Introduction
Pancreatic cancer (PC) is an aggressive disease and
is the fourth leading cause of cancer-associated mortality in the
western world, with an overall 5-year survival rate of <6%
(1). Surgical resection is the only
potentially curative treatment; however, since patients are often
diagnosed at advanced stages, only 15–20% of patients are
candidates for a pancreatectomy (2).
A prognostic evaluation is required for patients with resected
pancreatic cancer (3). Such
evaluations may distinguish patients with an improved prognosis
from those that require additional and more vigorous treatment
regimens (3).
The prognosis of patients in various types of cancer
is hypothesized to be closely associated with immune evasion by
tumor cells (4). Tumor cells may
escape from immune surveillance through various mechanisms
(5), which are dependent on the
balance between co-stimulatory and co-inhibitory signals (6,7). This
consists of signals from the peripheral membrane protein B7 family,
located on antigen presenting cells (APCs), when they interact with
cluster of differentiation (CD)-28, which is located on T cells.
The B7 family members (8), including
B7-H3 and B7-H4, are hypothesized to play a role in tumor immune
evasion, which in turn affects the prognosis of patients.
B7-H3 (9) is an
accessory co-stimulatory molecule in the B7/CD28 family, although
it is theorized to perform stimulatory and inhibitory functions.
The stimulatory properties of B7-H3 consist of promoting T cell
proliferation and interferon-γ production (10), while the inhibitory properties consist
of impairing type 1 T-helper cell responses and protecting cells
from natural killer cell-mediated lysis (11). In certain human malignant tumors,
including gastric cancer, the expression of B7-H3 has been revealed
to be associated with an improved prognosis of patients (12). By contrast, in non-small cell lung
cancer (13), clear-cell renal cell
carcinoma (14) and prostate cancer
(15), B7-H3 has been described as an
indicator of a poor prognosis. Yamato et al (16) reported that B7-H3 was expressed in PC,
and revealed that its expression was associated with aggressive
clinicopathological characteristics; however, the study did not
assess the association between B7-H3 expression and the survival
time of patients.
B7-H4 is a negative co-stimulatory molecule that
inhibits CD4+ and CD8+ T cell proliferation,
cell-cycle progression and interleukin-2 production, and renders
tumor cells refractory to apoptosis (17). In breast (18), non-small cell lung (12) and ovarian cancer (19), and renal cell carcinoma (20), aberrant expression of B7-H4 has been
demonstrated to be associated with a poor clinical outcome. B7-H4,
along with P53, was revealed to be a potential diagnostic marker
for PC, and the expression of B7-H4 was associated with adverse
pathological features, including an increased tumor grade (21). However, the prognostic value of B7-H4
expression in PC has not been fully elucidated.
The present study evaluated the expression of B7-H3
and B7-H4 in PC and normal tissue samples using immunohistochemical
analysis to determine whether B7-H3 and B7-H4 expression is an
independent predictor for the overall survival (OS) time in
patients with PC.
Materials and methods
Tissue samples
Pancreatic tumor samples and clinical data were
obtained from 40 patients with PC (26 men and 14 women; median age
at diagnosis, 54 years; range, 34–80 years), who underwent surgery
without pre-operative therapy between April 2000 and January 2009
at the Second Affiliated Hospital of Zhengzhou University
(Zhengzhou, Henan, China). The present study also obtained 10
normal pancreatic tissue samples, which acted as a control group
and were randomly selected during the same time period from benign
pancreatic tumor resections. The PC stage was classified according
to the 2002 American Joint Committee on Cancer
tumor-node-metastasis (TNM) staging system (22). Tumor cell differentiation was
determined using the 2000 World Health Organization classification
(23). Clinical data consisting of
the patient age, patient gender, tumor location, histopathological
type, histological grade, tumor stage and lymph node invasion was
collected from medical records. The median follow-up time was 58.5
months (range, 12–134 months), and the most recent follow-up
occurred on April 30, 2011. The present study was performed
subsequent to obtaining informed consent from all patients and
approval from the independent Institute Research Ethics Committee
of The First Affiliated Hospital of Soochow University (Suzhou,
China).
