Introduction
Colorectal cancer occurs in the colorectal mucosa
and colonic glands and is one of the most common types of malignant
tumor of the digestive system (1,2). The
worldwide incidence and mortality rates of colorectal cancer
increased significantly between 2009 and 2013; in 2013, a total of
142,820 new cases were diagnosed and 50,830 mortalities occurred as
a result of colorectal cancer (3).
Thus, this malignancy presents a serious threat to human health
(1,2).
Clinical and pathological data of patients with colorectal cancer
in China have demonstrated the following pathogenic
characteristics: The number of patients in cities is higher than
that in the countryside, indicating a clear urbanization trend; and
patients <30 years of age account for >10% of the total
number of patients, demonstrating a clear trend towards older age
(4). Surgical resection is currently
the main method used for the treatment of colorectal cancer.
However, as significant symptoms are often not present in the early
stages of the disease, patients are frequently diagnosed in the
later stages; by this time, the optimal period for surgery has
passed. Furthermore, metastasis or recurrence occurs in a large
number of patients following surgical resection, affecting the
prognosis of the patients. Therefore, the five-year survival rate
is low (64%), posing a serious threat to patient health (5,6).
The occurrence and pathogenesis of colorectal cancer
is a complex, multistep process, regulated by a number of different
genes (1). A total of 25% of patients
with colorectal cancer have a genetic history, which is associated
with familial adenomatous polyposis and hereditary non-polyposis
colorectal cancer (7). Results of
molecular pathology and colorectal cancer expression profile chip
screening have demonstrated that multiple genes serve regulatory
roles in the processes of colorectal cancer development (8). In order to identify possible gene target
therapies and individualized treatments, various studies have
focused on genes associated with the pathogenesis of colorectal
cancer and their mechanisms. Previous studies have employed various
methods to explore potential markers for the early diagnosis and
prognosis of colorectal cancer; however, at present, its primary
cause is unknown, and therefore further research is required
(9).
Recent studies have demonstrated that Silent
information regulator 1 (Sirt1) can regulate the deacetylation of
lysine residues of multiple proteins, which is dependent upon
nicotinamide adenine dinucleotide (NAD+). Sirt1, a
member of the Sirtuin family, is a type of class III histone
deacetylase (10). The Sirtuin family
regulates gene expression and is involved in the regulation of
various biological events in cells. In particular, Sirt1 is
important in cell survival, senescence, apoptosis, differentiation
and other metabolic processes (11).
Studies have demonstrated that the expression of Sirt1 is increased
in prostate cancer (12) and acute
myelocytic leukemia (13). Hida et
al (14) observed that the
expression of Sirt1 was also significantly enhanced in a variety of
types of non-melanoma skin cancer, including squamous cell
carcinoma, basal cell carcinoma, Bowen's disease and actinic
keratosis. The NAD+-dependent deacetylase, Sirt1, is
involved in cellular survival pathways, which ensure that the tumor
suppressor gene, p53, and members of the forkhead transcription
factor family remain deacetylated (15). Therefore, Sirt1 is considered to
promote cancer genes and it may be involved in the regulation of
tumor formation and initiation and developmental processes
(15).
In order to further explore the significance and
mechanism of Sirt1 in the pathogenesis of colorectal carcinoma, the
present study investigated the expression of Sirt1 in colorectal
carcinoma tissues and normal colorectal mucosa by means of the
immunohistochemistry streptavidin peroxidase (SP) method and
western blot analysis, and analyzed the associations between the
expression levels of Sirt1 and clinicopathological factors. The
results may provide novel ideas and inspiration for the research of
colorectal cancer etiology.
