Introduction
Gastric cancer (GC) is a leading cause of
cancer-associated mortality worldwide, is responsible for 700,000
mortalities annually, and remains highly prevalent in numerous
regions of Asia, Eastern Europe and South America (1). The majority of patients with GC are
diagnosed at an advanced stage of disease, and the overall 5-year
survival rate for patients with resectable GC remains low at
10–30%, despite clinical advances in surgery and therapy (2,3). The
elucidation of molecules and signaling pathways that drive
aggressive and metastatic GC is likely to offer novel avenues for
the early identification and therapeutic intervention of GC.
Tumor necrosis factor (TNF)-α-induced protein 8
(TIPE), also termed squamous cell carcinoma-stage 2 protein, cyclin
G2-1 and nuclear factor-κB-inducible death effector
domain-containing protein, was identified in a comparison between
the expression profile of a primary human head and neck squamous
cell carcinoma (HNSCC) cell line and the matching metastatic
HNSCC-derived cell line (4). TIPE is
a 21 kDa cytosolic TNF-α-inducible molecule and a member of the
Fas-associated death domain-like interleukin-1β-converting
enzyme-like inhibitory protein family of cell death inhibitory
proteins (5). The overexpression of
TIPE contributes to enhanced DNA synthesis, cell proliferation and
an inhibition of the activities of the apoptotic enzymes caspase 8
and caspase 3 (6,7). TIPE mRNA expression is induced by the
activation of transcription factor nuclear factor (NF)-κB and TNF-α
in human cancer cells, vascular endothelial cells and primary
rheumatoid arthritis synovial fibroblasts. The TNF-α-mediated
induction of TIPE mRNA may be reversed by IκBα, an inhibitor of
NF-κB, and the expression of TIPE in NF-κB-null cells inhibits
TNF-α-induced apoptosis (8).
Collectively, the aforementioned functional studies indicate that
TIPE is an oncogenic factor that is important for tumor
progression, and that the exogenous expression of TIPE enhances
cell survival.
With regard to the possible role of TIPE in
tumorigenesis and tumor progression, TIPE overexpression has
exhibited clinical relevance by frequently occurring in several
types of cancer tissues, including in lung, colon, prostate and
cervical cancer, esophageal squamous cell carcinoma and papillary
thyroid carcinoma (9,10). The exogenous expression of TIPE in
tumor cells is associated with enhanced proliferation, cell
migration and tumor growth (11). The
knockdown of TIPE expression in tumor cells reduces tumorigenicity,
which indicates that TIPE may be important in oncogenesis.
External-signal regulated kinase (ERK) is a cytosolic receptor
known to be pivotal in the nuclear protein import pathway in
numerous cancers. The ERK pathway is linked to cellular
proliferation, differentiation and apoptosis (12). Decoy receptor 3 (DcR3) is usually
expressed in tumor cells and competitively inhibits TNF signaling.
The overexpression of DcR3 in tumor cells protects the cells from
apoptosis (13). Therefore, DcR3 and
ERK are vital in the development of GC (14).
To the best of our knowledge, no studies on TIPE in
GC have been previously reported. The binding partners and
signaling pathways of TIPE are also unknown. In the present study,
the expression pattern of TIPE in GC and paracarcinoma tissues was
compared, the correlation between the expression levels of TIPE was
analyzed, and the clinical significance and expression of ERK and
DcR3 was assessed. The results indicated that TIPE may be important
in the progression of GC.
Patients and methods
Patients and tissues
A total of 30 GC tissues were obtained from 30
patients (27 male and 3 female) with stage III GC that had
previously undergone complete resection at the Zhongshan Hospital
(affiliated with Xiamen University; Xiamen, Fujian, China) between
the years 2012 and 2013. The paracarcinoma tissue that was selected
was located ≥5 cm away from the cancerous tissue. The tumor tissues
from which the DNA was isolated were fresh specimens that were
obtained during the resection surgery. DNA was isolated from the
tissues 20 min after resection. None of the patients had received
radiotherapy, chemotherapy or immunotherapy prior to the surgery.
All samples were obtained with patient consent and approval of the
Committee on Medical Ethics of Zhongshan Hospital, Xiamen
University.
Follow-up information was obtained from a review of
the patients' medical records. The mean age of the patients was
63.83 years (range, 43–88 years). The histological diagnosis and
grade of the differentiation were evaluated using hematoxylin and
eosin (H&E; Beyotime Institute of Biotechnology, Shanghai,
China)-stained sections, according to the World Health Organization
Guidelines of Classification (15).
All 30 specimens were revaluated with respect to histological
subtype, differentiation and tumor stage. The TNM staging system of
Union for International Cancer Control was used to classify
specimens (16).
