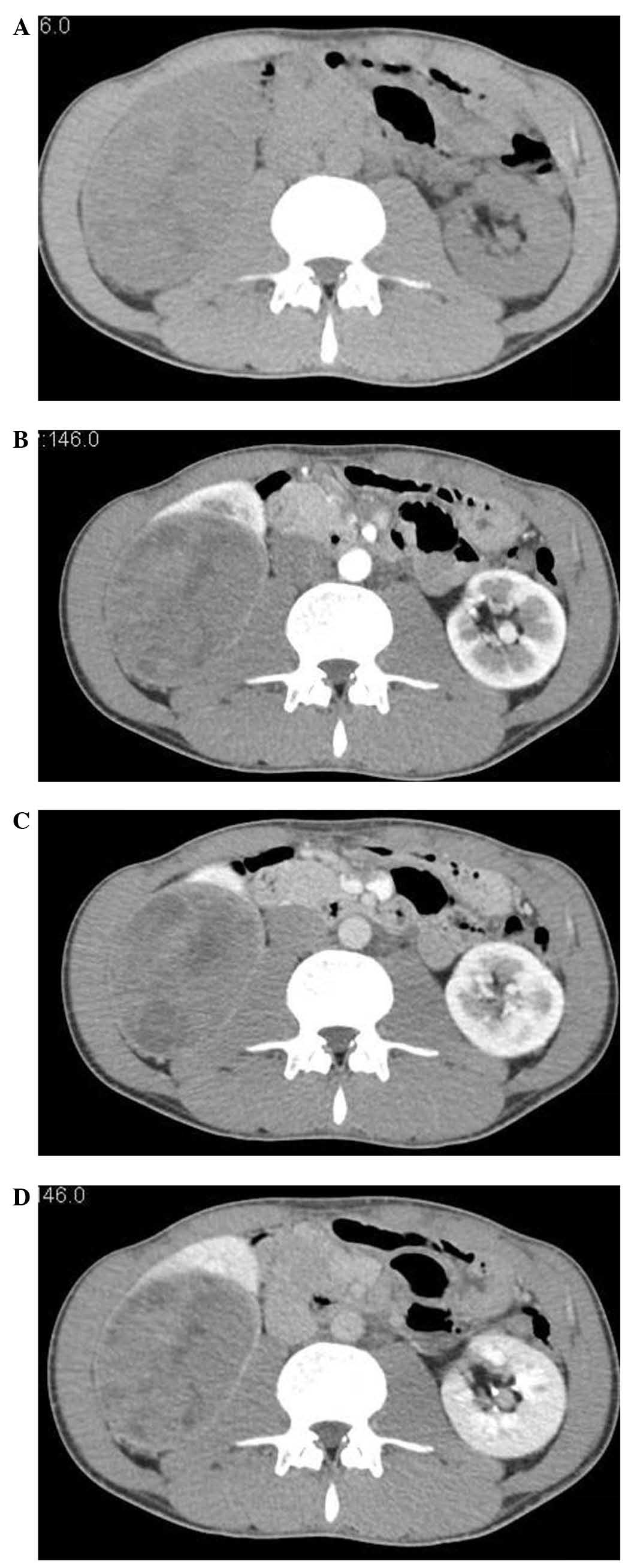
On non-contrast enhanced CT, tumors were considered
‘isodense’ if their density in HU was equal to that of the renal
parenchyma; ‘high’ if >30 HU; ‘mildly high’ if >10 HU; and
‘low’ if<10 HU, compared with the contralateral normal renal
parenchyma. The tumors were considered to be solid or cystic masses
based on the predominance. Tumor location was categorized as
medullary, cortical or exophytic based on the association of the
tumor with the renal parenchyma, and perinephric or renal sinus
fat. Thus, a medullary tumor, in which a component extended into
the renal pelvis, was considered to possess a medullary location.
Similarly, any tumor that was limited to the confines of the renal
contour was considered to have a cortical location; and an
exophytic location was assigned to any tumor extending beyond the
renal contour. The absence or presence of a tumor boundary was
assessed on the delayed phase of CT as a poorly or clearly defined
margin.
The attenuation of the tumor and the normal renal
medulla and cortex of the contralateral kidney were measured during
the 3 enhanced phases of CT. Intratumoral calcifications and cystic
components were excluded from the measured tumor area, which was
situated at the center of the mass and was defined as the region of
interest (ROI). In each phase, each 10-mm area within the ROI was
measured 4 times, and the mean value was calculated. The pattern of
enhancement of the tumor was defined as heterogeneous or
homogeneous, and its degree of enhancement was based on the HU
attenuation of the tumor, renal cortex and medulla.
