Introduction
The formation of cavities in tumors
characteristically occurs following anti-angiogenic therapy for
malignant lung lesions; however, cavitation is rarely observed in
colorectal cancer. Angiogenesis has been a therapeutic target
according to its importance in cancer development, and various
anti-angiogenic agents are in use in current cancer treatment. Thus
far, several studies have reported a frequency of ~20% cavity
formation following anti-angiogenic therapy (1–3).
Regorafenib is an oral multikinase inhibitor that
targets a broad range of angiogenic, stromal and oncogenic kinases,
and is approved for the treatment of colorectal cancer (4). In standard colorectal cancer therapy,
regorafenib is used as salvage therapy and, according to the
CORRECT trial, median overall survival is 6.4 months (4). Side effects observed in clinical trials
of regorafenib were manageable, and the major toxicities were
hand-foot skin reactions, diarrhea, hypertension and fatigue
(4). Cavity formation following
reforafenib treatment was reported for the first time as a
correspondence following a phase III trial (5), but the typical manifestation was not
demonstrated. The generally accepted mechanism for the development
of cavitation is rapid tumor growth, which outstrips the tumor
blood supply (1,2). At present, the clinical benefit of
cavitation remains unclear (2,3). Here, we
report the case of a 57-year-old male who was treated with
regorafenib as a single agent for pulmonary metastases from
colorectal cancer and developed extensive characteristic cavitation
followed by filling-in. Written informed consent was obtained from
the patient.
Case report
A 57-year-old male was diagnosed with sigmoid
colorectal cancer [stage IIA (T3N0M0), KRAS mutation-positive,
G12D] and underwent anterior resection. The patient's medical
history included subarachnoid hemorrhage at the age of 39, which
was treated by surgery, and hypertension at the same age. His
medication was Candesartan 8 mg/day.
One year after surgery, multiple lung metastases
were diagnosed and the patient commenced treatment with S-1,
oxaliplatin and bevacizumab. He received this regimen for 21 cycles
and was then switched to capecitabine, irinotecan and bevacizumab
due to progressive disease (PD). After receiving 14 cycles of this
combination, the patient was referred to the Keio Cancer Center,
Tokyo, Japan, for salvage line treatment. A computed tomography
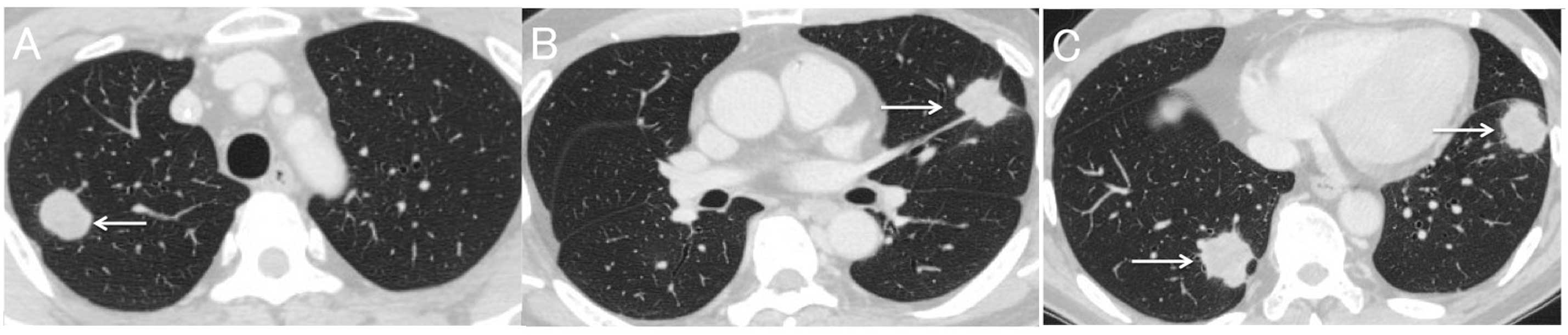
(CT) scan of the chest revealed several large masses in the lungs
(longest diameter, 36 mm; Fig. 1A–C)
and the patient commenced regorafenib 160 mg/day.