Materials and methods
Research subjects
The present study was approved by the Affiliated
Xinhua Hospital of Dalian University (Dalian, China) ethics
committee. Specimens were collected with informed consent, and the
investigation did not affect the disease diagnosis or follow-up
treatment of the patients. Specimens from 40 patients undergoing
surgical resection for the treatment of colorectal cancer were
collected between March 2010 and October 2012 at the Affiliated
Xinhua Hospital of Dalian University. The colorectal cancer tissue
specimens represented the experimental group, and the adjacent
normal mucosa tissues (>5 cm from tumor lesions) represented the
control group. The patients included in the study had not
previously received surgery for colorectal cancer, and did not
exhibit endocrine or immune system disease. Patients who had
received chemotherapy were excluded from the study. Hormonal
treatment was also not conducted within three months prior to the
surgery. Samples were acquired from the specimens within 30 min of
surgical removal, and each sample was divided into two. One part of
the sample was fixed in 10% formalin for the preparation of the
paraffin specimens, and the expression of Sirt1 was examined in
these samples by the immunohistochemical SP method. The second part
of the sample was stored in liquid nitrogen, and the expression of
Sirt1 protein in the fresh tissues was detected by western blot
analysis. The clinical and pathological data characteristics of the
patients were assessed, and the association between the expression
of Sirt1 and the clinical pathological data was analyzed. The
experimental group and the control group included a total of 80
samples from 40 patients, comprising 27 males and 13 females with a
mean age of 59.37±10.05 years. Hematoxylin and eosin staining
identified 12 cases of colon cancer and 28 cases of rectal cancer
in the experimental group, whilst the control group samples were
composed of normal mucosa. The 40 cases of colorectal cancer
consisted of 22 cases of predominantly ulcerative type and 18 cases
of exophytic type tumors, with no cases of polypoid type tumors
(16). According to histological
grading (17), there were 15 cases of
high differentiation, 19 cases of moderate differentiation and 6
cases of low differentiation. With regard to lymph node metastasis,
there were 19 cases without lymph node metastasis and 21 cases with
lymph node metastasis. According to Duke's classification (18), there were 18 cases of stage A+B and 22
cases of stage C+D.
Main reagents
The Sirt1 rabbit anti-human polyclonal antibody
(cat. no. sc-15404) was purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). The universal immunohistochemical
Streptavidin-Peroxidase staining kit (cat. no. SP-9000),
diaminobenzidine (DAB) developing liquid and mouse anti-human GAPDH
monoclonal antibody (cat. no. TA-08) were purchased from Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing, China). RIPA
strong lysis buffer, the protease inhibitor phenylmethanesulfonyl
fluoride (PMSF), a BCA Protein Assay kit and a Beyo ECL Plus kit
were purchased from Beyotime Institute of Biotechnology (Nanjing,
China).
Immunohistochemical staining of
paraffin sections using the SP method
Paraffin embedding and sectioning of colorectal
cancer and normal mucosa were conducted. Goat serum blocking
solution (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) was
applied dropwise at room temperature for 15 min. The Sirt1 primary
antibody (1:100) was added and incubated with samples at 4°C
overnight. A horseradish peroxidase-conjugated goat anti-mouse IgG
secondary antibody (cat. no. ZDR-5307; 1:300; Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd.) was added to the samples and
incubated at room temperature for 30 min. Each 4-µm section was
treated with 50 µl horseradish peroxidase-labeled streptavidin
working fluid (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.).
After the DAB liquid had developed for 3 min, the nuclei were
counterstained with hematoxylin (Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) for 40 sec, followed by dehydration. The
dehydrated sections were cover-slipped with neutral gum for
microscopic examination (Axiolab; Carl Zeiss AG, Oberkochen,
Germany). The positive staining graph included with the kit served
as the positive control, and phosphate-buffered saline replaced
Sirt1 and served as the negative control.
Photographs were captured from 5 arbitrary fields in
each section using a digital camera (Nikon COOLPIX S9500; Nikon
Corporation, Tokyo, Japan), and the double-blind method was used
for the data statistics. The percentage of positively stained cells
from the total cells in each field was scored as follows: <1%, 0
points; 1–20%, 1 point; 21–50%, 2 points; and >50%, 3 points.
The positive staining intensity was then scored as follows: No
coloring, 0 points; pale yellow, 1 point; brown-yellow, 2 points;
and sepia, 3 points. The product of these two scores served as the
overall section staining score.
Western blot analysis
Tissue proteins were extracted using the RIPA strong
lysis buffer and PMSF. The concentration of protein was measured
according to the instructions of the BCA kit. The protein samples
were analyzed by 10% SDS-PAGE. After transferring the samples to a
polyvinylidene fluoride membrane (Beyotime Institute of
Biotechnology), 5% skim milk powder was applied for blocking and
samples were incubated at 4°C overnight. After washing the membrane
with Tris-Buffered saline with Tween 20, the protein samples were
incubated with the polyclonal rabbit anti-human polyclonal Sirt1
(cat. no. sc-15404; Santa Cruz Biotechnology, Inc.) and monoclonal
mouse anti-human GAPDH antibodies (cat. no. TA-08; Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd.) at 4°C overnight,
followed by the horseradish peroxidase-conjugated goat anti-mouse
IgG secondary antibody (cat. no. ZDR-5307; Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd.) at 4°C overnight. Subsequently,
the membranes were placed in a chemiluminescence imaging instrument
(ImageQuant LAS 4000 Mini; GE Healthcare Bio-Sciences, Pittsburgh,
PA, USA), then exposure and image capture and analyses were
conducted (ImageQuant TL 1.0 software; GE Healthcare
Bio-Sciences).