Statistical analysis
Statistical analyses were performed with SPSS
statistical software, version 3.0 (SPSS Inc., Chicago, USA). The
numerical data were presented as the mean ± standard deviation.
One-way analysis of variance was used to compare the evaluated
characteristics. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The present study included 6 patients with RMC (3
females and 3 males), whose clinical data are presented in Table I. The mean age of the patients at the
time of diagnosis was 50.5 years (range, 22–72 years). Tumors were
located in the right kidney in 3 cases, and the left kidney in the
remaining 3 cases. The diameter of the tumors ranged from 2.90 to
10.50 cm (mean diameter, 7.48±3.25 cm), and tumor shape was
observed to be oval in 4 cases, and irregular in 2. Of the 6
patients, 2 presented with retroperitoneal lymph node metastasis,
and 3 with hydronephrosis. According to hemoglobin analyses, SC
hemoglobinopathy was present in only 1 case, whereas the presence
of SC trait was unknown for the remaining 5 cases, prior to
detection of the tumor. There was no bias towards any particular
location of the tumors or gender of the patients among the 6 cases
included in the study.
 | Table I.Clinical features of 6 Han Chinese
patients with renal medullary carcinoma. |
Table I.
Clinical features of 6 Han Chinese
patients with renal medullary carcinoma.
| Patient no. | Age (years) | Gender | Hemoglobin
status | Presentation |
|---|
| 1 | 72 |
Female | Sickle cell
trait | Renal mass |
| 2 | 38 | Male | Unknown | Flank pain,
hematuria |
| 3 | 57 | Male | Unknown | Left flank pain,
suspected renal calculus |
| 4 | 56 |
Female | Unknown | Abdominal pain |
| 5 | 58 |
Female | Unknown | Renal mass, fever,
back pain |
| 6 | 22 | Male | Unknown | Abdominal mass |
