Introduction
Superior mesenteric artery syndrome (SMAS) has
previously been described under various other names, including,
duodenal arterial mesenteric compression, duodenal ileus and Wilkie
syndrome (1–3). SMAS is caused by compression of the
third part of the duodenum by the superior mesenteric artery (SMA),
which takes its origin from the abdominal aorta at the level of the
first lumbar vertebra and crosses the duodenum (4,5). The exact
prevalence of SMAS worldwide remains unclear, however, the rate has
been estimated to be 0.013–0.3%, based on barium studies (6). Treatment is initially conservative,
which includes the insertion of a nasogastric tube, mobilization of
the patient to a prone, left lateral decubitus position,
administration of parenteral nutrition, fluid-electrolyte balance
correction and positive nitrogen balance to increase body weight
and restore the retroperitoneal fat tissue (7). In cases where conservative treatment has
failed, surgery including Treitz ligament division,
gastrojejunostomy, subtotal gastrectomy and Billroth II
gastrojejunostomy and duodenojejunostomy may be performed to avoid
the risk of duodenal atony and massive dilatation. Numerous
predisposing conditions for SMAS, including malignancies, burns,
prolonged bed rest, anorexia nervosa, malabsorption, anatomical
anomalies and surgical complications, have been identified to have
possible impacts on the angle between the SMA and the abdominal
aorta (7).
Primary small bowel adenocarcinoma is an uncommon
tumor, with non-specific symptoms that may cause a delay in
diagnosis and, consequently, a negative outcome (8–11). The
duodenum is most frequently involved, followed by the jejunum
(12). Small bowel adenocarcinomas
are rare, accounting for <2% of all tumors of the
gastrointestinal tract and ≤40% of all small bowel malignancies in
the USA (13). Furthermore, the
annual incidence is 1.2–6.5 cases per 1 million individuals. The
main treatment for small bowel adenocarcinoma is radical surgical
resection (14). The ability to
completely resect tumors is one of the most important prognostic
factors for survival, and adjuvant chemotherapy is required
(15). Small bowel adenocarcinoma
exhibits a poor prognosis at all stages of disease, with a 5-year
overall survival rate of 14–33% (16). A considerable number of patients with
small bowel carcinoma are diagnosed due to upper small bowel
obstruction (12). The present study
reports a case of a primary adenocarcinoma of the small intestine
causing SMAS. The aim of this report was to highlight that SMA
syndrome must be considered a symptom, rather than a disease;
therefore, determining the cause of SMA syndrome is important.
Case report
In August 2014, a 51-year-old man was admitted to
the Department of the Gastroenterology, Kunshan First People's
Hospital Affiliated to Jiangsu University (Kunshan, China) with
symptoms of anorexia, vomiting and epigastric abdominal pain
lasting for two weeks. During this two-week period, the patient's
body weight had reduced by 8 kg. The patient's past medical history
included an endoscopic resection of colon polyps one month earlier.
