Introduction
Primary hepatocellular carcinoma (HCC) is one of the
most common malignant tumors that result in a high mortality rate.
Liver transplantation (LT) is the treatment of choice for a select
group of patients with early-stage unresectable HCC (1). Studies of tumoral behavior in the
setting of transplantation have also identified additional
potential predictors of outcome, including tumor differentiation,
lobar distribution, serum α-fetoprotein (AFP) level, serum alkaline
phosphatase, tumor volume, etiology of liver disease and molecular
markers, such as loss of heterozygosity and gene expression
(2,3).
However, there is a high frequency of tumor recurrence following
LT. In these patients, tumor recurrence usually occurs within a
year of LT, and the survival time is several months (median, 12.2
months) at the time of relapse (4).
Currently, several factors have been indicated to contribute to HCC
recurrence. Tumor cells are occasionally not completely removed by
total hepatectomy, so recurrence may originate from residual
tumors. In addition, tumor cells may persist in a dormant state for
a long time prior to the induction of clinical metastases,
hematogenous spread, extrahepatic metastases and other sources of
tumor recurrence, since small metastatic lesions may easily be
missed by computed tomography (CT) scans, magnetic resonance
imaging and various radiographic examinations (4,5).
Therefore, an effective anti-cancer therapy following the surgical
removal of hepatic malignancy is required.
Increasing evidence indicates that adoptive
immunotherapy may decrease the recurrence and metastasis rates of
solid malignant tumors (6–9). Autologous activated immune cells,
including cytokine-induced killer (CIK) cells, are a unique
population of cytotoxic T lymphocytes that express natural killer
and T-cell markers, and may be generated from peripheral blood
mononuclear cells treated with interferon (IFN)-γ, anti-cluster of
differentiation (CD)3 monoclonal antibody, and interleukin (IL)-2.
These CIK cells have demonstrated increased proliferative and
cytotoxic activities against autologous and allogeneic malignant
cells (10,11). Previous studies also report that
adoptive immunotherapy using autologous CIK cells may decrease
tumor recurrence and metastasis in postsurgical and advanced liver
cancer patients (12,13). Therefore, autologous CIK cells may be
an effective therapeutic method for patients that are diagnosed
with HCC subsequent to LT.
The immune system of patients with LT may develop
elaborate and effective mechanisms to combat foreign agents. These
mechanisms are involved in the rejection of transplanted organs,
which are recognized as foreign substances by the recipient's
immune system. Liver allograft rejection is mediated by a primary
response of T lymphocytes, followed by the infiltration of the
graft and mixed inflammatory reactions. The proliferation of
mononuclear leukocytes inside the allograft is a prominent feature
of acute and chronic rejection (13).
Therefore, a large number of infused activated T cells may pose a
potential risk to the allograft.
The present study reports the treatment of a patient
that was diagnosed with primary HCC following LT, using a CIK cell
therapy that was generated in the current study. To the best of our
knowledge, the present study is the first case report to
demonstrate the feasibility and relatively low toxicity of the
program. Written informed consent was obtained from the patient's
family.
Case report
A 46-year-old male patient with a history of
hepatitis B for >10 years was diagnosed with HCC based on
typical imaging findings and elevated serum AFP levels (645 µg/l;
normal range, <25 µg/l) in July 2010. A routine CT scan revealed
a 8.5×7.3 cm mass in the right lobe of the liver. The patient was
admitted to the transplant ward of the First Central Hospital
(Tianjin, China). The patient underwent LT in August 2010.
Following surgery, the patient received oral immunosuppressive and
liver protection treatment. The immunosuppressive treatment
consisted of tacrolimus (FK506; 4 mg twice a day),
methylprednisolone (15 mg daily) and mycophenolate mofetil (0.75 g
twice a day). The post-operative blood levels of FK506 ranged
between 3.0 and 10.9 ng/ml. The examinations revealed that no acute
adverse event had occurred. The liver enzymes and total bilirubin
levels of the patient did not change significantly following the
immunossuppressive treatment compared with the levels prior to
surgery, which indicates that the transplanted liver demonstrated
normal function. Furthermore, no evidence of abnormality was
indicated by the liver ultrasound examination.
One month after surgery, in September 2010, the
patient was admitted in Tianjin Cancer Hospital (Tianjin, China).