Mass position
All RMCs were located in the medulla and invaded the
renal pelvis. The tumors extended to the renal cortex in 4 cases,
and to the perirenal tissue in 1 case.
Mass appearance
In all 6 cases, the tumor mass was predominantly
solid and heterogeneous, and presented cystic or necrotic
components. Punctate calcifications were detected in 1 case. A
poorly defined margin of the RMC was observed in 4 cases on the
delayed phase of CT, whereas the margin of the remaining 2 cases
appeared well-defined. No fibrous capsule was present in any of the
6 cases. In 1 case, the tumor had spread into the retroperitoneal
soft tissue, invaded the left renal artery, and developed regional
lymph node metastasis.
Attenuation of normal kidney and tumor
on unenhanced CT
The CT attenuation of the RMC was equal to that of
the normal renal cortex and medulla (42.3±2.7 vs. 40.7±3.6 and
41.2±3.9 HU, respectively; P>0.05). On unenhanced CT, the
attenuation of the solid part of the RMC was isodense, equal to
that of the normal renal parenchyma, in all 6 cases (Fig. 1).
Degree and pattern of CT
enhancement
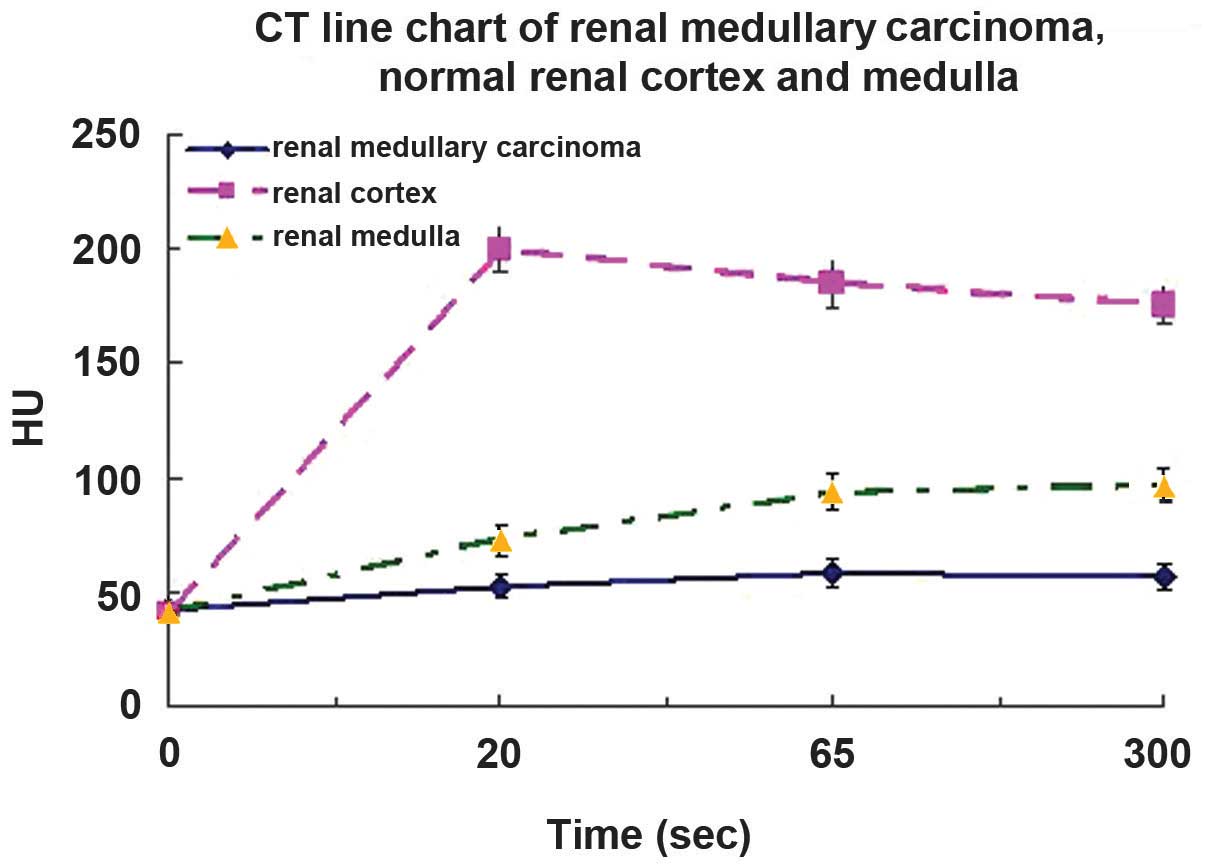
On dynamic contrast-enhanced CT scan, the
attenuation of RMC was markedly lower than that of the normal renal
cortex and medulla during all enhanced phases (P<0.05, n=6;
Table III and Fig. 2).
 | Table III.Attenuation of the renal tumors on
dynamic contrast-enhanced computed tomography scan, compared with
normal renal cortex and medulla. |
Table III.
Attenuation of the renal tumors on
dynamic contrast-enhanced computed tomography scan, compared with
normal renal cortex and medulla.
|
| Attenuation,
Hounsfield units |
|---|
|
|
|
|---|
| Phase | Renal medullary
carcinoma | Normal renal
cortex | Normal renal
medulla |