Statistical analysis
SPSS software version 22.0 (IBM SPSS, Armonk, NY,
USA) was used for statistical analysis. The data are expressed as
the mean ± standard deviation. A paired-samples t-test was employed
to analyze the expression of Sirt1 in the colorectal cancer tissues
and the normal mucosa tissues. One-way analysis of variance was
performed to analyze the differences in Sirt1 expression in the
tissues associated with the various clinical and pathological
variables. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of Sirt1 in
paraffin-embedded colorectal cancer and normal mucosa samples
detected by immunohistochemistry
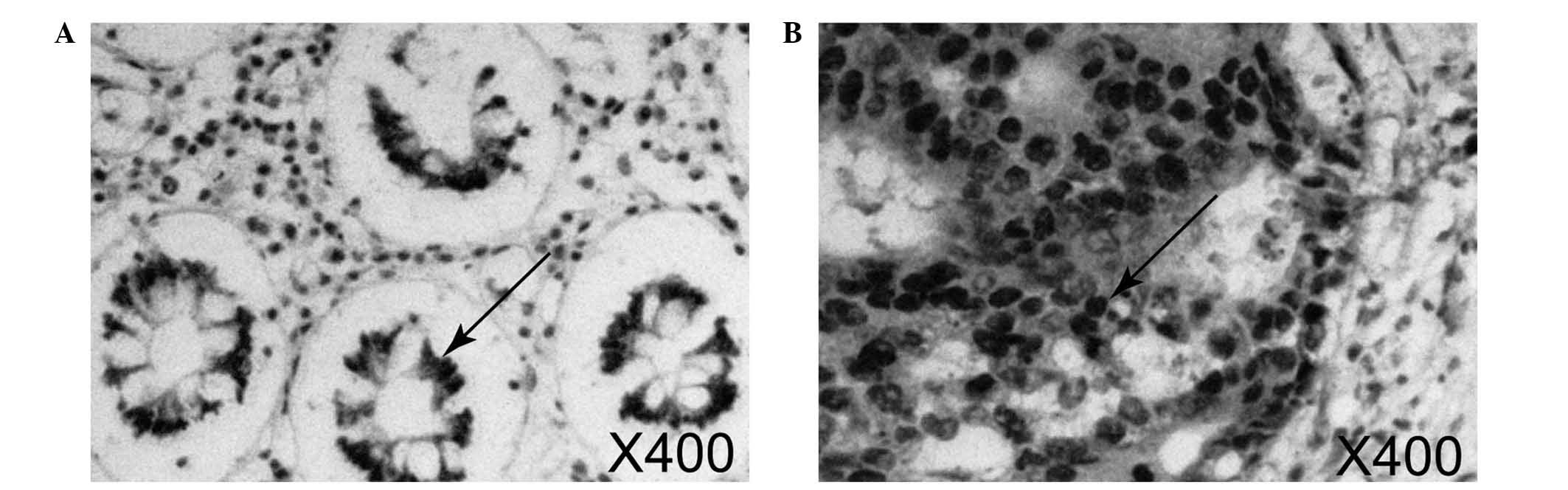
As indicated by the arrows in Fig. 1, Sirt1 was primarily expressed in the
nucleus, with stained nuclei indicating positive staining. As
illustrated in Fig. 1B, Sirt1 was
highly expressed in the colorectal cancer samples; the area of
positive staining was large. By contrast, the expression of Sirt1
was weaker and the area of positive staining was more limited in
normal mucosa samples (Fig. 1A).
The expression levels of Sirt1 in cancer tissues and
normal mucosa were analyzed according to the evaluation standards.
