Introduction
Malignant neoplasms in the small intestine account
for 1–2% of malignant neoplasms of the digestive organs (1). With regard to small intestinal
neoplasms, carcinoid tumors are most common, accounting for 31% of
all small intestinal neoplasms, followed by adenocarcinoma (30.1%),
lymphoma (16.3%) and gastrointestinal stromal tumors (7.1%)
(2). Duodenal adenosquamous
carcinomas (DASC) are extremely rare, and to date, only a small
number of cases have been published in the literature (3). Localized duodenal adenocarcinomas are
treated with surgery, including pancreatoduodenectomy and regional
lymph node dissection (4), and
adjuvant chemotherapies are often administered according to the
therapeutic strategy for colorectal cancer (5). In the case of DASCs, similar therapeutic
strategies are employed.
Intraluminal metastasis of malignant tumors to the
large vessels, such as the superior vena cava (SVC), is rarely
observed (6). To the best of our
knowledge, no cases of intraluminal metastasis to the SVC from
DASCs have been reported. Risk factors for venous thrombosis
include hypercoagulability due to underlying cancer, as well as the
presence of a central venous catheter (7), and these clinical characteristics may
also represent risk factors for intraluminal metastases of tumor
cells. Obstruction of the SVC may result in a lethal SVC syndrome
and thus, immediate treatment, as well as appropriate
identification of the causes is required.
The current study presents an extremely rare case of
SVC syndrome induced by the intraluminal metastasis of DASC.
Case report
In January 2013, a 76-year-old male underwent
placement of a cardiac pacemaker at Fukuoka Sanno Hospital
(Fukuoka, Japan) for the management of sick sinus syndrome. In May
2013, the patient presented at Kyushu University Hospital (Fukuoka,
Japan) with epigastric discomfort. Upper gastrointestinal endoscopy
demonstrated an ulcerating protruding lesion at the descending
section of the duodenum. Histological examination of a biopsy
specimen obtained from the lesion showed clusters of polygonal
atypical cells, with enlarged and pyknotic nuclei and
keratinization. Immunohistochemically, the tumor cells were
positive for keratin 903, AE1/AE3, p63 and cytokeratin 14, and
strongly focally positive for involucrin, which indicated squamous
cell carcinoma. Computed tomography (CT) and
fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT
revealed no metastasis. Therefore, a pancreatoduodenectomy and