Immunohistochemistry
Immunostaining was performed using an EliVision™
plus kit (Maixin Biotech, Inc., Fuzhou, Fujian, China), according
to the manufacturer's protocol. Formalin-fixed paraffin-embedded
tissue blocks were sliced in 3-µm sections and mounted on charged
glass slides (Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA).
Antigen retrieval was performed in citrate buffer (20 mmol/l; pH
6.0; Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China) at 120°C for
10 min. Endogenous peroxidase activity was blocked with 3.0%
hydrogen peroxide (Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China) for 10 min. Mouse anti-human B7-H3 (dilution, 1:100; clone,
7D7) (24) and mouse anti-human B7-H4
(dilution, 1:100; clone, 3C8) monoclonal antibodies (gifted from
Soochow University, Suzhou, China) (25) were used as the primary antibodies.
Negative controls were performed using mouse monoclonal
immunoglobulin G (NC-1390; Fuzhou Maixin Biotech Co., Ltd.) as the
primary antibody. For visualization, the sections were incubated
with 3,3′-diaminobenzidine solution (Fuzhou Maixin Biotech Co.,
Ltd.) and counterstained with hematoxylin (Amresco, LLC, Solon, OH,
USA).
Evaluation of immunostaining
B7-H3 and B7-H4 expression was calculated as the
percentage of tumor cells exhibiting immunoreactivity in the
cytoplasm or on the membrane, which was determined by counting the
number of B7-H3 or B7-H4-stained tumor cells out of 1,000 tumor
cells in each tissue section. Using an Olympus BH2 microscope
(Olympus Corporation, Tokyo, Japan), cell counts were performed at
a ×400 magnification in ≥5 randomly selected fields in tumor
sections. The intensity of the B7-H3 or B7-H4 expressing cells was
semi-quantitatively graded as previously described (13), according to the positive cell
percentage, as follows: 0, focal expression in <10%; +, focal
expression in 10–40%; ++, focal expression in 40–80%; and +++,
focal expression in >80%. For the purposes of the present
analysis, the samples were classified into 2 groups on the basis of
staining intensity, as follows: Negative, <10% expression; and
positive, 10–100% expression.
Statistical analysis
χ2 and Fisher's exact tests were used to
examine the association between B7-H3 and B7-H4 expression and
various clinicopathological parameters, whilst Spearman's rank
correlation coefficient was used to determine correlations. The
overall survival times of patients with or without B7-H3 and B7-H4
expression were compared using the Kaplan-Meier method of survival
time analysis and the log-rank test. For all patients, the survival
time was calculated from the date of pathological diagnosis to the
date of the patient succumbing to the disease or the date of the
last follow-up. The data from patients that were alive at the last
day of follow-up were censored. The median follow-up time was
calculated using only censored data. The median survival time was
also calculated. Hazard ratios (HR) and 95% confidence intervals
(CI) were estimated using univariate and multivariate Cox's
proportional-hazard models. All statistical analysis was calculated
with SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA),
and the P-values were two-tailed. P<0.05 was considered to
indicate a statistically significant difference.