His medical history and physical examination were suggestive of an
upper small bowel obstruction, with symptoms of recurrent bilious
vomiting, abdominal pain and upper abdominal distension. The
initial blood and urine examinations were within normal ranges. A
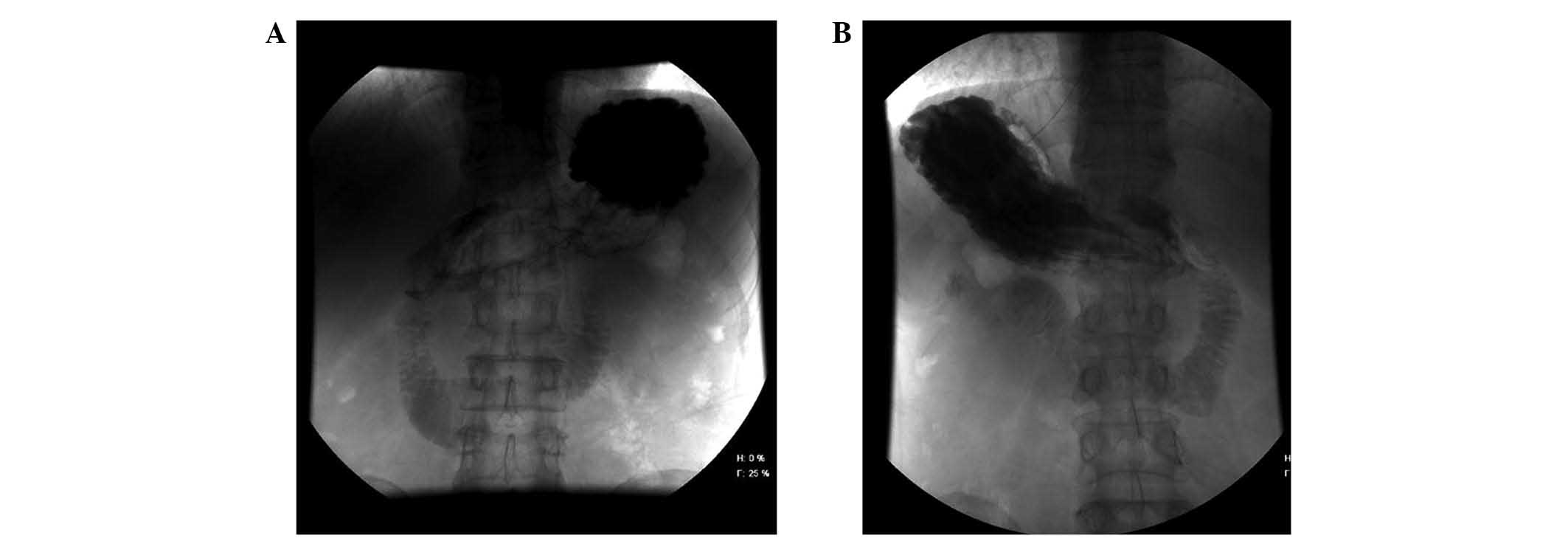
digital gastrointestinal X-ray machine (PLD7600; Philips,
Amsterdam, The Netherlands) was used to perform diatrizoate
angiography; diatrizoate (Lunan Pharmaceutical Group Co., Ltd.,
Shandong, China) revealed dilation of the proximal duodenum, and
stenosis of its third part in the supine position (Fig. 1A). In the prone position, the contrast
medium passed through the obstructed part of the distal side
(Fig. 1B). Contrast-enhanced
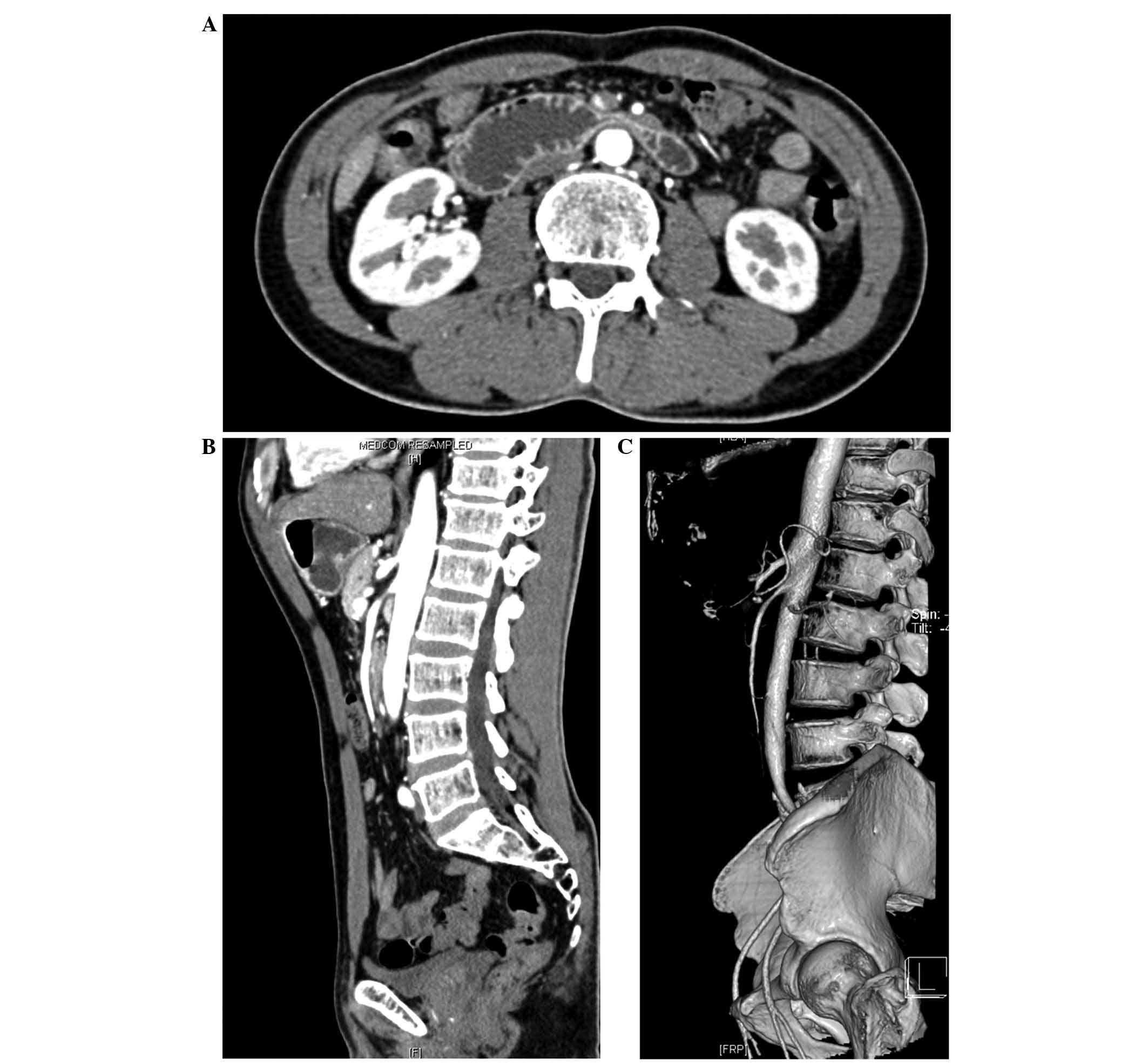
abdominal computed tomography (CT) scanning (SOMATOM Sensation
Cardiac; Siemens AG, Munich, Germany) demonstrated compression of
the duodenum between the aorta and SMA, as well as a distended
duodenal bulb due to compression of the third portion of the
duodenum (Fig. 2A), an
aortomesenteric distance of 8 mm and a reduction of the
aortomesenteric angle to ~20° (Fig. 2B
and C). Based on the history of symptoms, clinical appearance,
the diatrizoate and CT results, SMAS was suspected. A nasogastric
tube was inserted and the patient was treated with proton pump
inhibitors [Pantoprazole Sodium for Injection (40 mg, daily);
Yangtze River Pharmaceutical Group Co., Ltd., Jiangsu, China] and
total parenteral nutritional was commenced; however, the symptoms
did not improve, and >800 ml of bilious gastric fluid was
drained from the patient each day.