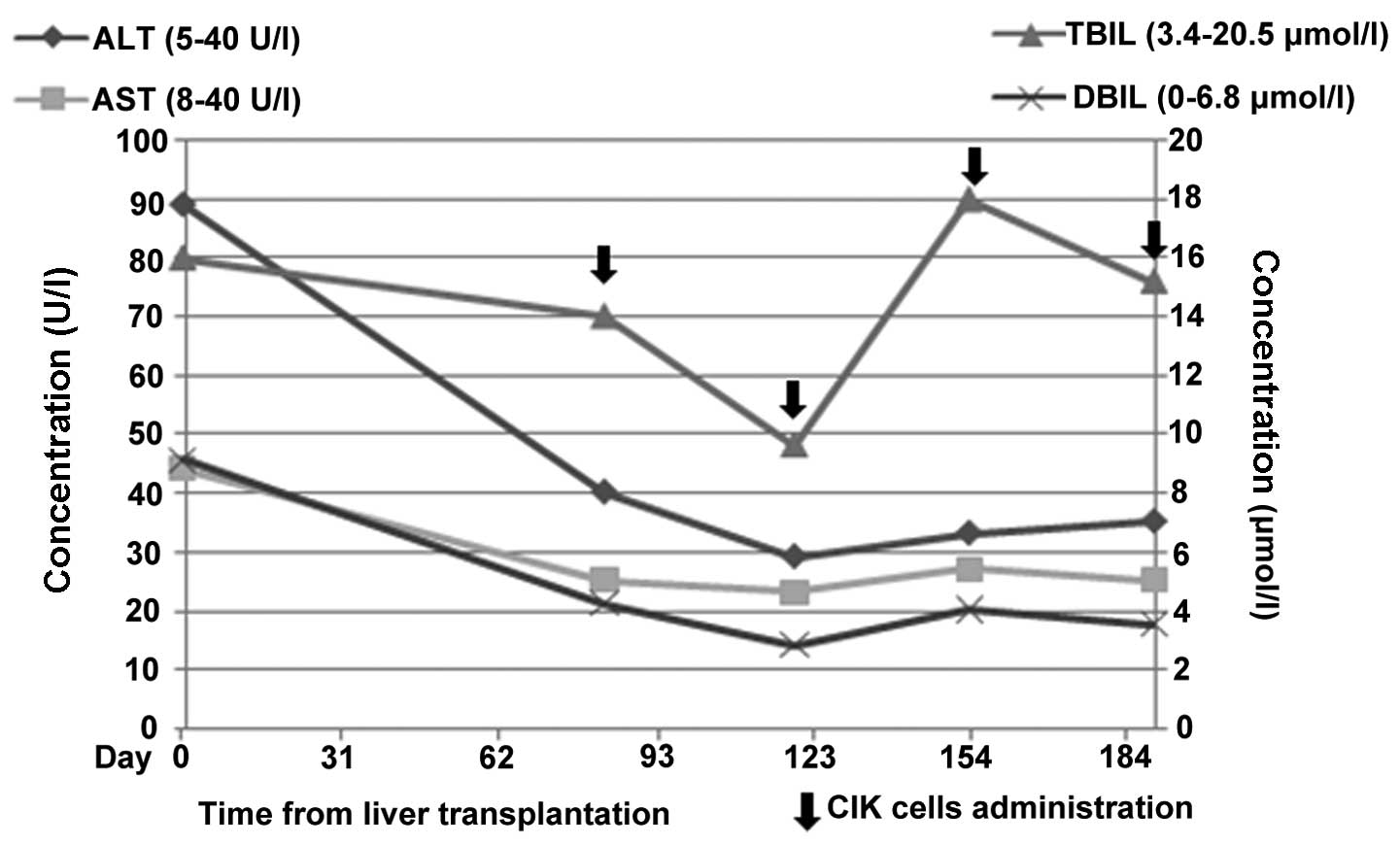
Here, the patient received 4 cycles of treatment with CIK cells at
an interval of 1 month subsequent to LT (Fig. 1). For each treatment, the patient was
treated with 2 intravenous infusions of >5×109 CIK
cells at 1-day intervals. During and subsequent to the CIK cell
transfusion, no infusion-associated toxicity or secondary acute
host-versus-graft disease (HVGD) occurred. The most common adverse
events reported following the infusions were chill, fever,
headache, nausea and vomiting. The patient experienced a slight
fever subsequent to 3 out of the 4 infusions. The fever was treated
with Brufen (5 mg/kg, every 4–6 h until the patient's temperature
returned to normal levels). No serious side effects were indicated
according to the National Cancer Institute-Common Terminology
Criteria for Adverse Events (http://evs.nci.nih.gov/ftp1/CTCAE/About.html). The
clinical examinations for liver failure, such as blood tests, liver
ultrasounds and CT examinations, were performed to examine for
HVGD. The functional indicators of the transplanted liver were the
liver volume, bile secretion, liver enzymes and total bilirubin. It
was found that the blood levels of liver enzymes, including
aspartate aminotransferase, alanine aminotransferase, γ-glutamyl
transferase, total bilirubin and direct bilirubin, did not change
significantly (Fig. 1; Table I). No increased liver volume was
indicated upon quantification with ultrasound or CT examination.