Results
B7-H3 and B7-H4 expression in PC
tissue samples
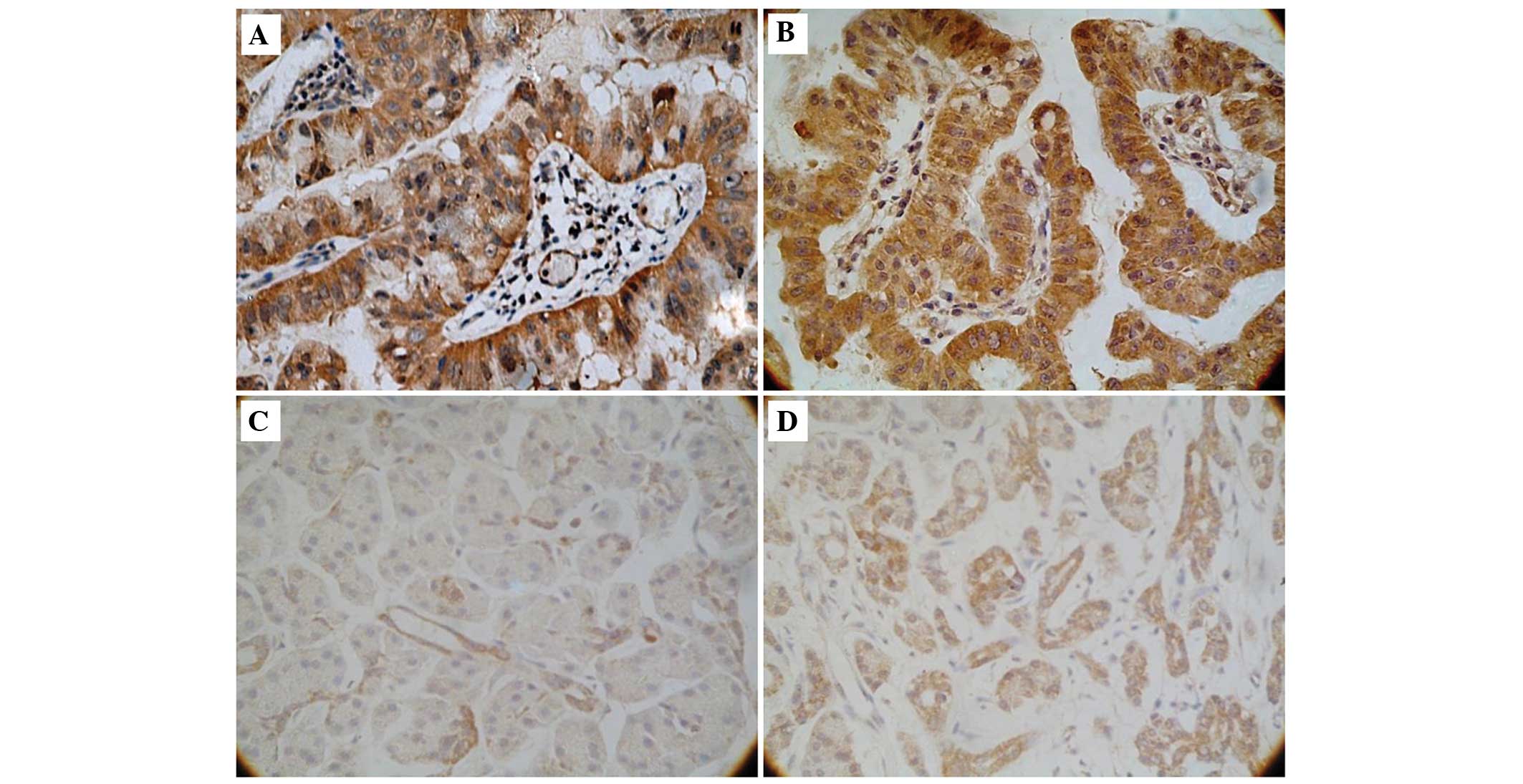
Staining for B7-H3 and B7-H4 was observed in the
cell cytoplasm and membrane in cancerous and non-cancerous cells.
B7-H3 expression was increased in PC tissue samples (35 out of 40
patients, 88%; Fig. 1A) compared with
normal tissue samples (3 out of 10 patients, 30%; Fig. 1C; P<0.01). Similarly, B7-H4
expression was increased in PC tissue samples (30 out of 40
patients, 75%; Fig. 1B) compared with
normal tissue samples (2 out of 10 patients, 20%; Fig. 1D; P<0.01).