dissection of the second lymph node group, according to the General
Rules for the Study of Pancreatic Cancer (8), were performed in June 2013. The diameter
of the resected tumor was 5 cm, and the tumor was located
separately from the duodenal papilla. Post-operative
histopathological examination revealed adenosquamous carcinoma of
the duodenum [pT4N1M0, stage IIIA, according to the TNM
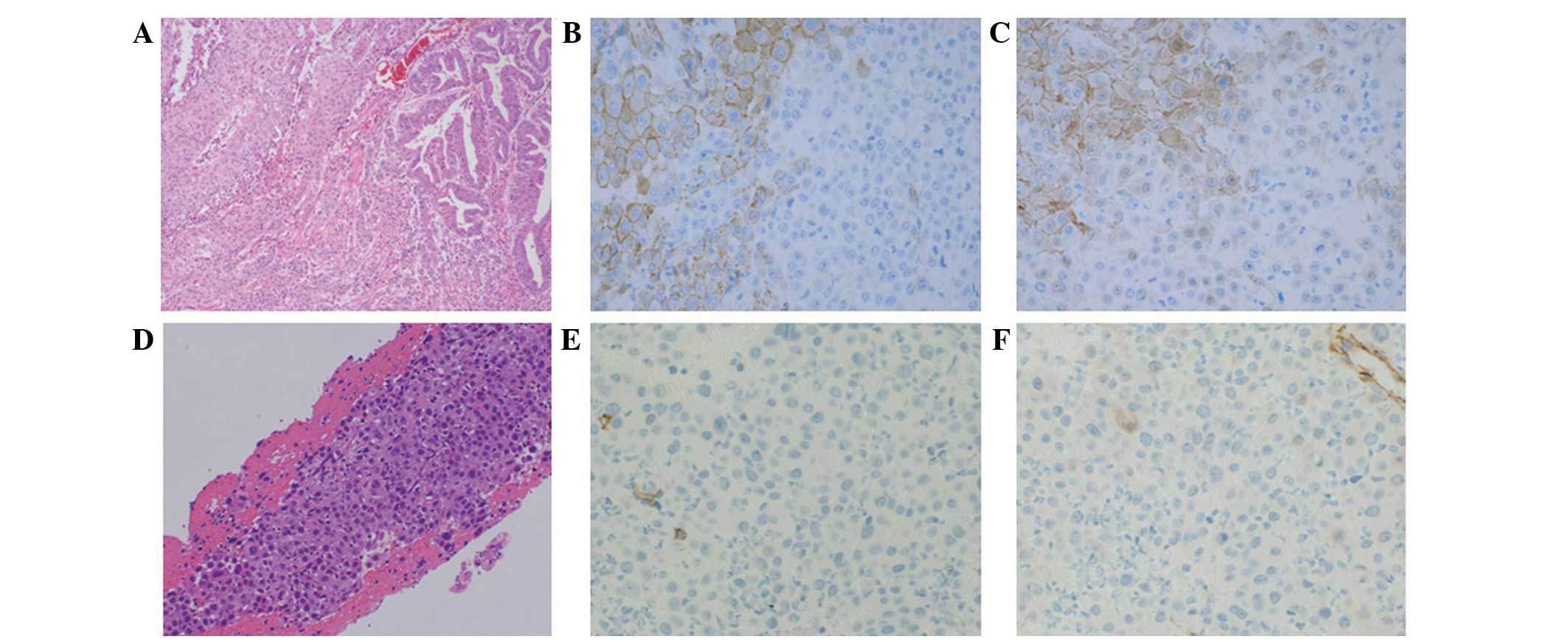
Classification of Malignant Tumours (9)] (Fig. 1A).
The tumor was mainly composed of three types of tissue: i)
Adenocarcinoma proliferating in a glandular pattern, ii) squamous
cell carcinoma with apparent squamous differentiation and iii)
poorly-differentiated carcinoma, which exhibited no features of
adenocarcinoma or squamous cell carcinoma. Immunohistochemically,
the adenocarcinoma and squamous cell carcinoma components were
positive for membranous E-cadherin and β-catenin. By contrast, the
poorly-differentiated carcinoma components were negative for
E-cadherin and β-catenin (Fig. 1B and
C). The patient underwent 12 cycles of adjuvant chemotherapy
with a modified FOLFOX6 regimen, consisting of fluorouracil (400
mg/m2 bolus, followed by 2,400 mg/m2 46 h
continuous infusion, day 1), leucovorin (200 mg, day 1) and
oxaliplatin (85 mg/m2, day 1), which was repeated every
14 days and administered via a central venous catheter. No
recurrent disease was observed for 5 months after the completion of
treatment. However, in July 2014, the patient was admitted to
Kyushu University Hospital following the sudden onset of severe
pain at the back right of the ear, edema of the right side of the
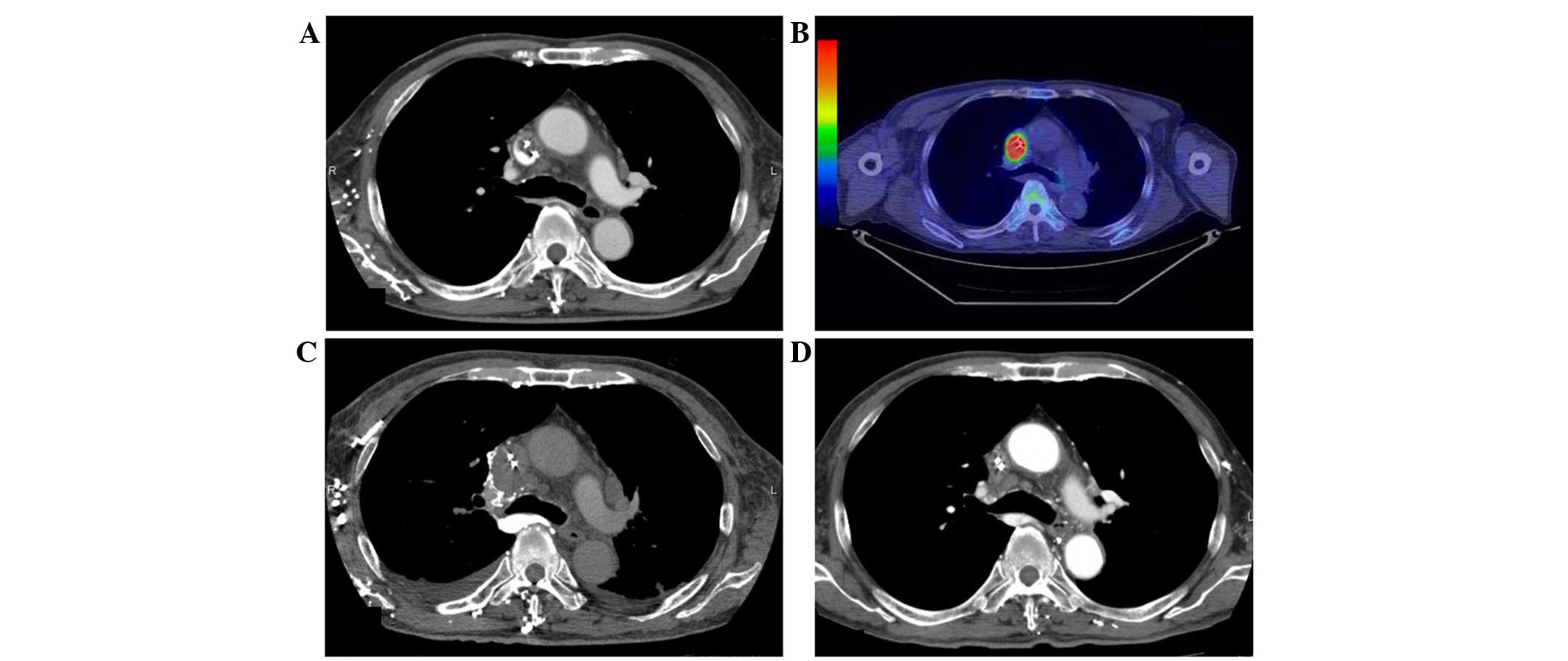
face and right jugular vein dilatation. Enhanced CT revealed
filling defects extending from the SVC to the right brachiocephalic
vein, indicating catheter-induced venous thrombosis (Fig. 2A). The central venous catheter was
removed immediately, and anticoagulant therapy with heparin (17,500
units for 10 days) followed by warfarin (2 mg/day, continuously)
was administered, however, the patient's symptoms were not
ameliorated. Percutaneous transluminal aspiration of the thrombus
and balloon dilatation of the vein using catheters were also
performed, however, the symptoms showed no improvement.
Pathological examination of the thrombus aspiration identified
metastatic carcinoma, which exhibited similar features to that
observed in the poorly-differentiated component of the primary
tumor identified in the duodenum (Fig.