|---|
| Cortical | 52.6±4.8 | l99.5±9.7 | 72.7±6.4 |
| Medullary | 58.6±5.7 |
184.6±10.8 | 93.5±7.8 |
| Delayed | 56.8±6.1 | 175.7±8.5 | 96.5±7.9 |
Surgical/gross observation and
follow-up
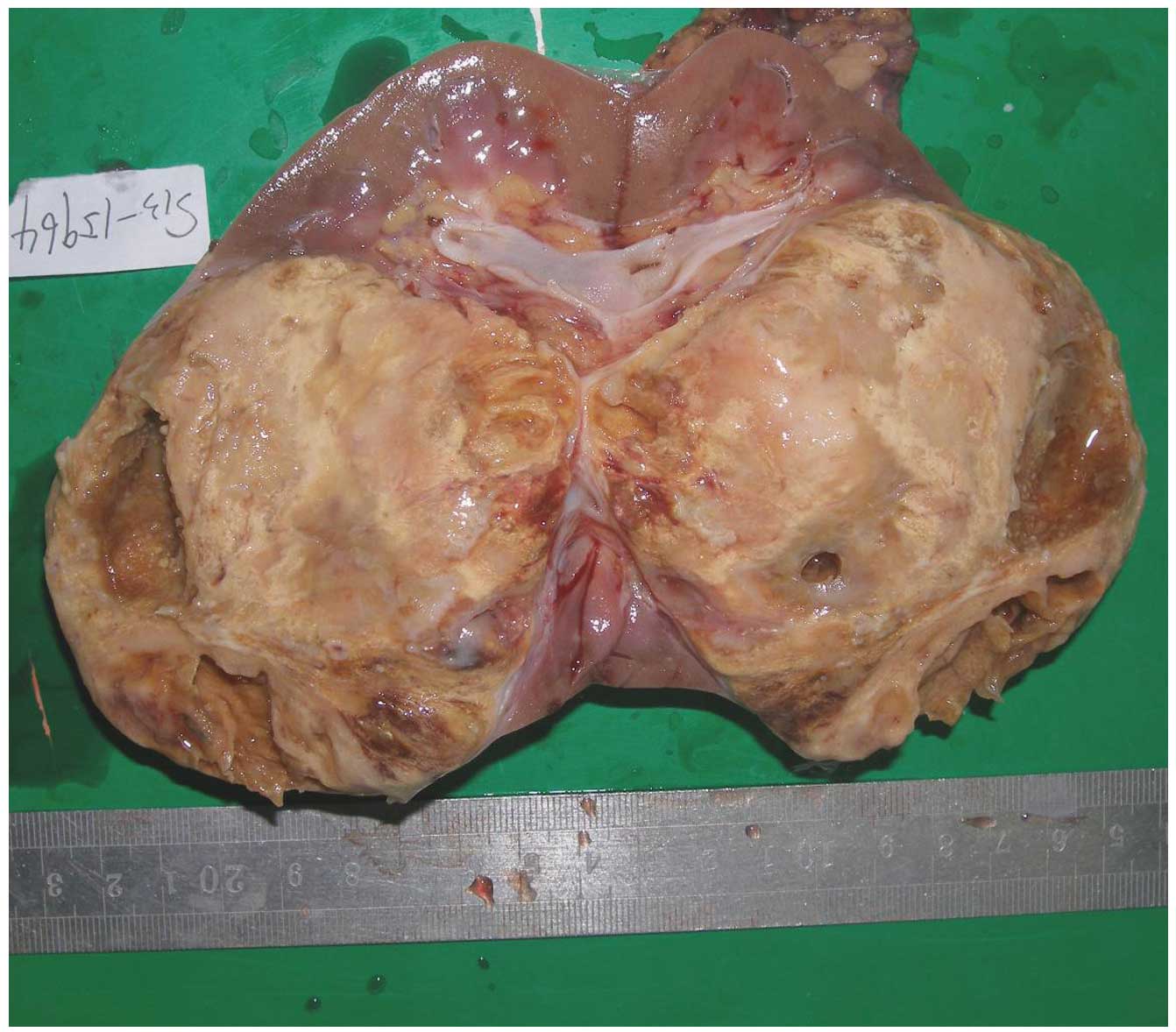
All 6 patients with RMC underwent surgery. In 4
cases, the masses were oval, and in 2 cases, the masses were
irregular in shape and exhibited regional lymph node metastasis.
The tumor masses were firm or rubbery with white-to-grey color, and
occupied the medulla (Fig. 3). All 6
tumors had invaded into the renal pelvis or calyx.
Despite the total nephrectomy performed, the
patients presented a poor outcome. Consequently, chemotherapy
and/or immunotherapy was administered post-surgically in 5 cases:
One case received sunitinib at a dose of 50 mg/day, administered in
one 6-week cycle of daily oral therapy for 4 weeks, followed by 2
weeks off. After the cycle, abdominal CT images revealed an
enlargement of the previously observed retroperitoneal lymph nodes.
The patient was then treated with two 7-week cycles of
high-dose-intensity MVAC (methotrexate, vinblastine, doxorubicin,
and cisplatin), which was initially described by Sternberg et
al (14,15). Doses of 30 mg/m2
methotrexate and 3.0 mg/m2 vinblastine were administered
by intravenous infusion on days 1 and 15. Doses of 30
mg/m2 doxorubicin and 70 mg/m2 cisplatin were
administered by intravenous infusion on days 2 and 16 of 28-day
cycles. The other cases were treated with 1–3 cycles of
high-dose-intensity MVAC. By contrast, 1 case diagnosed with
increasing metastatic burden did not receive adjuvant therapy. The
clinical condition of the patients rapidly deteriorated following
diagnosis of RMC. The median survival time of the 6 cases from the
time of diagnosis was 11 weeks.