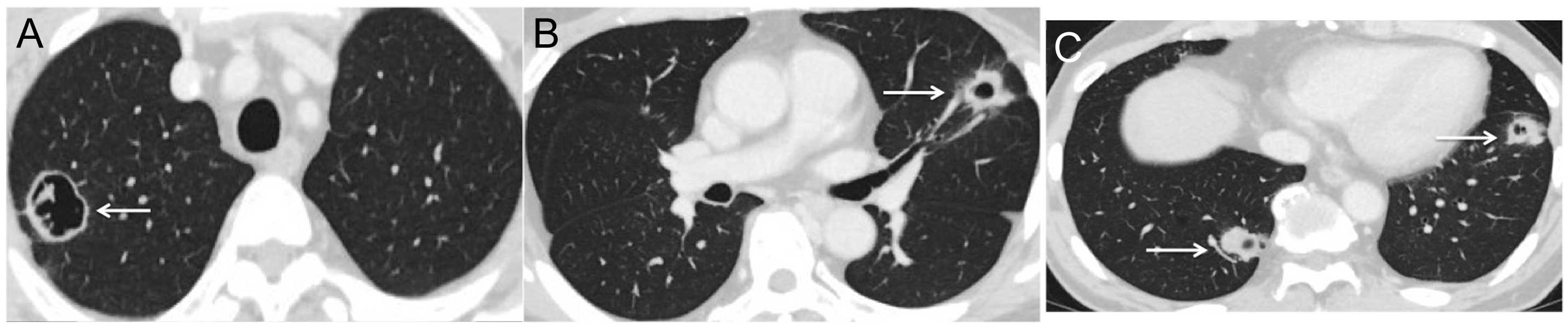
Following the first two cycles, a notable response
was demonstrated on CT, with shrinkage and characteristic
cavitation being visible in all metastatic deposits (Fig. 2A–C). The decrease in the size of
tumors was calculated as 38%, and there were associated decreases
in the serum concentrations of the tumor markers carcinoembryonic
antigen and CA19-9. The patient was evaluated as having a partial
response. The toxicities experienced were hand-foot syndrome and
hypertension, which were tolerated, and chest pain and hemoptysis
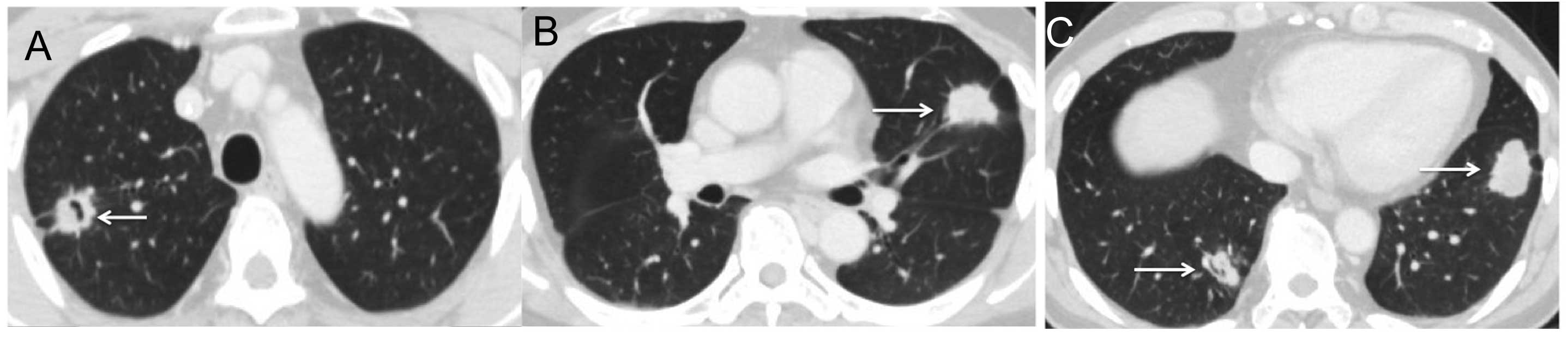
were not observed. After the patient had received eight cycles, the
metastatic lesions had enlarged by 27% and the cavities had
disappeared through filling-in, resulting in an evaluation of PD
(Fig. 3A–C).