1D). Immunohistochemistry revealed that the tumor cells were
negative for E-cadherin (Fig. 1E) and
β-catenin (Fig. 1F). FDG-PET/CT
revealed metabolically active nodules in the SVC at locations
identical to those of the tumors identified on CT (Fig. 2B) and in the first thoracic vertebrae.
The patient's symptoms rapidly worsened with respiratory discomfort
and hypoxemia. Repeated enhanced CT scans performed at 3-week
intervals showed rapid enlargement of the malignant thrombosis in
the SVC extending to the right atrium (Fig. 2C), however, no pulmonary emboli were
identified. Surgical resection of the tumor thrombus was attempted,
however, surgery was terminated due to the patient's general
condition and the identification of distant metastases. Immediate
palliative radiotherapy (30 Gy in 10 fractions) was administered to
the tumor thrombus, in addition to the continuation of
anticoagulant therapy. The patient's systemic condition gradually
improved following radiotherapy, with a decline in supplementary
oxygen requirements. Subsequently, 5 cycles of chemotherapy with
irinotecan (150 mg/m2, day 1, every 14 days) plus
cetuximab (400 mg/m2, day 1 of cycle 1, followed by 250
mg/m2, every 7 days), which is a standard regimen for
advanced colorectal cancer, was administered based on histological
findings revealing that the primary tumor cells highly expressed
the epidermal growth factor receptor and possessed a wild-type KRAS
phenotype. A CT scan performed after two cycles of chemotherapy
(November 2014) revealed a significant reduction of the tumor
thrombus in the SVC (Fig. 2D). During
treatment with irinotecan and cetuximab, the patient developed
grade 3 pneumonia and grade 4 sepsis [according to the Common
Terminology Criteria for Adverse Events (10)]. Thus, cetuximab single therapy was
continuously performed for 11 cycles considering these adverse
events. In March 2015, a CT scan revealed regrowth of the tumor
thrombus in the SVC and FDG-PET/CT also revealed metabolically
active nodules in the SVC. Palliative radiotherapy (40 Gy in 20
fractions) was administered to the tumor thrombus. The pacemaker
leads were then removed, due to the possibility of the leads
providing a nidus for tumor attachment and growth. Chemotherapy
with paclitaxel single therapy (80 mg/m2 paclitaxel on
days 1, 8 and 15, every 28 days) for 1 cycle. The physical
condition of the patient had deteriorated after 1 cycle, and the
patient succumbed 13 months after the diagnosis of intraluminal SVC
metastasis.