Pathological findings
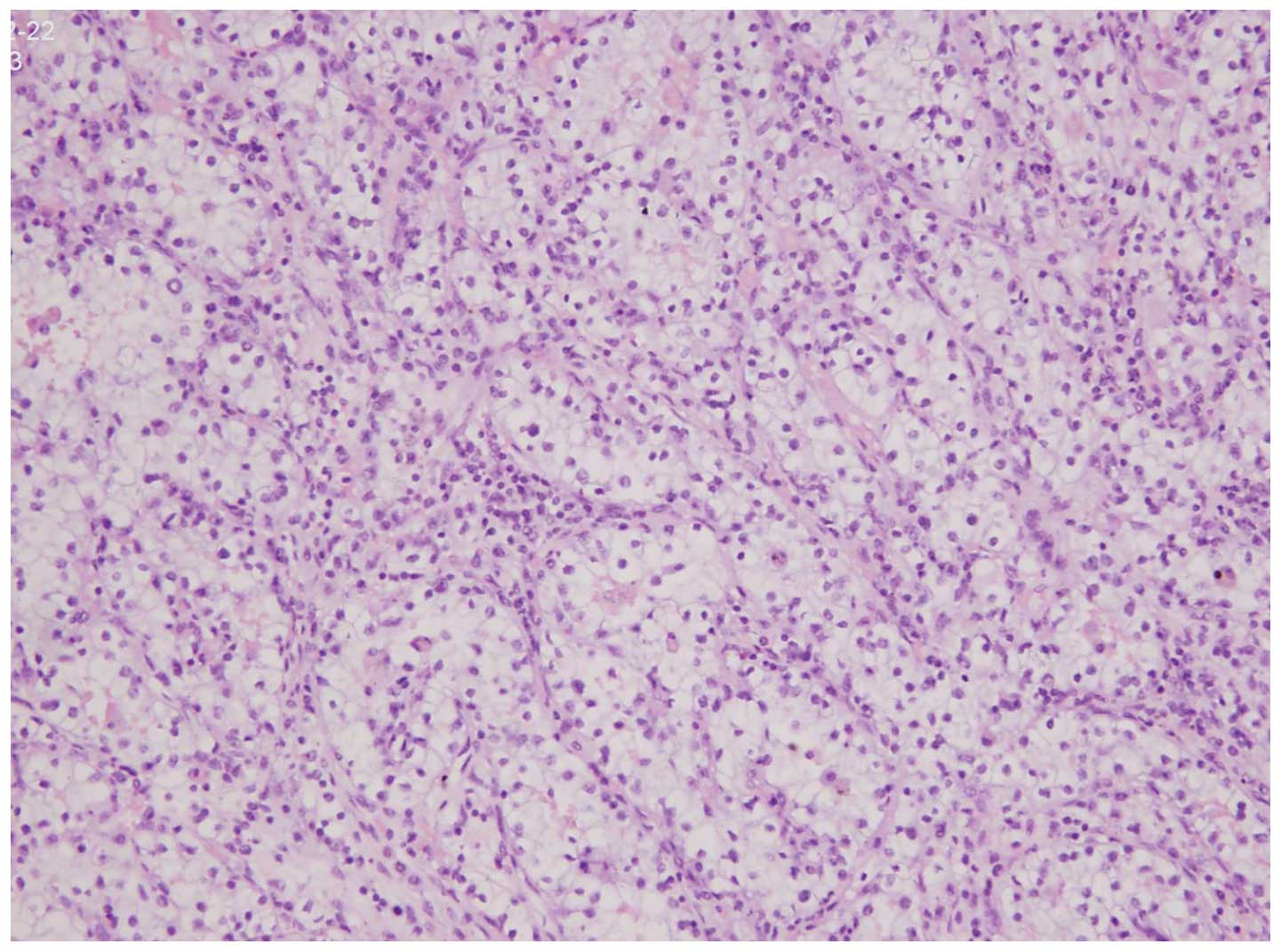
All 6 tumors displayed similar microscopic
characteristics, including a cribriform architecture and stromal
desmoplasia (Fig. 4). The reticular
pattern of growth consisted of tumor cell aggregates forming spaces
of different sizes. An adenoid-cystic pattern of growth was further
identified in 5 tumors. Necrosis was noted in all cases.
Immunohistochemical studies confirmed homogeneous expression of low
molecular weight cytokeratin CAM 5.2, and co-expression of vimentin
and epithelial membrane antigen in all tumors.
Discussion
According to the 2004 WHO classification of renal
tumors, RMC is a rare subtype of renal cell carcinoma that was
first described as the ‘seventh sickle cell nephropathy’ (1,16). The age
range of the patients in the present study was 22–72 years. This
population is older than that reported by Davis et al
(1) (range, 11–39 years), possibly
due to the fact that the patients of the present study belonged to
the Han Chinese population, in which SC hemoglobinopathies are
rare. Accordingly, only 1 of the 6 cases in the present study was
diagnosed with SC hemoglobinopathy by hemoglobin analysis.