Furthermore, no clinical responses, such as tiredness, fever and
hepatic or gastrointestinal symptoms, were observed.
 | Table I.Liver function examination. |
Table I.
Liver function examination.
| Indicator (normal
range) | Before
transplant | After transplant | After CIK-1 | After CIK-2 | After CIK-3 | After CIK-4 |
|---|
| ALT, U/l (5–40) | 48 | 89 | 40 | 29 | 33 | 35 |
| AST, U/l (8–40) | 62 | 44 | 25 | 23 | 27 | 25 |
| ALP, U/l
(40–150) | 177 | 218 | 68 | 48 | 59 | 51 |
| GGT, U/l (11–50) | 232 | 312 | 73 | 56 | 63 | 59 |
| TBIL, µmol/l
(3.4–20.5) | 15.8 | 16.0 | 14.0 | 9.6 | 18.0 | 15.2 |
| DBIL, µmol/l
(0–6.8) | 6.0 | 9.1 | 4.2 | 2.8 | 4.0 | 3.5 |
| LDH, U/l
(109–245) | 170 | 155 | – | – | – | – |
| ALB, g/l (35–55) | 35 | 38 | 48.8 | 46.2 | 48.2 | 46.1 |
| TBA, g/l (60–83) | 70.0 | 67.0 | 76.6 | 70.0 | 73.8 | 71.7 |
The CIK cells were prepared as described in previous
studies (5,14,15).
Briefly, peripheral blood mononuclear cells were collected from the
patient using a Cobe Spectra Apheresis System (CaridianBCT,
Lakewood, CO, USA) and cultured in Invitrogen AIM-V medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA), containing 50 ng/ml
anti-CD3 antibody (mouse anti-human, clone OKT3; cat. no.
16-0037-85; eBioscience, Inc., San Diego, CA, USA) to stimulate CIK
cell growth, 100 U/ml recombinant human IL-1α (eBioscience) and
1,000 U/ml recombinant human IFN-γ (Peprotech, Rocky Hill, NJ, USA)
at 37°C with 5% CO2 for 24 h. Then, 300 U/ml recombinant
human IL-2 (Beijing SL Pharmaceutical Co.,Ltd., Beijing, China) was
added to the media. Fresh medium containing IL-2 (300 U/ml) and
IFN-γ (1,000 U/ml) was added to the culture media every 5 days. The
CIK cells were harvested and analyzed for phenotype and
cytotoxicity subsequent to 14 days of culture.