Discussion
Advanced duodenal adenocarcinomas often metastasize
to the lymph nodes, liver and lung, however, intraluminal
metastasis to the large vessels, such as the SVC, are extremely
rare (11). Furthermore, intraluminal
metastasis to the SVC from DASCs has not been reported previously.
To date, only a limited number of cases of malignant neoplasms of
the skin (12,13), prostate (14), lung (15), thyroid (16), thymus (17) and colon (18) with intraluminal metastases to the SVC
have been reported (Table I). We
hypothesize that metastases to the large vessels are extremely rare
due to the high volume and speed of blood flow, and the thick
sub-endothelial layer, which prevent invasion by tumor cells. For
the treatment of tumor thrombosis in the large vessels, various
therapeutic strategies, including irradiation of the tumor and
systemic chemotherapy, have been employed to control tumor growth.
Notably, in the present case, treatment with 30 Gy of radiotherapy
significantly improved the patient's systemic symptoms and
suppressed the otherwise aggressive tumor growth.
 | Table I.Reported cases of intraluminal
superior vena cava metastasis. |
Table I.
Reported cases of intraluminal
superior vena cava metastasis.
| First author
(ref.) | Age,
years/gender | Primary site | Histology | Advanced/relapse | Other lesions | Treatment |
|---|
| Blanco et al
(12) | 72/M | Skin | Melanoma | Relapse | Left axillary mass
Lung | CTx, RT, AC |
| Ghattas et al
(13) | 42/F | Skin | Melanoma | Relapse | None | Surgery, CTx |
| Takeda et al
(14) | 60/M | Prostate | Adenocarcinoma | Advanced | Lymph node | Endocrine therapy,
AC |
| Wang et al
(15) | 61/M | Lung | Adenocarcinoma | Advanced | Lymph node | RT, CTx |
| Murphy et al
(16) | 75/F | Thyroid | Poorly-differentiated
thyroid carcinoma | Relapse | Lung | RT |
| Matsuno et al
(17) | 65/F | Thymus | Sarcomatoid | Advanced | None | Surgery, AC,
carcinoma adjuvant CTx |
| Alzand et al
(18) | 54/F | Colon | Adenocarcinoma | Relapse | None | Surgery |
Intraluminal metastasis to the SVC in the present
case may have been present at the time of the initial diagnosis,
although it was not identified by radiological examination. Evident
metastasis in the SVC appeared surrounding a lead of the cardiac
pacemaker and surrounding the central vein catheter, suggesting
that these leads and the catheter itself may have contributed to
the onset of this extremely rare condition. Symptomatic upper
extremity and central vein thrombosis attributed to pacemaker leads
occurs in 1–3% of patients with permanent pacemakers (19). The pathogenesis of pacemaker
lead-related thrombosis may involve local inflammation induced by
foreign-body reactions (20) and
endothelial cell injury as a result of lead-activated local
hypercoagulability (19). Similarly,
the pacemaker leads and/or the central venous catheter may provide
a nidus for tumor attachment and growth.