Multiplication of the staining percentage and staining intensity
scores were conducted to calculate the mean overall section
staining values of the samples in the experimental and control
groups. A significant difference was identified between the two
groups (P<0.05; Table I).
 | Table I.Sirt1 staining score as detected by
the immunohistochemical streptavidin peroxidase method. |
Table I.
Sirt1 staining score as detected by
the immunohistochemical streptavidin peroxidase method.
| Group | Cases | Sirt1 staining
score |
|---|
| Normal tissues | 40 | 1.15±0.419 |
| Cancer tissues | 40 |
2.62±0.537a |
The associations between Sirt1 expression and
clinicopathological variables are presented in Table II. The mean staining score for Sirt1
was 2.35±0.347 (±standard deviation) and 2.89±0.561 in male and
female patients, respectively. However, no significant differences
in Sirt1 expression were identified between males and females
(P=0.084). The mean staining score for Sirt1 was 1.64±0.401 and
2.91±0.514 in patients aged <50 and ≥50 years, respectively, and
this difference was determined to be statistically significant
(P<0.0001). The mean staining score for Sirt1 in 22 patients
with ulcerative type colorectal cancer was 1.58±0.462, while in 18
patients with exophytic type the mean staining score for Sirt1 was
2.93±0.618. This difference was statistically significant
(P<0.0001). The mean staining score for Sirt1 in 15 patients
with highly differentiated cancer was 1.46±0.471, whereas in the 25
patients exhibiting medium and low differentiation, the mean
staining score was 2.79±0.630, and this difference was found to be
statistically significant (P<0.0001). Regarding tissue
differentiation, the 19 patients with superficial muscular layer
differentiation exhibited a mean Sirt1 staining score of
1.60±0.513, whereas the 21 patients exhibiting whole layer
differentiation exhibited a mean score of 2.97±0.437, and this
difference was determined to be statistically significant
(P<0.0001). The mean staining score for Sirt1 in 21 patients
with lymph node metastasis was 1.53±0.428, and in 19 patients with
lymph node metastasis the score was 2.95±0.643. This difference was
also determined to be statistically significant (P<0.0001). The
mean Sirt1 staining score for the 19 patients with A+B Duke's stage
cancer was 1.49±0.501, whereas the mean Sirt1 staining score of the
21 patients with C+D Duke stage cancer was 2.92±0.713, and this
difference was statistically significant (P<0.0001). Therefore,
the present results revealed that older age, lower tissue
differentiation, deeper depth of invasion, lymph node metastasis
and higher Duke's stage are associated with higher Sirt1 expression
(P<0.0001). Furthermore, in terms of morphological type, Sirt1
expression is higher in exophytic compared with ulcerating tumors
(P<0.0001); however, Sirt1 expression is not significantly
associated with patient gender (P=0.084).
 | Table II.Association between Sirt1 expression