Association between B7-H3 and B7-H4
expression and clinicopathological parameters
The association between tumor cell B7-H3 and B7-H4
expression and clinicopathological parameters is revealed in
Table I. The present study
demonstrated that positive B7-H3 expression in PC tissue samples
was associated with an early TNM stage (P<0.01). Positive B7-H4
expression was associated with tumors located in the body and tail
of the pancreas, compared with tumors located in the head of the
pancreas (P<0.01), and lymph node metastasis (P=0.02). The
expression of either protein was not associated with the age of the
patient at the time of diagnosis (≤54 vs. >54 years), gender,
histological grade, or histopathological type. In addition, the
present study identified a positive correlation between B7-H3
expression and B7-H4 expression in PC tissue samples (r=0.37;
P=0.02).
 | Table I.Association between
clinicopathological parameters and B7-H3 and B7-H4 expression in
pancreatic cancer tissue samples. |
Table I.
Association between
clinicopathological parameters and B7-H3 and B7-H4 expression in
pancreatic cancer tissue samples.
|
|
| B7-H3 expression | B7-H4 expression |
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | n | Present, n | Not present, n | P-value | Present, n | Not present, n | P-value |
|---|
| Total | 40 | 35 | 5 |
| 30 | 10 |
| Age at diagnosis,
years |
|
|
| 0.63 |
|
| 0.47 |
| ≤54
years | 23 | 21 | 2 |
| 16 | 7 |
| >54
years | 17 | 14 | 3 |
| 14 | 3 |
| Gender |
|
|
|
0.64 |
|
|
0.25* |
|
Male | 26 | 22 | 4 |
| 18 | 8 |
|
Female | 14 | 13 | 1 |
| 12 | 2 |
| Tumor location in
pancreas |
|
|
|
0.32 |
|
| <0.01* |
|
Head | 26 | 24 | 2 |
| 16 | 10 |
| Body or
tail | 14 | 11 | 3 |
| 14 | 0 |
| Histological
grade |
|
|
|
0.33 |
|
| 1.00 |
|
Well | 10 | 10 | 0 |
| 8 | 2 |
|
Moderate | 14 | 11 | 3 |
| 10 | 4 |
|
Poor | 16 | 14 | 2 |
| 12 | 4 |
| Histopathological
type |
|
|
|
0.43a |
|
| 0.26 |
| Ductal
adenocarcinoma | 36 | 31 | 5 |
| 28 | 8 |
|
Mucinous
cystadenocarcinoma | 4 | 4 | 0 |
| 2 | 2 |
| Tumor stage |
|
|
| <0.01 |
|
| 0.10 |
| T1 | 9 | 5 | 4 |
| 8 | 1 |
| T2 | 11 | 11 | 0 |
| 5 | 6 |
| T3 | 12 | 12 | 0 |
| 10 | 2 |
| T4 | 8 | 7 | 1 |
| 7 | 1 |
| Lymph node
metastasis |
|
|
|
0.03a |
|
| 0.02 |
| N0 | 17 | 17 | 0 |
| 9 | 8 |
| N1 | 20 | 15 | 5 |
| 18 | 2 |
| TNM stage |
|
|
| <0.01 |
|
| 0.73 |
| I | 11 | 11 | 0 |
| 8 | 3 |
| II | 15 | 15 | 0 |
| 10 | 5 |
|
III | 8 | 7 | 1 |
| 7 | 1 |
| IV | 6 | 2 | 4 |
| 5 | 1 |
Association between B7-H3 and B7-H4
expression and OS time
At the time of the final follow-up, 34 out of 40
patients had succumbed to PC; of the remaining 6 patients, 3 were
alive and 3 were lost to follow-up. The patients with tumors that
expressed B7-H4 had a poorer OS time compared with the patients
with tumors that did not express B7-H4 (P=0.01). Other prognostic
factors were also demonstrated to decrease the OS time, including
tumors located in the body and tail of the pancreas compared with
tumors located in the head of the pancreas (P<0.01) and lymph
node metastasis (P=0.02). The patients with tumors that did not
express B7-H3 and B7-H4 had an improved prognosis compared with the
patients with tumors that did express 1 or 2 of the proteins
(P<0.01).