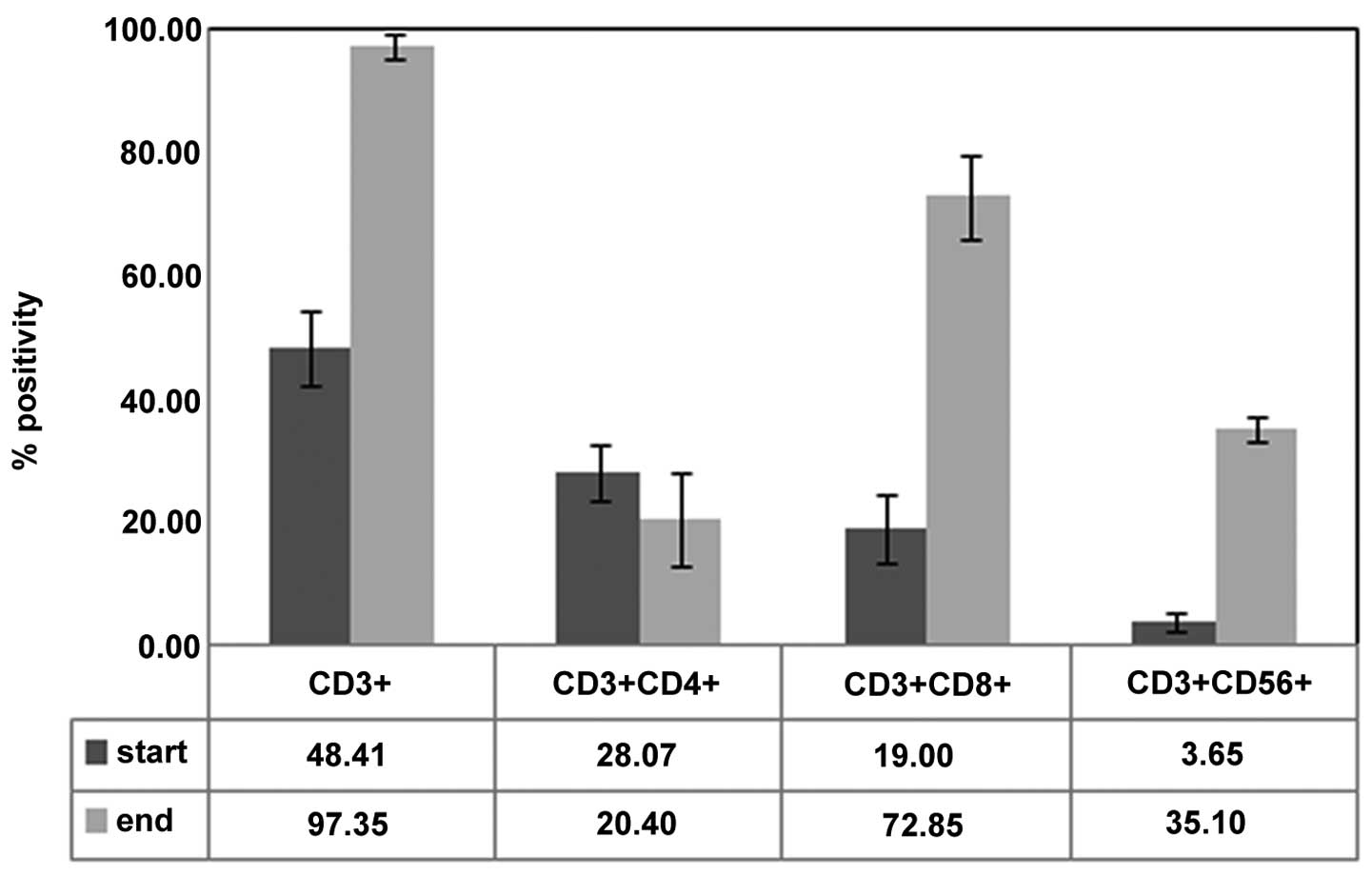
The phenotypic analysis of the prepared cells prior
to culture and subsequent to 14 days of culture demonstrated the
expansion of CD3+/CD56+ cells. The mean
percentages of the CD3+,
CD3+/CD4+, CD3+/CD8+
and CD3+/CD56+ cell subsets were 48.41±6.19,
28.07±4.76, 19.00±5.54 and 3.65±1.41%, respectively, prior to
culture and 97.35±2.19, 20.40±7.50, 72.85±7.00 and 35.00±9.90%,
respectively, subsequent to culture. All P-values were <0.05
(Fig. 2). The in vitro
cytotoxicity of the CIK preparations was analyzed at the end of the
culture period. The CIK cells were mixed with K562 cells at
effector-to-target ratios of 40:1, 20:1 and 10:1. The mean values
of cytotoxicity of these CIK cell ratios against the K562 cells
were 35.00±9.90, 22.10±9.76 and 14.50±9.76%, respectively. All
products were free of bacterial and fungal contamination, free of
Mycoplasma and contained <5 EU/ml endotoxin. The patient
succumbed to the disesase in November 2013.