and clinicopathological data of patients with colorectal
cancer. |
Table II.
Association between Sirt1 expression
and clinicopathological data of patients with colorectal
cancer.
| Variable | Cases | Sirt1 staining
score | P-value |
|---|
| Gender |
|
| 0.084 |
| Male | 27 | 2.35±0.347 |
|
|
Female | 13 | 2.89±0.561 |
|
| Age, years |
|
| <0.0001 |
|
<50 | 16 | 1.64±0.401 |
|
|
≥50 | 24 | 2.91±0.514 |
|
| Morphological
type |
|
| <0.0001 |
|
Ulcerative | 22 | 1.58±0.462 |
|
|
Exophytic | 18 | 2.93±0.618 |
|
| Tissue
differentiation |
|
| <0.0001 |
|
High | 15 | 1.46±0.471 |
|
| Medium
or low | 25 | 2.79±0.630 |
|
| Depth of
invasion |
|
| <0.0001 |
| Shallow
muscle layer | 19 | 1.60±0.513 |
|
| Whole
layer | 21 | 2.97±0.437 |
|
| Lymph node
metastasis |
|
| <0.0001 |
|
Yes | 21 | 1.53±0.428 |
|
| No | 19 | 2.95±0.643 |
|
| Duke's stage |
|
| <0.0001 |
|
A+B | 19 | 1.49±0.501 |
|
|
C+D | 21 | 2.92±0.713 |
|
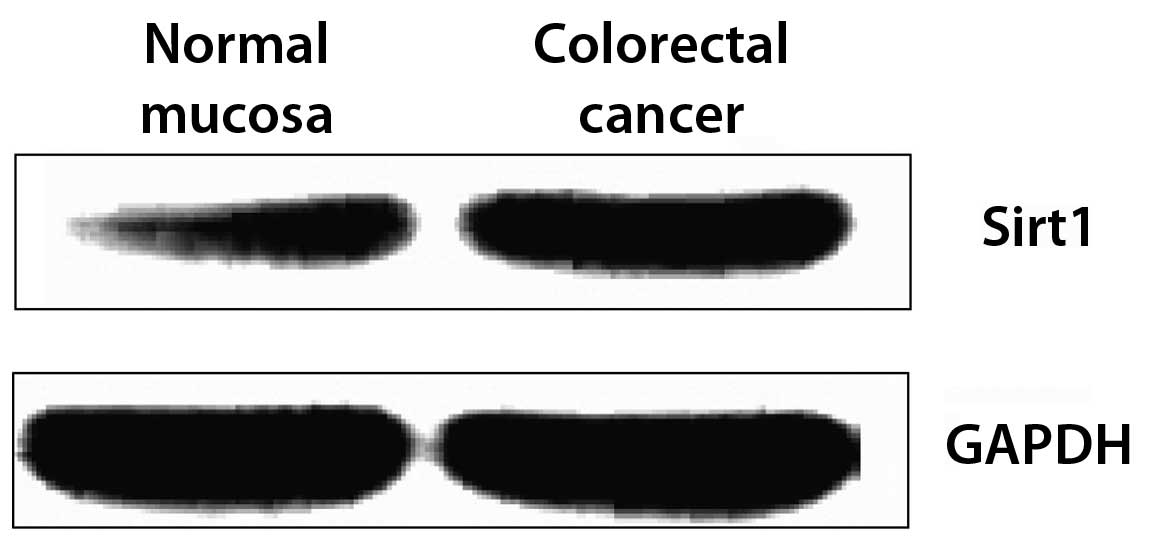
Expression of Sirt1 protein in fresh
colorectal cancer and normal mucosa tissue samples detected by
western blotting
According to the detection results acquired by the
SP immunohistochemistry method, Sirt1 may be an important factor in
colorectal cancer, as indicated by the significantly increased
expression of Sirt1 in the paraffin-embedded carcinoma specimens.
To verify this observation, the expression of Sirt1 protein in
fresh tumor tissues and normal tissues was analyzed simultaneously
by western blot analysis. Expression was analyzed in 40 tissue
specimens in each group, and the expression levels of Sirt1 were
presented as the gray value of Sirt1/GAPDH. The results
demonstrated that Sirt1 expression in colorectal cancer tissue was
elevated compared with that of normal mucosa (Fig. 2; Table
III). This difference was significant (P<0.05).
 | Table III.Gray value of the Sirt1 protein as
detected by western blot analysis. |
Table III.
Gray value of the Sirt1 protein as
detected by western blot analysis.
| Group | Cases | Sirt1/GAPDH gray
value |
|---|
| Normal tissues | 40 | 0.72±0.327 |
| Tumor tissues | 40 |
1.21±0.245a |
Discussion
The occurrence and pathogenesis of colorectal cancer
constitute a complex process regulated by numerous genes (1). Under the conditions of different
stimuli, abnormal transcription and translation of multiple genes
induces changes in the expression of signaling proteins.
Subsequently, signal conduction pathways become uncontrolled,
leading to an imbalance in cell growth, survival, differentiation
and proliferation (19). The protein
acetylation/deacetylation cycle is also important in the process of
gene expression and regulation (20).
Protein acetylation and deacetylation are catalyzed by histone
acetyltransferases and histone deacetylases, and Sirt1 belongs to
the class III histone deacetylases and is a member of the Sirtuin
family. This family catalyzes the deacetylation of lysine residues
on various proteins in a NAD+-dependent manner (10,21).