Discussion
In the present study, we report the case of a
patient with a characteristic manifestation of multiple pulmonary
metastases of colorectal cancer that responded to regorafenib with
cavity formation.
The generally accepted mechanism for the development
of cavitation is a tumor growth so rapid that it outstrips the
tumor blood supply, forming central necrosis and inhibiting
tumor-associated vasculature (1,2). The
cavity formation caused by regorafenib may be attributable to the
same mechanisms. With regard to the correlation between clinical
benefit and cavitation, there are no significant differences in
progression-free or overall survival between subjects with and
without cavitation reported in lung cancer (2,3).
This unique morphological response not only occurs
in lung cancer but also in liver metastasis in colorectal cancer.
However, liver metastasis is replaced by fibrosis and necrosis, and
is observed in <5% of all tumors (6). Significantly, morphological changes have
been reported in association with pathological response and overall
survival in liver metastasis of colorectal cancer (6). However, the association between these
morphological changes and clinical benefit remains
controversial.
The well-known consequences of cavitation include
pulmonary hemorrhage and pneumothorax treated with anti-angiogenic
therapy (7,8); however, the association between
hemoptysis and cavitation is controversial (2,3,6). Given that regorafenib caused cavitation
in our case, bleeding events and pneumothorax could have
occurred.
In conclusion, early tumor cavitation in lung
metastasis demonstrates the predictive potential of regorafenib in
colorectal cancer. Attention should also be paid to the possibility
of hemoptysis, pneumothorax and chest pain occurring with
cavitation, which are associated with the use of other
anti-angiogenic inhibitors (7,8). Research
into the correlation between cavitation and efficacy is ongoing as
a subgroup analysis in the CORRECT trial (4). Further investigation of the toxicity
profile is required in the post-marketing setting.
References
|
1
|
Crabb SJ, Patsios D, Sauerbrei E, Ellis
PM, Arnold A, Goss G, Leighl NB, Shepherd FA, Powers J, Seymour L
and Laurie SA: Tumor cavitation: impact on objective response
evaluation in trials of angiogenesis inhibitors in non-small-cell
lung cancer. J Clin Oncol. 27:404–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nishino M, Cryer SK, Okajima Y, Sholl LM,
Hatabu H, Rabin MS, Jackman DM and Johnson BE: Tumoral cavitation
in patients with non-small-cell lung cancer treated with
antiangiogenic therapy using bevacizumab. Cancer Imaging.
12:225–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marom EM, Martinez CH, Truong MT, Lei X,
Sabloff BS, Munden RF, Gladish GW, Herbst RS, Morice RC, Stewart
DJ, et al: Tumor cavitation during therapy with antiangiogenesis
agents in patients with lung cancer. J Thorac Oncol. 3:351–357.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: CORRECT Study Group: Regorafenib monotherapy for previously
treated metastatic colorectal cancer (CORRECT): An international,
multicentre, randomised, placebo-controlled, phase 3 trial. Lancet.
381:303–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ricotta R, Sartore-Bianchi A, Verrioli A,
Vanzulli A and Siena S: Regorafenib for metastatic colorectal
cancer. Lancet. 381:15372013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chun YS, Vauthery JN, Boonsirikamchai P,
Maru DM, Kopetz S, Palavecino M, Curley SA, Abdalla EK, Kaur H,
Charnsangavej C and Loyer EM: Association of computed tomography
morphologic criteria with pathologic response and survival in
patients treated with bevacizumab for colorectal liver metastases.
JAMA. 302:2338–2344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho YJ, Murgu SD and Colt HG: Bronchoscopy
for bevacizumab-related hemoptysis. Lung Cancer. 56:465–468. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verschoor AJ and Gelderblom H:
Pneumothorax as adverse event in patients with lung metastases of
soft tissue sarcoma treated with pazopanib: a single reference
centre case series. Clin Sarcoma Res. 4:142014. View Article : Google Scholar : PubMed/NCBI
|