Following two weeks of treatment, it was concluded
that conservative measures had failed, and the patient was
transferred to the Department of Gastrointestinal Surgery, Kunshan
First People's Hospital Affiliated to Jiangsu University, for
additional treatment. A laparotomy was performed. During surgery, a
duodenal obstruction was confirmed exactly at the point of crossing
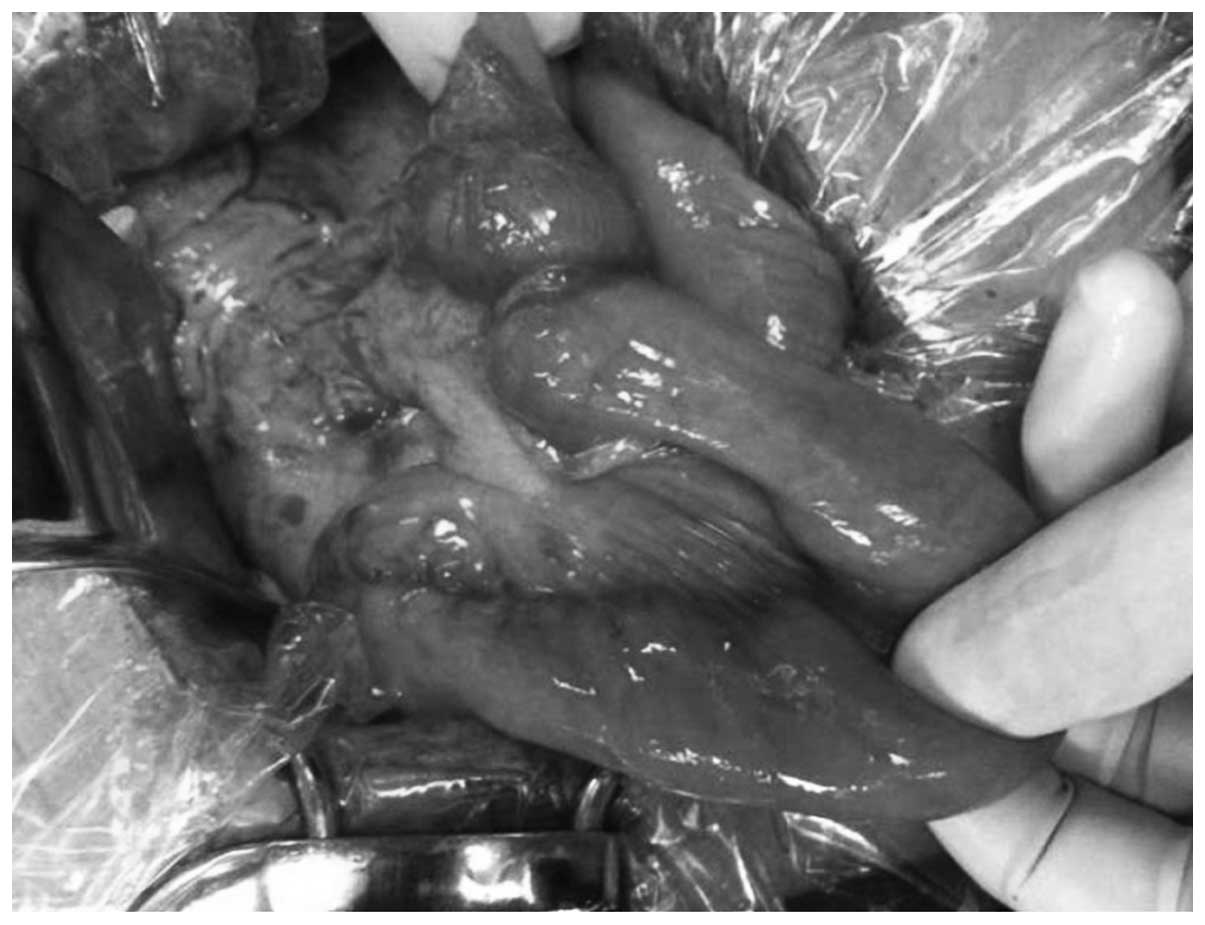
by the SMA. However, macroscopic examination revealed a type 2
tumor extension with serosal infiltration at the duodenal-jejunal
junction, which was causing complete bowel obstruction (Fig. 3). Laparotomy did not reveal direct
invasion into the pancreas, peritoneal dissemination or distant
metastasis. The ligament of Treitz was separated from the duodenum.
Rapid histological diagnosis during surgery revealed
moderately-differentiated adenocarcinoma. Partial resection of the
duodenum and jejunum, accompanied by lymph node dissection along
the superior mesenteric artery, was performed. The inferior
pancreaticoduodenal artery and 1st jejunal artery were ligated for
lymphadenectomy. End to end anastomosis was performed between the
duodenum and jejunum.