Multiple studies have demonstrated that the possible
regulatory mechanism of Sirt1 as a cancer gene is associated with
tumor protein p53 (22). The p53
protein is a tumor suppressor protein that serves diverse roles in
multiple physiological processes within the body. Following a
decrease in its expression levels, or if gene mutation occurs, the
p53 protein can no longer fulfill these roles, thus increasing the
risk of cancer (23). Sirt1 may
induce p53 loss-of-function through the deacetylation of p53 at the
Lys382 residue, which is contained in the C-terminus, consequently
losing the ability to suppress tumor formation (24). Furthermore, studies have also
demonstrated that, under conditions of DNA damage and oxidative
stress, the overexpression of Sirt1 may inhibit the p53-regulated
cell cycle and lead to the arrest of cell replication and cell
apoptosis (25). In addition, the
deacetylase activity of Sirt1 was observed to decrease when Sirt1
mutants were transcribed and translated using the site-directed
mutagenesis method in a model of carcinogenesis, which increased
the sensitivity of cells to DNA damage and the oxidative stress
response (26). Further studies
demonstrated that the addition of a specific inhibitor of Sirt1, 5′
adenosine monophosphate-activated protein kinase, into human
hepatoma HepG2 and PLC/PRF/5 cells led to significant reductions in
the activity and function of Sirt1, resulting in increased
acetylation and transcriptional activity of the p53 protein
(27,28). In addition, previous studies noted
that Sirt1 expression in primary colorectal carcinoma was
significantly increased, suggesting that Sirt1 is key in the
occurrence and development of intestinal tumors (29). In the present study it was observed
that Sirt1 expression in the primary colorectal cancer tissues were
all significantly increased.
There are notable effects of living habits, diet
structure and geographical regions on the incidence of
gastrointestinal tract cancer (7,30).
Therefore, in order to minimize the effect of external factors on
the experimental results, the current study used colorectal cancer
specimens and normal mucosa samples from the same patients as the
experimental and control groups, respectively. The results
demonstrated that, compared with that of normal mucosa tissues, the
expression of Sirt1 in colorectal cancer tissues was significantly
increased. Although gender did not affect the expression of Sirt1,
significant differences in the expression of Sirt1 in colorectal
specimens were observed between patients of different age groups,
with significantly increased expression in patients aged ≥50 years.
This may be associated with the putative role of Sirt1 as the
longevity gene (31). Studies have
indicated that the specific activators of Sirt1 contribute to the
protection of cardiovascular function and prolong life (32). Therefore, the greater the age of the
patient, the higher the expression of Sirt1 may be. The increased
expression of Sirt1 was more evident in the process of cancer
cells. Due to the fact that patients with polypoid type and
adhesive type tumors were fewer in number, along with the greater
difficulty of accessing the normal mucosa in such patients, the
present research was conducted using tumors of ulcerative and
exophytic gross morphological types. In addition, as the ulcerative
type tumors with low differentiation accounted for a large
proportion of the sample, the degree of malignancy was high. By
contrast, the proportions of high and low differentiation in
patients with the exophytic type tumor were comparable, and the
degree of malignancy was low. It was observed that the expression
of Sirt1 increased significantly in the patients with the
ulcerative type. This is consistent with the observations for
degree of tissue differentiation; increased Sirt1 expression was
enhanced in the group of patients with medium and low
differentiation. The depth of invasion, lymph node metastasis and
Duke's stage are also indices reflecting the degree of cancer
progression and malignancy (33).
According to these three indices, it was observed that the
expression of Sirt1 was elevated more significantly in cases with a
higher degree of malignancy and further progression of colorectal
carcinoma. Therefore, these results provide strong evidence of
Sirt1 as a cancer-associated gene in colorectal cancer, laying the
foundation for follow-up research.
However, opposing opinions have also been presented.
One study suggested that the Sirt1 protein may serve inhibitory
roles in the occurrence and development processes of colon cancer
(34). In addition, animal tumor
models have shown that Sirt1 may function as an anti-oncogene,
acting as a tumor-inhibitory factor (35). Leko et al overexpressed the
Sirt1 protein in APCMin/+ mice, and the risk of cancer
of the colon was significantly reduced (36). The mechanism underlying this may be
that the overexpressed Sirt1 acts as a deacetylase, leading to the
deacetylation of β-catenin in the cytoplasms of cells, thereby
resulting in its nuclear localization and loss of its normal
function (37). Furthermore, the
expression level of Sirt1 protein in the nucleus and its
deacetylation activity have been demonstrated to be negatively
correlated with the expression of β-catenin (38).
In conclusion, the association between Sirt1 and the
occurrence and development of cancer, and the related mechanisms,
are unclear at present. The leading theory is that the activation
of the Sirt1 protein can increase the risk of cancer, based on the
evidence that Sirt1 can deacetylate and inactivate the tumor
suppressor gene p53 (26). The
present investigation provides a novel direction for investigation
into the early diagnosis and targeted treatment of colorectal
cancer.