The most significant clinical challenge presented by
RMC is that chemo-, immuno- and radiotherapy have all been
unsuccessful for the treatment of RMC, based on previous studies
(4,5,17–22). Therefore, an early diagnosis may
improve the survival rates of patients with RMC. Although RMC has
been relatively well described in previous pathological studies
(23–25), the imaging data published in these
studies are limited, particularly regarding histopathological
examinations. In routine clinical work, a correct imaging diagnosis
of RMC is difficult, which is mostly likely due to the low
morbidity and level of awareness of this disease (26). The data of the present study suggest
that the following imaging characteristics may aid in identifying
RMC accurately: RMC tends to poorly circumscribed, solitary and
heterogeneous mass, which arises from the renal medulla, and
presents lower enhancement compared with that of the cortex and
medulla in all phases of CT. In addition, RMC exhibits an
infiltrative appearance, and usually extends into the renal pelvis
and peripelvic tissues.
Histopathologically, RMC is known to arise from the
renal medulla, as reported in previous pathological studies
(23,24). Since other renal tumors, including
parts of clear cell renal cell carcinoma, transitional cell
carcinoma, rhabdoid tumor and renal lymphoma may also involve the
medulla, differentiating RMC from such other tumors may be
challenging based solely on the location of the tumor (26). However, a number of imaging and
clinical features may facilitate this differentiation: The majority
of cortical clear cell renal cell carcinomas (~94%) have an
expansible appearance, and display exophytic growth that disrupts
the reniform contour (27). Their
degree of enhancement is usually higher than that of the normal
renal cortex (28). These findings
suggest that distinguishing between RMCs and other renal tumors
with a rich blood supply based on their different enhancement on CT
may be feasible. Transitional cell carcinomas arise from the
collecting system, however, gross hematuria and hydronephrosis are
common at the initial staging (29).
Rhabdoid tumor of the kidney usually occurs in patients under 3
years of age, although its appearance is similar to that of RMC
(30,31). Renal lymphoma is usually bilateral and
multifocal, and mostly occurs in patients with non-Hodgkin's
lymphoma, although it is evident only in ~5% of patients at
presentation (32,33).
The results of the present study indicate that RMCs
are heterogeneous masses with an isodense parenchyma on unenhanced
CT scan, which is considered to be due to their stromal
desmoplasia. On pathology and surgery, satellite lesions in the
renal cortex and intratumoral hemorrhage were observed in 5 cases
and necrosis was observed in all the cases. This combination of
traits differs from that of solid tumors that exhibit high
attenuation, such as clear cell renal cell and papillary
carcinomas, oncocytomas and angiomyolipomas with minimal fat
(34).
In the present study, the enhancement of RMCs on CT
was lower than that of the renal cortex and medulla during all
enhanced phases of CT, which was hypothesized to be due to the rare
hypovascular features of this type of tumor. This pattern of
enhancement is atypical of tumors with a rich blood supply,
including renal angiomas, renal angiomyolipomas with minimal fat
and clear cell renal cell carcinomas, whose degree of enhancement
is normally higher than that of the normal renal cortex (35). Therefore, these findings support the
concept that distinguishing between RMCs and renal tumors with a
rich blood supply based on their different enhancement on CT may be
relatively easy.
Due to their similar characteristics, RMC was often
misclassified in the past as an aggressive form of collecting duct
carcinoma, prior to being recognized as a separate entity. Both
RMCs and collecting duct carcinomas present an infiltrative
pattern, are biologically aggressive, arise from the medulla, and
are considered to derive from proliferating cells of the collecting
duct epithelium, although collecting duct carcinoma is not
associated with hemoglobinopathies (23,36,37).
Therefore, it is often difficult to distinguish between these 2
types of tumors by using exclusively dynamic contrast-enhanced CT
imaging.
In conclusion, the presence of an infiltrative,
heterogeneous mass with necrosis, which arises from the renal
medulla and displays lower enhancement than that of the renal
cortex and medulla during all phases of CT, suggests the diagnosis
of RMC.
Acknowledgements
The present study was supported by a grant from the
scientific research programs of Fujian provincial Health and Family
Planning Commission for young scholars (2014-01-45). The authors
are grateful to Dr Xianying Zhen and Dr Kaige Wu, for their advice
and support at various stages of the project.