For histological analysis, tissue sections (4-mm)
were incubated with 4% paraformaldehyde (Generay Biotech Co., Ltd.,
Shanghai, China) for 15 min at room temperature and washed twice
with Tris-buffered saline (TBS)/0.1% saponin (Generay Biotech Co.,
Ltd.) for 4 min each. The sections were then incubated with
TBS/0.3% H2O2/0.1% Saponin and 0.02%
NaN3 (Generay Biotech Co., Ltd.) for 30 min to block
endogenous peroxidase activity. Next, the sections were washed
three times with TBS/saponin for 3 min each then incubated with
goat serum [dilution, 1:100; Hangzhou MultiSciences (Lianke)
Biotech Co., Ltd., Hangzhou, China] in TBS/saponin for 20 min to
block non-specific binding sites. The slides were incubated with an
appropriate antibody for immunoblotting, including carcinoembryonic
antigen (clone E-4; mouse anti-human monoclonal; catalog no.
sc-48374), cytokeratin 20 (clone G-20; goat anti-human polyclonal;
catalog no. sc-17113), cytokeratin 7 (clone N-20; goat anti-human
polyclonal; catalog no. sc-17116) and cytokeratin 19 (clone M-17;
goat anti-human polyclonal; catalog no. sc-33111) (dilution, 1:500;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
The slides were then washed four times with TBS/saponin followed by
incubation with biotinylated secondary antibody, horseradish
peroxidase-labeled goat anti-mouse (catalog no. sc-2005) and goat
anti-human IgG (catalog no. sc-2457) (dilution, 1:1,000; Santa Cruz
Biotechnology, Inc.) for 30 min. Avidin-biotin-peroxidase reagents
[Hangzhou MultiSciences (Lianke) Biotech Co., Ltd.] were then
added, and the resulting peroxidase activity was revealed following
incubation with 0.5 mg/ml horseradish peroxidase substrate solution
[Hangzhou MultiSciences (Lianke) Biotech Co., Ltd.]. The slides
were then washed four times in TBS. Tissues were then stained with
hematoxylin and eosin and analyzed using an optical microscope
(DSX100; Olympus Corporation, Tokyo, Japan). The histological
report determined a diagnosis of moderately-differentiated
adenocarcinoma, partially composed of poorly-differentiated cells
that were perforating the visceral peritoneum, according to the
tumor-node-metastasis classification of malignant tumors (American
Joint Committee on Cancer) (17).
Additionally, 5/18 lymph nodes demonstrated microscopic metastasis.
The definitive diagnosis was primary adenocarcinoma of the
duodenal-jejunal junction, T4N1M0, stage III (17). The postoperative course was uneventful
and the patient was discharged on postoperative day 12.
Discussion
SMAS is an uncommon type of upper intestinal
obstruction (18). The
pathophysiological process of this syndrome, resulting in a
decrease in aortomesenteric angle, is commonly regarded as being
due to a decrease in retroperitoneal fat following acute weight
loss (19). There are a number of
known aetiologies for SMAS, including malignancies and
malabsorption syndromes. Diagnosis of SMAS is dependent on the
barium meal findings of duodenal dilation, retention of barium
within the duodenum and characteristic vertical linear extrinsic
pressure in the third part of the duodenum (19). Previously, angiographic measurement of
the aortomesenteric angle was considered the gold standard of
diagnosis; an aortomesenteric angle of <22–25° and a distance of
<8 mm were observed to correlate well with SMAS (20). However, due to the invasive nature of
angiography, CT scanning or upper gastrointestinal series are now
more commonly used for the diagnosis of SMAS (20).
Primary adenocarcinoma of the small intestine is
40–60 times less frequent compared with colon cancer (21). The diagnosis of small bowel
adenocarcinoma is frequently delayed as its symptoms and signs are
non-specific. It may develop in any location, but is more frequent
in proximal segments, particularly the duodenum and upper jejunum
(22).
The current study reported a case of primary
adenocarcinoma of the small intestine presenting as SMAS. The
patient received conservative therapy for two weeks in the
gastroenterology department for the treatment of SMAS; however, the
upper gastrointestinal obstructive symptoms demonstrated no
significant improvement. Laparotomy revealed complete obstruction
of the duodenal-jejunal junction by a primary small intestine
adenocarcinoma; how the present patient subsequently developed SMAS
is unclear. A plausible explanation may be that the significant
weight loss induced by the tumor, as well as a reduction in the
angle at which the SMA branched from the aorta, led to compression
of the third portion of the duodenum.
In conclusion, the present case highlights that SMAS
may be considered as a symptom of a disease, rather than a primary
diagnosis. Thus, research investigating the cause of the condition
is required. In patients with SMAS, if conservative treatment
fails, surgery should then be considered as the next available
option.