References
|
1
|
Azer SA: Overview of molecular pathways in
inflammatory bowel disease associated with colorectal cancer
development. Eur J Gastroenterol Hepatol. 25:271–281. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raskov H, Pommergaard HC, Burcharth J and
Rosenberg J: Colorectal carcinogenesis - update and perspectives.
World J Gastroenterol. 20:18151–18164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Newman NA, Votanopoulos KL, Stewart JH,
Shen P and Levine EA: Cytoreductive surgery and hyperthermic
intraperitoneal chemotherapy for colorectal cancer. Minerva Chir.
67:309–318. 2012.PubMed/NCBI
|
|
6
|
Hompes D, D'Hoore A, Van Cutsem E, Fieuws
S, Ceelen W, Peeters M, Van der Speeten K, Bertrand C, Legendre H
and Kerger J: The treatment of peritoneal carcinomatosis of
colorectal cancer with complete cytoreductive surgery and
hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) with
oxaliplatin: A Belgian multicentre prospective phase II clinical
study. Ann Surg Oncol. 19:2186–2194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu Z, Shrubsole MJ, Smalley WE, Wu H, Chen
Z, Shyr Y, Ness RM and Zheng W: Association of meat intake and
meat-derived mutagen exposure with the risk of colorectal polyps by
histologic type. Cancer Prev Res (Phila). 4:1686–1697. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piepoli A, Tavano F, Copetti M, Mazza T,
Palumbo O, Panza A, di Mola FF, Pazienza V, Mazzoccoli G, Biscaglia
G, et al: MiRNA expression profiles identify drivers in colorectal
and pancreatic cancers. PLoS One. 7:e336632012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Linnekamp JF, Wang X, Medema JP and
Vermeulen L: Colorectal cancer heterogeneity and targeted therapy,
A case for molecular disease subtypes. Cancer Res. 75:245–249.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blander G and Guarente L: The Sir2 family
of protein deacetylases. Annu Rev Biochem. 73:417–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Casatta N, Porro A, Orlandi I, Brambilla L
and Vai M: Lack of Sir2 increases acetate consumption and decreases
extracellular pro-aging factors. Biochim Biophys Acta.
1833:593–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huffman DM, Grizzle WE, Bamman MM, Kim JS,
Eltoum IA, Elgavish A and Nagy TR: SIRT1 is significantly elevated
in mouse and human prostate cancer. Cancer Res. 67:6612–6618. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bradbury CA, Khanim FL, Hayden R, Bunce
CM, White DA, Drayson MT, Craddock C and Turner BM: Histone
deacetylases in acute myeloid leukaemia show a distinctive pattern
of expression that changes selectively in response to deacetylase
inhibitors. Leukemia. 19:1751–1759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hida Y, Kubo Y, Murao K and Arase S:
Strong expression of a longevity-related protein, SIRT1, in Bowen's
disease. Arch Dermatol Res. 299:103–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stünkel W, Peh BK, Tan YC, Nayagam VM,
Wang X, Salto-Tellez M, Ni B, Entzeroth M and Wood J: Function of
the SIRT1 protein deacetylase in cancer. Biotechnol J. 2:1360–1368.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bass P, Carr N and Du Boulay C: Pathology:
A Core Text of Basic Pathological Processes with Self-Assessment
(2nd). Edinburgh: Churchill Livingstone. 2004.
|
|
17
|
Yang J, Guo R, Kang A, Chen X, Su B, Huang
X, Jin Y and Li Z: A novel histological typing and grading-scale
system of colorectal cancer. Nan Fang Yi Ke Da Xue Xue Bao.
34:169–173. 2014.(In Chinese). PubMed/NCBI
|
|
18
|
Dukes CE: The classification of cancer of
the rectum. J Pathol. 35:323–332. 1932. View Article : Google Scholar
|
|
19
|
Chueca E, Lanas A and Piazuelo E: Role of
gastrin-peptides in Barrett's and colorectal carcinogenesis. World
J Gastroenterol. 18:6560–6570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fadri-Moskwik M, Weiderhold KN, Deeraksa
A, Chuang C, Pan J, Lin SH and Yu-Lee LY: Aurora B is regulated by
acetylation/deacetylation during mitosis in prostate cancer cells.