The primary tumor in the present case was diagnosed
as an adenosquamous carcinoma, which is rarely found among
malignant tumors of the small intestine (3). It is not known whether adenosquamous
carcinoma of the small intestine has a higher metastatic potential
to distant organs than other histological types. With regard to
colorectal tumors, significant differences in metastatic potential
have been identified between adenocarcinomas and adenosquamous
carcinomas (21). Therefore, in the
present case, the histological characteristics of the tumor may
correlate with the metastatic capacity. Notably, pathohistological
examination of the SVC thrombus revealed similar features to those
of the poorly-differentiated component of the primary tumor in the
duodenum. Immunohistochemical examination of the
poorly-differentiated carcinoma components of the primary tumor in
the duodenum were negative for the expression of E-cadherin and
β-catenin, which was consistent with that observed in the SVC
thrombus. Adenocarcinomas of the small intestine, which possess
specific markers of epithelial-mesenchymal transition, such as
E-cadherin negativity, or vimentin and/or fibronectin positivity,
are significantly associated with an undifferentiated histology and
poor clinical outcomes (22). Loss of
the E-cadherin protein was identified in 41.8% of small intestinal
adenocarcinomas, and aberrant β-catenin protein expression was
found in 40.7% of small intestinal adenocarcinomas (23). Furthermore, 24% of small intestinal
adenocarcinomas exhibit the two phenotypes and were found to
closely correlate with a poorly-differentiated histology (23). The decreased expression of E-cadherin
may be associated with the loss of the intercellular junctional or
cellular polarity of cancer cells and thus, theoretically, this may
increase the metastatic potential of tumor cells (24–26).
Additionally, the activation of β-catenin in tumor cells as a
result of the loss of E-cadherin expression may induce the
expression of metastasis-related genes, including Snail and Twist
(27). The tumor phenotype (wild-type
KRAS) in the present case may have induced an increased possibility
of metastasis and also contributed to the intraluminal metastasis.
A recent study showed that the KRAS mutation is associated with
poor prognosis in colorectal cancer (28). However, whether KRAS mutation status
correlates with poor prognosis and the metastatic capacity of
duodenum carcinoma remains unclear.
Overall, aggressive intraluminal SVC metastasis as a
result of adenosquamous carcinoma of the duodenum may be associated
with the placement of artificial devices in the large vessels, as
well as the development of an aggressive metastatic phenotype in
the tumor cells. The patient in the present case exhibited
intraluminal superior vena cava metastasis from adenosquamous
carcinoma of the duodenum. Radiotherapy, chemotherapy and
anticoagulant therapy effectively suppressed tumor growth. Thus,
the present study indicates that patients with advanced cancer
harboring distant metastases to rare organ locations should undergo
multidisciplinary therapy in accordance with the pathogenesis of
the metastasis, with the type of therapy selected based on patient
factors, as well as tumor factors.
References
|
1
|
Guo X, Mao Z, Su D, Jiang Z and Bai L: The
clinical pathological features, diagnosis, treatment and prognosis
of small intestine primary malignant tumors. Med Oncol. 31:9132014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hatzaras I, Palesty JA, Abir F, et al:
Small-bowel tumors: Epidemiologic and clinical characteristics of
1260 cases from the Connecticut tumor registry. Arch Surg.
142:229–235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wada T, Mizuno K, Itoh K, et al:
Adenosquamous carcinoma of the jejunum. J Gatroenterol. 38:786–790.
2003. View Article : Google Scholar
|
|
4
|
Cecchini S, Correa-Gallego C, Desphande V,
Ligorio M, Dursun A, Wargo J, Fernàndez-del Castillo C, Warshaw AL
and Ferrone CR: Superior prognostic importance of perineural
invasion vs. lymph node involvement after curative resection of
duodenal adenocarcinoma. J Gastrointest Surg. 16:113–120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Overman MJ, Kopetz S, Lin E, Abbruzzese JL
and Wolff RA: Is there a role for adjuvant therapy in resected
adenocarcinoma of the small intestine. Acta Oncol. 49:474–479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Otten TR, Stein PD, Patel KC, Mustafa S
and Silbergleit A: Thromboembolic disease involving the superior
vena cava and branchiocephalic veins. Chest. 123:809–812. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rice TW, Rodriguez RM and Light RW: The
superior vena cava syndrome: Clinical characteristics and evolving
etiology. Medicine (Baltimore). 85:37–42. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Japan Pancreas Society: General Rules for
the Study of Pancreatic Cancer (6th). Kanehara, Tokyo: 2009.
|
|
9
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours (7th). Hoboken, NJ:
Wiley-Blackwell. 2009.
|
|
10
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE). version 4.0.
http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdfAccessed.