References
|
1
|
Ylinen P, Kinnunen J and Höckerstedt K:
Superior mesenteric artery syndrome. A follow-up study of 16
operated patients. J Clin Gastroenterol. 11:386–391. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hines JR, Gore RM and Ballantyne GH:
Superior mesenteric artery syndrome. Diagnostic criteria and
therapeutic approaches. Am J Surg. 148:630–632. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siddiqui MN, Ahmad T and Jaffary A:
Retroperitoneal fungal abscess presenting as superior mesenteric
artery syndrome. Postgrad Med J. 72:433–434. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sinagra E, Montalbano LM, Linea C, Giunta
M, Tesè L, La Seta F, Malizia G, Orlando A, Marasà M and D'Amico G:
Delayed-onset superior mesenteric artery syndrome presenting as
oesophageal peptic stricture. Case Rep Gastroenterol. 6:94–102.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agrawal S and Patel H: Superior mesenteric
artery syndrome. Surgery. 153:601–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agrawal GA, Johnson PT and Fishman EK:
Multidetector CT of superior mesenteric artery syndrome. J Clin
Gastroenterol. 41:62–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Welsch T, Büchler MW and Kienle P:
Recalling superior mesenteric artery syndrome. Dig Surg.
24:149–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Halfdanarson TR, McWilliams RR, Donohue JH
and Quevedo JF: A single-institution experience with 491 cases of
small bowel adenocarcinoma. Am J Surg. 199:797–803. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruiz-Tovar J, Martínez-Molina E, Morales V
and Sanjuanbenito A: Primary small bowel adenocarcinoma. Cir Esp.
85:354–359. 2009.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reynolds I, Healy P and Mcnamara DA:
Malignant tumours of the small intestine. Surgeon. 12:263–270.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaiyasate K, Jain AK, Cheung LY, Jacobs
MJ and Mittal VK: Prognostic factors in primary adenocarcinoma of
the small intestine: 13-year single institution experience. World J
Surg Oncol. 6:122008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Overman MJ: Recent advances in the
management of adenocarcinoma of the small intestine. Gastrointest
Cancer Res. 3:90–96. 2009.PubMed/NCBI
|
|
13
|
Raghav K and Overman MJ: Small bowel
adenocarcinomas - existing evidence and evolving paradigms. Nat Rev
Clin Oncol. 10:534–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Delaunoit T, Neczyporenko F, Limburg PJ
and Erlichman C: Small bowel adenocarcinoma: A rare but aggressive
disease. Clin Colorectal Cancer. 4:241–248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chaaya A and Heller SJ: Introduction to
small bowel tumors. Tech Gastrointest Endosc. 14:88–93. 2012.
View Article : Google Scholar
|
|
16
|
Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY,
Bennett CL and Talamonti MS: Small bowel cancer in the United
States: Changes in epidemiology, treatment, and survival over the
last 20 years. Ann Surg. 249:63–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours (7th). Wiley Blackwell.
153–159. 2009.
|
|
18
|
Yakan S, Calıskan C, Kaplan H, Deneclı AG
and Coker A: Superior mesenteric artery syndrome: A rare cause of
intestinal obstruction. Diagnosis and surgical management. Indian J
Surg. 75:106–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahmed AR and Taylor I: Superior mesenteric
artery syndrome. Postgrad Med J. 73:776–778. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Molina Rodríguez JL, Martí Obiol R, Mozos
López F and Ortega Serrano J: Superior mesenteric artery syndrome.
Cir Esp. 90:532012.(In Spanish). PubMed/NCBI
|
|
21
|
Aparicio T, Zaanan A, Svrcek M,
Laurent-Puig P, Carrere N, Manfredi S, Locher C and Afchain P:
Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis
and treatment. Dig Liver Dis. 46:97–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu Y, Fröbom R and Lagergren J: Incidence
patterns of small bowel cancer in a population-based study in
Sweden: Increase in duodenal adenocarcinoma. Cancer Epidemiol.
36:e158–e163. 2012. View Article : Google Scholar : PubMed/NCBI
|