Following adjustments for tumor location and lymph
node metastasis, the patients with tumors that expressed the 2
proteins B7-H3 and B7-H4 were at an increased risk of mortality
compared with the patients with tumors that expressed only 1
protein or did not express either proteins (HR, 0.17; 95% CI,
0.03–0.94; P=0.04; Table II). In
addition, patients with tumors that expressed B7-H4 were at an
increased risk of mortality compared with the patients with tumors
that did not express B7-H4 (HR, 0.15; 95% CI, 0.03–0.80; P=0.03;
Table II).
 | Table II.Multivariate backward stepwise Cox's
proportional hazards analyses demonstrating clinicopathological
parameters that were associated with a poorer overall survival time
in patients with pancreatic cancer. |
Table II.
Multivariate backward stepwise Cox's
proportional hazards analyses demonstrating clinicopathological
parameters that were associated with a poorer overall survival time
in patients with pancreatic cancer.
| Clinicopathological
parameter | HR | 95% CI | P-value |
|---|
| B7-H3 and B7-H4
expression | 0.17 | 0.03–0.94 | 0.04 |
| B7-H4
expression | 0.15 | 0.03–0.80 | 0.03 |
| Lymph node
metastasis | 1.77 |
0.15–20.77 | 0.65 |
| Tumor located in
body or tail of pancreas | 0.73 | 0.17–3.20 | 0.68 |
Discussion
B7-H3 and B7-H4 are two novel members of the B7
superfamily of peripheral membrane proteins that are expressed on
APCs and have been implicated in tumor immunogenicity and cancer
development (13,26,27). The
present study demonstrated that B7-H3 and B7-H4 were expressed in
the cytoplasm and membrane of cancerous and non-cancerous
pancreatic cells, and that B7-H4 expression or the combination of
B7-H3 and B7-H4 expression in cancerous cells was associated with a
decreased overall survival time, independent of other prognostic
factors that are also associated with B7-H3 and B7-H4 expression.
Although other studies have demonstrated an association between
B7-H3 or B7-H4 expression and clinicopathological parameters, in
particular that increased B7-H1 expression in PC tissues is
associated with a decreased overall survival time (28), the present study identified that an
expression of B7-H4 and a simultaneous combined expression of B7-H3
and B7-H4 are independent predictors of a poor prognosis in
patients with PC.
Previous evidence (29) indicates that B7 family molecules are
expressed in PC and that their interactions with corresponding
receptors are directly associated with the T cell engagement of an
antigen. An upregulation of B7-H3 and B7-H4 expression in tumor
tissue was detected in non-small cell lung (13) and prostate cancer (15) in previous studies; however, only a few
studies have evaluated B7-H3 or B7-H4 expression levels in PC
(30). Yamato et al (16) identified that B7-H3 expression in PC
was associated with an advanced tumor stage and lymph node
metastasis, and Awadallah et al (21) reported that B7-H4 was expressed on
cytological specimens, which were obtained by endoscopic
ultrasound-guided fine needle aspiration and surgical specimens
from patients with PC. The overall expression of B7-H4 in benign
tissues was relatively decreased compared with that observed in the
majority of carcinoma patients. In the survival and correlation
analyses of 24 patients (17 surgically resected cases and 7 biopsy
cases) that succumbed to pancreatic adenocarcinoma, there was no
statistically significant association identified between patient
survival rate and the proportion of cells that stained positively
for B7-H4 expression (P=0.55) or the intensity of B7-H4 staining
(P=0.26) (21). The present study
demonstrated that B7-H3 and B7-H4 expression were associated with
certain clinicopathological features of patients with PC, including
the increased expression of B7-H3 in an early TNM stage (stage I or
II; P<0.01), the increased expression of B7-H4 associated with
tumors located in the body and tail of the pancreas compared to
those located in the head of the pancreas (P<0.01) and lymph
node metastasis (P=0.02).