Discussion
To the best of our knowledge, the present study is
the first to report adoptive cell transfer in a post-LT patient
with HCC. In the current study, large numbers of CIK cells were
produced in a 2-week time period and safely administered to a
patient that underwent LT. In the case of infusion-associated
toxicity or secondary acute HVGD, no additional CIK infusions would
be administered. In all patients, after a minimum of 28 days,
additional CIK infusions may be performed at the same time interval
and up to the best clinical response, emergence of toxicity or
occurrence of HVGD. The present study has demonstrated the
feasibility and relatively low toxicity of the program.
CIK cells are ex vivo-expanded T lymphocytes
that express natural killer and T-cell markers. Expanded cells
induce the non-major histocompatibility complex-restricted lysis of
tumor cells or allografts (6–9). The adverse effects of intravenous
infusion of autologous CIK cells and the status of the donor's
liver following the infusion of large numbers of lymphocytes are
the major concerns of CIK therapy subsequent to LT in the present
study. Whether CIK cells trigger the rejection of the donated liver
has been a growing concern. However, no serious CIK-associated
adverse effects were observed in the present patient. Certain
slight adverse effects may occur following symptomatic treatment,
but the clinical indicators of rejective responses were normal in
the current patient.
Immunosuppressive medical treatments that are
directed against T cells, including FK506 and cyclosporin A (CsA),
inhibit T-cell responses (16). The
present patient was treated with FK506 during CIK cell therapy to
investigate how immunosuppressive drugs may affect CIK cell
therapy. Certain previous studies have reported that CIK cells
contain cytolytic granules that exhibit perforin and granzyme
activity that are released into the extracellular space upon
binding with susceptible target cells or following the
cross-linking of CD3 with an anti-CD3 monoclonal antibody adhered
to plastic (17–20). The use of immunosuppressive drugs,
such as CsA and FK506, prevented the degranulation of CIK cells
that is induced by CD3-TCR stimulation; however, the drugs were
unable to block the cytotoxicity that was triggered by the
interaction with tumor targets. In addition, the degranulation
induced by target cells was unaffected by CsA and FK506 (6–16,21). Therefore, the use of immunosuppressive
drugs may not affect the efficacy of CIK. The prepared CIK cells in
the present study remained effective against liver cancer cells.
Therefore, the present study demonstrates that additional patients
are required to assess the relatively low toxicity and feasibility
of CIK infusion in LT patients.
Acknowledgements
The authors woudl like to thank Dr Fang Yan
(Vanderbilt University, Nashville, TN, USA) for the suggestions
made during the manuscript preparation. The present study was
supported by a grant from the National Natural Science Funds of
China (no. 81402362).
Glossary
Abbreviations
Abbreviations:
|
LT
|
liver transplantation
|
|
CIK
|
cytokine-induced killer cell
|
|
HCC
|
hepatocellular carcinoma
|
|
HVGD
|
host-versus-graft disease
|
|
TBIL
|
total bilirubin
|
|
DBIL
|
direct bilirubin
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
References
|
1
|
Saidi RF and Hejazi Kenari SK: Liver
transplantation for hepatocellular carcinoma: Past, present and
future. Middle East J Dig Dis. 5:181–192. 2013.PubMed/NCBI
|
|
2
|
Silva MF and Sherman M: Criteria for liver
transplantation for HCC: What should the limits be? J Hepatol.
55:1137–1147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Decaens T, Roudot-Thoraval F, Badran H,
Wolf P, Durand F, Adam R, Boillot O, Vanlemmens C, Gugenheim J,
Dharancy S, et al: Impact of tumour differentiation to select
patients before liver transplantation for hepatocellular carcinoma.
Liver Int. 31:792–801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sapisochin G, Goldaracena N, Astete S,
Laurence JM, Davidson D, Rafael E, Castells L, Sandroussi C, Bilbao
I, Dopazo C, et al: Benefit of treating hepatocellular carcinoma
recurrence after liver transplantation and analysis of prognostic
factors for survival in a large Euro-American series. Ann Surg
Oncol. 22:2286–2294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schlitt HJ, Neipp M, Weimann A, Oldhafer
KJ, Schmoll E, Boeker K, Nashan B, Kubicka S, Maschek H, Tusch G,
et al: Recurrence patterns of hepatocellular and fibrolamellar
carcinoma after liver transplantation. J Clin Oncol. 17:324–331.