FASEB J. 26:4057–4067. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim SJ, Ao Z, Warnock G and McIntosh CH:
Incretin-stimulated interaction between β-cell Kv1.5 and Kvβ2
channel proteins involves acetylation/deacetylation by CBP/SirT1.
Biochem J. 451:227–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hishida T, Nozaki Y, Nakachi Y, Mizuno Y,
Iseki H, Katano M, Kamon M, Hirasaki M, Nishimoto M, Okazaki Y and
Okuda A: Sirt1, p53, and p38(MAPK) are crucial regulators of
detrimental phenotypes of embryonic stem cells with Max expression
ablation. Stem Cells. 30:1634–1644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Castro RE, Ferreira DM, Afonso MB,
Borralho PM, Machado MV, Cortez-Pinto H and Rodrigues CM:
miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat
liver and activated by disease severity in human non-alcoholic
fatty liver disease. J Hepatol. 58:119–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zannini L, Buscemi G, Kim JE, Fontanella E
and Delia D: DBC1 phosphorylation by ATM/ATR inhibits SIRT1
deacetylase in response to DNA damage. J Mol Cell Biol. 4:294–303.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Wang P, Liu X, He D, Liang C and Yu
Y: Exogenous NAD(+) supplementation protects H9c2 cardiac myoblasts
against hypoxia/reoxygenation injury via Sirt1-p53 pathway. Fundam
Clin Pharmacol. 28:180–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marfe G, De Martino L, Tafani M,
Irno-Consalvo M, Pasolini MP, Navas L, Papparella S, Gambacurta A
and Paciello O: A multicancer-like syndrome in a dog characterized
by p53 and cell cycle-checkpoint kinase 2 (CHK2) mutations and
sirtuin gene (SIRT1) down-regulation. Res Vet Sci. 93:240–245.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee CW, Wong LL, Tse EY, Liu HF, Leong VY,
Lee JM, Hardie DG, Ng IO and Ching YP: AMPK promotes p53
acetylation via phosphorylation and inactivation of SIRT1 in liver
cancer cells. Cancer Res. 72:4394–4404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kriegl L, Vieth M, Kirchner T and Menssen
A: Up-regulation of c-MYC and SIRT1 expression correlates with
malignant transformation in the serrated route to colorectal
cancer. Oncotarget. 3:1182–1193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schweiger MR, Hussong M, Röhr C and
Lehrach H: Genomics and epigenomics of colorectal cancer. Wiley
Interdiscip Rev Syst Biol Med. 5:205–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chong ZZ, Shang YC, Wang S and Maiese K:
SIRT1: New avenues of discovery for disorders of oxidative stress.
Expert Opin Ther Targets. 16:167–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hubbard BP, Gomes AP, Dai H, Li J, Case
AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, et al:
Evidence for a common mechanism of SIRT1 regulation by allosteric
activators. Science. 339:1216–1219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yao X, Zhao G, Yang H, Hong X, Bie L and
Liu G: Overexpression of high-mobility group box 1 correlates with
tumor progression and poor prognosis in human colorectal carcinoma.
J Cancer Res Clin Oncol. 136:677–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Firestein R, Blander G, Michan S,
Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S,
de Cabo R, Fuchs C, et al: The SIRT1 deacetylase suppresses
intestinal tumorigenesis and colon cancer growth. PLoS One.
3:e20202008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yeung F, Hoberg JE, Ramsey CS, Keller MD,
Jones DR, Frye RA and Mayo MW: Modulation of NF-kappaB-dependent
transcription and cell survival by the SIRT1 deacetylase. EMBO J.
23:2369–2380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leko V, Park GJ, Lao U, Simon JA and
Bedalov A: Enterocyte-specific inactivation of SIRT1 reduces tumor
load in the APC(+/min) mouse model. PLoS One. 8:e662832013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
et al: Small molecule activators of sirtuins extend
Saccharomyces cerevisiae lifespan. Nature. 425:191–196.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Simic P, Zainabadi K, Bell E, Sykes DB,
Saez B, Lotinun S, Baron R, Scadden D, Schipani E and Guarente L:
SIRT1 regulates differentiation of mesenchymal stem cells by
deacetylating β-catenin. EMBO Mol Med. 5:430–440. 2013. View Article : Google Scholar : PubMed/NCBI
|