The prognostic role of B7-H3 in cancer is unclear.
In gastric cancer (12), an increased
B7-H3 expression is revealed to be associated with an improved
prognosis compared with a decreased B7-H3 expression; however, in
non-small cell lung cancer (13),
renal cell carcinoma (14), and
prostate cancer (15), B7-H3 served
as a marker of poor prognosis or disease metastasis. In the present
study, B7-H3 was more likely to be expressed in PC tissue compared
with normal pancreatic tissue, which is consistent with a previous
study (16). Although the present
findings did not demonstrate any survival benefit from the presence
of B7-H3 expression, it was observed that B7-H3 expression was
increased in late-stage tumors (T2-T4) compared to early-stage
tumors (T1) and in tumors that had not metastasized to the lymph
nodes compared with tumors that had metastasized (P<0.01 and
P=0.03, respectively). These results suggest that B7-H3 may be
involved in the development of human PC. However, it is unclear why
B7-H3 expression was not a predictor of OS time, considering its
positive correlation with B7-H4 expression as well as the decreased
OS time observed in patients with tumors that were positive for a
combination of B7-H3 and B7-H4 expression. The inconsistent
association between B7-H3 expression and prognosis in PC and other
cancers may be partly explained by an unknown receptor for B7-H3
(27,31) and the complex tumor microenvironment
(32).
The present study revealed that the presence of
solitary B7-H4 expression or a combination of B7-H3 and B7-H4
expression in human PC may serve as independent predictors of poor
prognosis. Mugler et al (33)
and Simon et al (34)
hypothesized that B7-H4 may induce immune evasion through the
suppression of T cell activation and cytokine secretion and the
development of cytotoxicity. In vitro and in vivo
studies (35) have suggested that
B7-H4 overexpression in numerous tumor types may allow tumors to
avoid eliciting an antitumor immune response. Although the present
results suggest that solitary B7-H4 expression and a combination of
B7-H3 and B7-H4 expression may be prognostic factors for PC, the
exact mechanisms by which expression of these proteins leads to a
decreased survival time remains to be clarified.
A limitation of the present study was the relatively
small sample size, which may lead to an overestimation of the
magnitude of the association between variables. Considering the
potential prognostic value of B7-H3 and B7-H4 in patients with PC,
which would aid in the clarification of the most effective
treatments to administer to patients, additional retrospective
studies of a larger scale are required to confirm the association
that the present study observed between the expression of B7-H3 and
B7-H4 and the clinical outcomes in PC patients.
In conclusion, the present findings indicate that
B7-H3 and B7-H4 are more likely to be expressed in PC tissue
compared with normal pancreatic tissue, and that solitary B7-H4
expression and a combination of B7-H3 and B7-H4 expression are
associated with a decreased OS time in patients with PC. Therefore,
solitary B7-H4 and a combined B7-H3 and B7-H4 expression in PC
tissues may be useful independent predictors of prognosis.
Large-scale studies are required to validate these findings.
Acknowledgements
The authors would like to thank Dr Lingchuan Guo
(The First Affiliated Hospital of Soochow University, Suzhou,
China) for providing technical assistance and Ms. Erica Goodoff
(The University of Texas MD Anderson Cancer Center, Houston, USA)
for editing the manuscript. The present study was supported by the
National Natural Science Foundation of China (grant nos. 30901765,
81272542 and 81101867), Medical Scientific Research Project of
Jiangsu Provincial Bureau of Health (grant no. Z201206), China
International Medical Foundation (grant no. CIMF-F-H001-057),
Special Foundation of Wu Jieping Medical Foundation for Clinical
Scientific Research (grant no. 320.6753.1225), Science and
Education for Health Foundation of Suzhou for Youth (grant no.
SWKQ1003) and Science and Technology Project Foundation of Suzhou
(grant no. SYS201112).