References
|
1
|
Davis CJ Jr, Mostofi FK and Sesterhenn IA:
Renal medullary carcinoma. The seventh sickle cell nephropathy. Am
J Surg Pathol. 19:1–11. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Skolarus TA, Serrano MF, Berger DA,
Bullock TL, Yan Y, Humphrey PA and Kibel AS: The distribution of
histological subtypes of renal tumors by decade of life using the
2004 WHO classification. J Urol. 179:433–444. 2008. View Article : Google Scholar
|
|
3
|
Lopez-Beltran A, Scarpelli M, Montironi R
and Kirkali Z: 2004 WHO classification of the renal tumors of the
adults. Eur Urol. 49:798–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schaeffer EM, Guzzo TJ, Furge KA, Netto G,
Westphal M, Dykema K, Yang X, Zhou M, Teh BT and Pavlovich CP:
Renal medullary carcinoma: Molecular, pathological and clinical
evidence for treatment with topoisomerase-inhibiting therapy. BJU
Int. 106:62–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hakimi AA, Koi PT, Milhoua PM, Blitman NM,
Li M, Hugec V, Dutcher JP and Ghavamian R: Renal medullary
carcinoma: The Bronx experience. Urol. 70:878–882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sathyamoorthy K, Teo A and Atallah M:
Renal medullary carcinoma in a patient with sickle-cell disease.
Nat Clin Pract Urol. 3:279–283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simpson L, He X, Pins M, Huang X, Campbell
SC, Yang XJ, Perlman EJ and Bergan RC: Renal medullary carcinoma
and ABL gene amplification. J Urol. 173:1883–1888. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khan A, Thomas N, Costello B, Jobling L,
de Kretser D, Broadfield E and O'Shea S: Renal medullary carcinoma:
Sonographic, computed tomography, magnetic resonance and
angiographic findings. Eur J Radiol. 35:1–7. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Selby DM, Simon C, Foley JP, Thompson IM
and Baddour RT: Renal medullary carcinoma: Can early diagnosis lead
to long-term survival? J Urol. 163:12382000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blitman NM, Berkenblit RG, Rozenblit AM
and Levin TL: Renal medullary carcinoma: CT and MRI features. AJR
Am J Roentgenol. 185:268–272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Swartz MA, Karth J, Schneider DT,
Rodriguez R, Beckwith JB and Perlman EJ: Renal medullary carcinoma:
Clinical, pathologic, immunohistochemical, and genetic analysis
with pathogenetic implications. Urology. 60:1083–1089. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neville A and Hatem SF: Renal medullary
carcinoma: Unsuspected diagnosis at stone protocol CT. Emerg
Radiol. 14:245–247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steele EL and MacLennan GT: Renal
medullary carcinoma. J Urol. 174:14492005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sternberg CN, de Mulder P, Schornagel JH,
Theodore C, Fossa SD, van Oosterom AT, Witjes JA, Spina M, van
Groeningen CJ, Duclos B, et al: EORTC Genito-Urinary Cancer Group:
Seven year update of an EORTC phase III trial of high-dose
intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in
advanced urothelial tract tumours. Eur J Cancer. 42:50–54. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sternberg CN, de Mulder PH, Schornagel JH,
Théodore C, Fossa SD, van Oosterom AT, Witjes F, Spina M, van
Groeningen CJ, de Balincourt C and Collette L: European
Organization for Research and Treatment of Cancer Genitourinary
Tract Cancer Cooperative Group: Randomized phase III trial of
high-dose-intensity methotrexate, vinblastine, doxorubicin, and
cisplatin (MVAC) chemotherapy and recombinant human granulocyte
colony-stimulating factor versus classic MVAC in advanced
urothelial tract tumors: European Organization for Research and
Treatment of Cancer Protocol no. 30924. J Clin Oncol. 19:2638–2646.
2001.PubMed/NCBI
|
|
16
|
Lopez-Beltran A, Scarpelli M, Montironi R
and Kirkali Z: 2004 WHO classification of the renal tumors of the
adults. Eur Urol. 49:798–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Strouse JJ, Spevak M, Mack AK, Arceci RJ,
Small D and Loeb DM: Significant responses to platinum-based
chemotherapy in renal medullary carcinoma. Pediatr Blood Cancer.
44:407–411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walsh A, Kelly DR, Vaid YN, Hilliard LM
and Friedman GK: Complete response to carboplatin, gemcitabine, and
paclitaxel in a patient with advanced metastatic renal medullary
carcinoma. Pediatr Blood Cancer. 55:1217–1220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ronnen EA, Kondagunta GV and Motzer RJ:
Medullary renal cell carcinoma and response to therapy with
bortezomib. J Clin Oncol. 24:e142006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maroja Silvino MC, Venchiarutti Moniz CM,
Munhoz Piotto GH, Siqueira S, Galapo Kann A and Dzik C: Renal
medullary carcinoma response to chemotherapy: a referral center
experience in Brazil. Rare Tumors. 5:e442013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stahlschmidt J, Cullinane C, Roberts P and
Picton SV: Renal medullary carcinoma: Prolonged remission with
chemotherapy, immunohistochemical characterisation and evidence of
bcr/abl rearrangement. Med Pediatr Oncol. 33:551–557. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walsh AM, Fiveash JB, Reddy AT and
Friedman GK: Response to radiation in renal medullary carcinoma.