November 10–2015
|
|
11
|
Zaanan A, Costes L, Gauthier M, et al:
Chemotherapy of advanced small-bowel adenocarcinoma: A multicenter
AGEO study. Ann Oncol. 21:1786–1798. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blanco P, Ly S, Beylot Barry M, Laurent F,
Roques X, Doutre M and Beylot C: Surgical treatment of an
endovascular metastatic melanoma of the superior vena cava.
Dermatology. 199:156–157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghattas S, Howle J, Wang W, Kefford R and
Gruenewald S: Intravascular metastatic melanoma: A difficult
diagnosis. Australas J Dermatol. 54:141–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takeda T, Saitoh M and Takeda S: Superior
vena cava syndrome caused by an intravascular thrombosis due to
underlying prostate carcinoma. Intern Med. 47:2007–2009. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Liang J, Wang W, Ouyang H and Wang
L: Malignant thrombosis of the superior vena cava caused by
non-small-cell lung cancer treated with radiation and erlotinib: A
case with complete and prolonged response over 3 years. Onco
Targets Ther. 6:749–753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murphy C, Schwalb H, Berlangieri S and Eek
R: Intraluminal superior vena cava metastasis in a patient with
poorly differentiated thyroid carcinoma. J Clin Oncol. April
21–2014.(Epub ahead of print).
|
|
17
|
Matsuno Y, Takama N, Yasuhara K, Koyano T,
Obayashi T, Sasaki T, Kanesawa N and Kurabayashi M: Long-survival
case of thymic carcinoma with superior vena cava tumor thrombus.
Ann Thorac Surg. 94:1729–1731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alzand BS, Geyik Z, Dannert R and Cheriex
EC: Superior vena cava syndrome as a complication of colon
carcinoma. Int J Cardiol. 132:e45–e47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spittell PC and Hayes DL: Venous
complications after insertion of a transvenous pacemaker. Mayo Clin
Proc. 67:258–265. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krug H and Zerbe F: Major venous
thrombosis. A complication of transvenous pacemaker electrodes. Br
Heart J. 44:158–161. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cagir B, Nagy MW, Topham A, Rakinic J and
Fry RD: Adenosquamous carcinoma of the colon, rectum, and anus:
Epidemiology, distribution, and survival characteristics. Dis Colon
Rectum. 42:258–263. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim A, Bae YK, Gu MJ, Kim JY, Jang KY, Bae
HI, Lee HJ and Hong SM: Epithelial-mesenchymal transition phenotype
is associated with patient survival in small intestinal
adenocarcinoma. Pathology. 45:567–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee HJ, Lee OJ, Jang KT, Bae YK, Chung JY,
Eom DW, Kim JM, Yu E and Hong SM: Combined loss of E-cadherin and
aberrant β-catenin protein expression correlates with a poor
prognosis for small intestinal adenocarcinomas. Am J Clin Pathol.
139:167–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kase S, Sugio K, Ymazaki K, Okamoto T,
Yano T and Sugimachi K: Expression of E-cadherin and beta-catenin
in human non-small cell lung cancer and the clinical significance.
Clin Cancer Res. 6:4789–4796. 2000.PubMed/NCBI
|
|
25
|
Liu S, Liao G, Ding J, Ye K, Zhang Y, Zeng
L and Chen S: Dysregulated expression of snail and E-cadherin
correlates with gastrointestinal stromal tumor metastasis. Eur J
Cancer Prev. 23:329–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fernebro E, Bendahl PO, Dictor M, Persson
A, Fernö M and Nilbert M: Immunohistochemical patterns in rectal
cancer: Application of tissue microarray with prognostic
correlations. Int J Cancer. 111:921–928. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sorbye H, Dragomir A, Sundström M,
Pfeiffer P, Thunberg U, Bergfors M, Aasebø K, Eide GE, Ponten F,
Qvortrup C and Glimelius B: High BRAF mutation frequency and marked
survival differences in subgroups according to KRAS/BRAF mutation
status and tumor tissue availability in a prospective
population-based metastatic colorectal cancer cohort. PLoS One.
29:e01310462015. View Article : Google Scholar
|