Glossary
Abbreviations
Abbreviations:
|
PC
|
pancreatic cancer
|
|
OS
|
overall survival
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katz MH, Pisters PW, Evans DB, Sun CC, Lee
JE, Fleming JB, Vauthey JN, Abdalla EK, Crane CH, Wolff RA, et al:
Borderline resectable pancreatic cancer: The importance of this
emerging stage of disease. J Am Coll Surg. 206:833–846. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geer RJ and Brennan MF: Prognostic
indicators for survival after resection of pancreatic
adenocarcinoma. Am J Surg. 165:68–72. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagaraj S, Gupta K, Pisarev V, Kinarsky L,
Sherman S, Kang L, Herber DL, Schneck J and Gabrilovich DI: Altered
recognition of antigen is a mechanism of CD8+ T cell tolerance in
cancer. Nat Med. 13:828–835. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zou W: Immunosuppressive networks in the
tumour environment and their therapeutic relevance. Nat Rev Cancer.
5:263–274. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greenwald RJ, Freeman GJ and Sharpe AH:
The B7 family revisited. Annu Rev Immunol. 23:515–548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Subudhi SK, Alegre ML and Fu YX: The
balance of immune responses: Costimulation verse coinhibition. J
Mol Med Berl. 83:193–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Driessens G, Kline J and Gajewski TF:
Costimulatory and coinhibitory receptors in anti-tumor immunity.
Immunol Rev. 229:126–144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chapoval AI, Ni J, Lau JS, Wilcox RA,
Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K and Chen L:
B7-H3: A costimulatory molecule for T cell activation and IFN-gamma
production. Nat Immunol. 2:269–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prasad DV, Nguyen T, Li Z, Yang Y, Duong
J, Wang Y and Dong C: Murine B7-H3 is a negative regulator of T
cells. J Immunol. 173:2500–2506. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suh WK, Gajewska BU, Okada H, Gronski MA,
Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, et
al: The B7 family member B7-H3 preferentially down-regulates T
helper type 1-mediated immune responses. Nat Immunol. 4:899–906.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu
KF, Zhao JM, Zhang GB and Zhang XG: Relationship between
co-stimulatory molecule B7-H3 expression and gastric carcinoma
histology and prognosis. World J Gastroenterol. 12:457–459.
2006.PubMed/NCBI
|
|
13
|
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R,
Lefvert AK and Wang X: B7-H3 and B7-H4 expression in non-small-cell
lung cancer. Lung Cancer. 53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crispen PL, Sheinin Y, Roth TJ, Lohse CM,
Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich
BC, et al: Tumor cell and tumor vasculature expression of B7-H3
predict survival in clear cell renal cell carcinoma. Clin Cancer
Res. 14:5150–5157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zang X, Thompson RH, Al-Ahmadie HA, Serio
AM, Reuter VE, Eastham JA, Scardino PT, Sharma P and Allison JP:
B7-H3 and B7x are highly expressed in human prostate cancer and
associated with disease spread and poor outcome. Proc Natl Acad Sci
USA. 104:19458–19463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamato I, Sho M, Nomi T, Akahori T,
Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H and Nakajima Y:
Clinical importance of B7-H3 expression in human pancreatic cancer.
Br J Cancer. 101:1709–1716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prasad DV, Richards S, Mai XM and Dong C:
B7S1, a novel B7 family member that negatively regulates T cell
activation. Immunity. 18:863–873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tringler B, Zhuo S, Pilkington G, Torkko
KC, Singh M, Lucia MS, Heinz DE, Papkoff J and Shroyer KR: B7-H4 is
highly expressed in ductal and lobular breast cancer. Clin Cancer
Res. 11:1842–1848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tringler B, Liu W, Corral L, Torkko KC,
Enomoto T, Davidson S, Lucia MS, Heinz DE, Papkoff J and Shroyer
KR: B7-H4 overexpression in ovarian tumors. Gynecol Oncol.