Rare Tumors. 3:e322011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amin MB, Smith SC, Agaimy A, Argani P,
Compérat EM, Delahunt B, Epstein JI, Eble JN, Grignon DJ, Hartmann
A, et al: Collecting duct carcinoma versus renal medullary
carcinoma: An appeal for nosologic and biological clarity. Am J
Surg Pathol. 38:871–874. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Q, Galli S, Srinivasan R, Linehan WM,
Tsokos M and Merino MJ: Renal medullary carcinoma: Molecular,
immunohistochemistry, and morphologic correlation. Am J Surg
Pathol. 37:368–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prasad SR, Humphrey PA, Catena JR, Narra
VR, Srigley JR, Cortez AD, Dalrymple NC and Chintapalli KN: Common
and uncommon histologic subtypes of renal cell carcinoma: Imaging
spectrum with pathologic correlation. Radiographics. 26:1795–1810.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blitman NM, Berkenblit RG, Rozenblit AM
and Levin TL: Renal medullary carcinoma: CT and MRI features. AJR
Am J Roentgenol. 185:268–272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshimitsu K, Irie H, Tajima T, Nishie A,
Asayama Y, Hirakawa M, Nakayama T, Kakihara D and Honda H: MR
imaging of renal cell carcinoma: Its role in determining cell type.
Radiat Med. 22:371–376. 2004.PubMed/NCBI
|
|
28
|
Zhu Q, Zhu W, Wang Z and Wu J: Clinical
and CT imaging features of mucinous tubular and spindle cell
carcinoma. Chin Med J (Engl). 127:1278–1283. 2014.PubMed/NCBI
|
|
29
|
Strobel SL, Jasper WS, Gogate SA and
Sharma HM: Primary carcinoma of the renal pelvis and ureter.
Evaluation of clinical and pathologic features. Arch Pathol Lab
Med. 108:697–700. 1984.PubMed/NCBI
|
|
30
|
Farmakis SG and Siegel MJ: Rhabdoid tumor:
An aggressive renal medullary tumor of childhood. J Comput Assist
Tomogr. 39:44–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Z, Chen J, Zhou J, Liu Y, Feng Z,
Tang L and Jin Y: Clinicopathological study and diagnosis of
rhabdoid tumor of kidney combined with metanephric adenoma. Chin
Med J (Engl). 127:4290–4291. 2014.PubMed/NCBI
|
|
32
|
Ganeshan D, Iyer R, Devine C, Bhosale P
and Paulson E: Imaging of primary and secondary renal lymphoma. AJR
Am J Roentgenol. 201:712–719. 2013. View Article : Google Scholar
|
|
33
|
Navas Martínez MC, Molina Escudero R, Soto
Delgado M, Jiménez Romero ME and Jiménez Jiménez J: Bilateral
primary renal lymphoma: Case report and bibliographic. Arch Esp
Urol. 64:904–907. 2011.PubMed/NCBI
|
|
34
|
Swartz MA, Karth J, Schneider DT,
Rodriguez R, Beckwith JB and Perlman EJ: Renal medullary carcinoma:
clinical, pathologic, immunohistochemical, and genetic analysis
with pathogenetic implications. Urol. 60:1083–1089. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hindman N, Ngo L, Genega EM, Melamed J,
Wei J, Braza JM, Rofsky NM and Pedrosa I: Angiomyolipoma with
minimal fat: Can it be differentiated from clear cell renal cell
carcinoma by using standard MR techniques? Radiology. 265:468–477.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soto Delgdo M, Pedrero Márquez G, Arroyo
Maestre JM and Beardo Villar P: Collecting duct carcinoma of the
kidney. A contribution of 4 new cases. Arch Esp Urol. 67:714–717.
2014.PubMed/NCBI
|
|
37
|
Kwon KA, Oh SY, Kim HY, Kim HS, Lee HY,
Kim TM, Lim HY, Lee NR, Lee HJ, Hong SH and Rha SY: Clinical
features and treatment of collecting duct carcinoma of the kidney
from the Korean cancer study group genitourinary and gynecology
cancer committee. Cancer Res Treat. 46:141–147. 2014. View Article : Google Scholar : PubMed/NCBI
|