1999.PubMed/NCBI
|
|
6
|
Ren X, Yu J, Liu H, Zhang P, An X, Zhang N
and Hao X: Th1 bias in PBMC induced by multicycles of auto-CIKs
infusion in malignant solid tumor patients. Cancer Biother
Radiopharm. 21:22–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Wang C, Yu J, Cao S, Wei F, Zhang W,
Han Y and Ren XB: Dendritic cell-activated cytokine-induced killer
cells enhance the anti-tumor effect of chemotherapy on non-small
cell lung cancer in patients after surgery. Cytotherapy.
11:1076–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu L, Zhang W, Qi X, Li H, Yu J, Wei S,
Hao X and Ren X: Randomized study of autologous cytokine-induced
killer cell immunotherapy in metastatic renal carcinoma. Clin
Cancer Res. 18:1751–1759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li R, Wang C, Liu L, Du C, Cao S, Yu J,
Wang SE, Hao X, Ren X and Li H: Autologous cytokine-induced killer
cell immunotherapy in lung cancer: A phase II clinical study.
Cancer Immunol Immunother. 61:2125–2133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishimura R, Baker J, Beilhack A, Zeiser
R, Olson JA, Sega EI, Karimi M and Negrin RS: In vivo
trafficking and survival of cytokine-induced killer cells resulting
in minimal GVHD with retention of antitumor activity. Blood.
112:2563–2574. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Linn YC, Lau LC and Hui KM: Generation of
cytokine-induced killer cells from leukaemic samples with in
vitro cytotoxicity against autologous and allogeneic leukaemic
blasts. Br J Haematol. 116:78–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takayama T, Sekine T, Makuuchi M, Yamasaki
S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi
Y, et al: Adoptive immunotherapy to lower postsurgical recurrence
rates of hepatocellular carcinoma: A randomised trial. Lancet.
356:802–807. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hui D, Qiang L, Jian W, Ti Z and Da-Lu K:
A randomized, controlled trial of postoperative adjuvant
cytokine-induced killer cells immunotherapy after radical resection
of hepatocellular carcinoma. Dig Liver Dis. 41:36–41. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dollinger MM, Howie SE, Plevris JN, Graham
AM, Hayes PC and Harrison DJ: Intrahepatic proliferation of ‘naive’
and ‘memory’ T cells during liver allograft rejection: Primary
immune response within the allograft. FASEB J. 12:939–947.
1998.PubMed/NCBI
|
|
15
|
Sangiolo D, Mesiano G, Carnevale-Schianca
F, Piacibello W, Aglietta M and Cignetti A: Cytokine induced killer
cells as adoptive immunotherapy strategy to augment graft versus
tumor after hematopoietic cell transplantation. Expert Opin Biol
Ther. 9:831–840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lowdell MW, Lamb L, Hoyle C, Velardi A and
Prentice HG: Non-MHC-restricted cytotoxic cells: Their roles in the
control and treatment of leukaemias. Br J Haematol. 114:11–24.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kägi D, Ledermann B, Bürki K, Seiler P,
Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM and Hengartner H:
Cytotoxicity mediated by T cells and natural killer cells is
greatly impaired in perforin-deficient mice. Nature. 369:31–37.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heusel JW, Wesselschmidt RL, Shresta S,
Russell JH and Ley TJ: Cytotoxic lymphocytes require granzyme B for
the rapid induction of DNA fragmentation and apoptosis in
allogeneic target cells. Cell. 76:977–987. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Dai H, Li H, Lv H, Wang T, Fu X
and Han W: Growth of human colorectal cancer SW1116 cells is
inhibited by cytokine-induced killer cells. Clin Dev Immunol.
2011:6214142010.PubMed/NCBI
|
|
20
|
Wang X, Yu W, Li H, Yu J, Zhang X, Ren X
and Cao S: Can the dual-functional capability of CIK cells be used
to improve antitumor effects? Cell Immunol. 287:18–22. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mehta BA, Schmidt-Wolf IG, Weissman IL and
Negrin RS: Two pathways of exocytosis of cytoplasmic granule
contents and target cell killing by cytokine-induced
CD3+CD56+ killer cells. Blood. 86:3493–3499.
1995.PubMed/NCBI
|