100:44–52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krambeck AE, Thompson RH, Dong H, Lohse
CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC and Kwon
ED: B7-H4 expression in renal cell carcinoma and tumor vasculature:
Associations with cancer progression and survival. Proc Natl Acad
Sci USA. 103:10391–10396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Awadallah NS, Shroyer KR, Langer DA,
Torkko KC, Chen YK, Bentz JS, Papkoff J, Liu W, Nash SR and Shah
RJ: Detection of B7-H4 and p53 in pancreatic cancer: Potential role
as a cytological diagnostic adjunct. Pancreas. 36:200–206. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: Exocrine pancreas. AJCC Cancer
Staging Manual (6th). (New York, NY). Springer. 157–164. 2002.
|
|
23
|
Klöppel G, Hruban RH, Longneck DS, Adler
G, Kern SE and Partanen TJ: Tumours of the exocrine pancreas. In:
World Health Organization Classification of Tumours. Pathology and
Genetics of Tumours of the Digestive System. Hamilton SR and
Aaltonen LA: 2:(Lyon). IARC Press. 219–252. 2000.
|
|
24
|
Zhou YH, Chen YJ, Ma ZY, Xu L, Wang Q,
Zhang GB, Xie F, Ge Y, Wang XF and Zhang XG: 4IgB7-H3 is the major
isoform expressed on immunocytes as well as malignant cells. Tissue
Antigens. 70:96–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan
M, Shan BE, Lu BF and Zhang XG: B7-H4 expression associates with
cancer progression and predicts patient's survival in human
esophageal squamous cell carcinoma. Cancer Immunol Immunother.
60:1047–1055. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fauci JM, Straughn JM Jr, Ferrone S and
Buchsbaum DJ: A review of B7-H3 and B7-H4 immune molecules and
their role in ovarian cancer. Gynecol Oncol. 127:420–425. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yi KH and Chen L: Fine tuning the immune
response through B7-H3 and B7-H4. Immunol Rev. 229:145–151. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen XL, Yuan SX, Chen C, Mao YX, Xu G and
Wang XY: Expression of B7-H1 protein in human pancreatic carcinoma
tissues and its clinical significance. Ai Zheng. 28:1328–1332.
2009.(In Chinese). PubMed/NCBI
|
|
29
|
Nomi T, Sho M, Akahori T, Hamada K, Kubo
A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M and
Nakajima Y: Clinical significance and therapeutic potential of the
programmed death-1 ligand/programmed death-1 pathway in human
pancreatic cancer. Clin Cancer Res. 13:2151–2157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qian Y, Hong B, Shen L, Wu Z, Yao H and
Zhang L: B7-H4 enhances oncogenicity and inhibits apoptosis in
pancreatic cancer cells. Cell Tissue Res. 353:139–151. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roth TJ, Sheinin Y, Lohse CM, Kuntz SM,
Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML,
et al: B7-H3 ligand expression by prostate cancer: A novel marker
of prognosis and potential target for therapy. Cancer Res.
67:7893–7900. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP,
Wu C and Zheng L: Activated monocytes in peritumoral stroma of
hepatocellular carcinoma foster immune privilege and disease
progression through PD-L1. J Exp Med. 206:1327–1337. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mugler KC, Singh M, Tringler B, Torkko KC,
Liu W, Papkoff J and Shroyer KR: B7-H4 expression in a range of
breast pathology: Correlation with tumor T cell infiltration. Appl
Immunohistochem Mol Morphol. 15:363–370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Simon I, Katsaros D, de la Longrais
Rigault I, Massobrio M, Scorilas A, Kim NW, Sarno MJ, Wolfert RL
and Diamandis EP: B7-H4 is over-expressed in early-stage ovarian
cancer and is independent of CA125 expression. Gynecol Oncol.
106:334–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi IH, Zhu G, Sica GL, Strome SE,
Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K and Chen L: Genomic
organization and expression analysis of B7-H4, an immune inhibitory
molecule of the B7 family. J Immunol. 171:4650–4654. 2003.
View Article : Google Scholar : PubMed/NCBI
|