Immunohistochemistry
The surgically excised tumor specimens were fixed
with 10% neutral formalin (Shanghai Jianglai Bio-Technology Co.,
Ltd., Shanghai, China), embedded in paraffin (ZhongYou Chem,
Xiamen, China), cut in a microtome (RM2235; Leica Biosystems
Nussloch GmbH, Nussloch, Germany) to the desired thickness of 4 µm,
and stained with H&E to confirm the tumor. Immunohistochemical
staining for TIPE was performed using the streptavidin-peroxidase
(ZSGB-BIO, Beijing, China) complex method. In brief, tissue
sections were deparaffinized in xylene and rehydrated in a series
of graded alcohol (100%, 95% and 80% for 5 min) using standard
procedures. The sections were placed into an enamel cylinder
(Shanghai Heqi Glassware Co., Ltd., Shanghai, China) that contained
10 mmol/l sodium citrate (pH 6.0; Sigma-Aldrich, St. Louis, MO,
USA), which was heated using a gas burner at 95°C for 5 min for
antigen unmasking. The sections were then immersed in 3% hydrogen
peroxide (Sangon Biotech Co., Ltd., Shanghai, China) to remove the
endogenous peroxidase. The sections were incubated with normal goat
serum (Shanghai Haoran Bio Technologies Co., Ltd., Shanghai, China)
to reduce nonspecific binding and then incubated at 4°C overnight
with rabbit anti-human TIPE polyclonal antibody (cat. no. ab166804;
dilution, 1:200; Abcam, Cambridge, MA, USA). The sections were then
washed with phosphate-buffered saline and incubated for 30 min with
biotinylated goat anti-rabbit secondary antibody at 37°C. The
substrate 3,3′-diaminobenzidine tetrahydrochloride, which was
dissolved in ultrapure water, was added in order to aid
visualization of the expression.
RNA extraction and reverse
transcription (RT)-polymerase chain reaction (PCR)
The total RNA was extracted from cells using TRIzol
(Thermo Fisher Scientific, Waltham, MA, USA), in order to measure
the TIPE/ERK/DcR3 gene copy number. The total RNA was extracted
from the tissue using the TRIzol total RNA extraction reagent
(CWbio, Beijing, China). The RNA quality and concentration were
measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher
Scientific). RT and PCR were performed using the Takara
PrimeScript™ RT-PCR kit (Takara Biotechnology Co., Ltd. Dalian,
Liaoning, China) and the 2xEasyTaq® PCR super mix (TransGen,
Beijing, China). Template cDNA was synthesized from 2.0 µg of the
total RNA using RT-PCR, which was performed using the Biometra
TAdvanced Thermal Cycler (Biometra GmbH, Göttingen, Germany).
Briefly, in a total volume of 20 µl, the samples were incubated at
94°C for 3 min, followed by 30 cycles of incubation at 94°C for 30
sec, 56°C for 30 sec and 72°C for 1 min, and then held at 72°C for
5 min. The sequences of the primer pairs are shown in Table I. The RT-PCR results were analyzed
using gel electrophoresis, the imaging outputs of which were
compared using a grayscale analysis against the corresponding
β-actin, and recorded for basic statistical analysis.
 | Table I.Primer sequences used for reverse
transcription polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription polymerase chain reaction.
| Primer pair | Sequence, 5′-3′ | Fragment size, base
pairs |
|---|
| TIPE |
| 132 |
| F |
CCCAGGGAAGTGGCTACAGA |
|
| R |
GCCTCCTTCTTGTTCTGGGT |
|
| ERK1 |
| 205 |
| F |
CCTGCTCATCAACACCACC |
|
| R |
CGTAGCCACATACTCCGTCA |
|
| ERK2 |
| 180 |
| F |
TCTTCCAGCCCTCCTTCCTG |
|
| R |
CGTTTCTGCGCCGTTAGGT |
|
| DcR3 |
| 161 |
| F |
GCAAAGCCAAGGATTCCCCCTG |
|
| R |
GGCACTGCTCTGAGCTGGAGCTG |
|
| β-actin |
| 164 |
| F |
AGCCATGTACGTAGCCATCC |
|
| R |
ACCCTCATAGATGGGCACAG |
|
Statistical analysis
All analyses were performed using SPSS version 20.0
for Windows (IBM SPSS, Armonk, NY, USA). The data were presented as
the mean ± standard deviation. The significance of the difference
between groups was assessed using the Student's two-tailed
t-test. Spearman's rank correlation coefficient was used to
analyze the correlation between TIPE, ERK and DcR3 expression.
P<0.05 was considered to indicate a statistically significant
difference. All mean values were calculated from at least three
independent experiments.
Results
Location of TIPE in stage III GC
patients
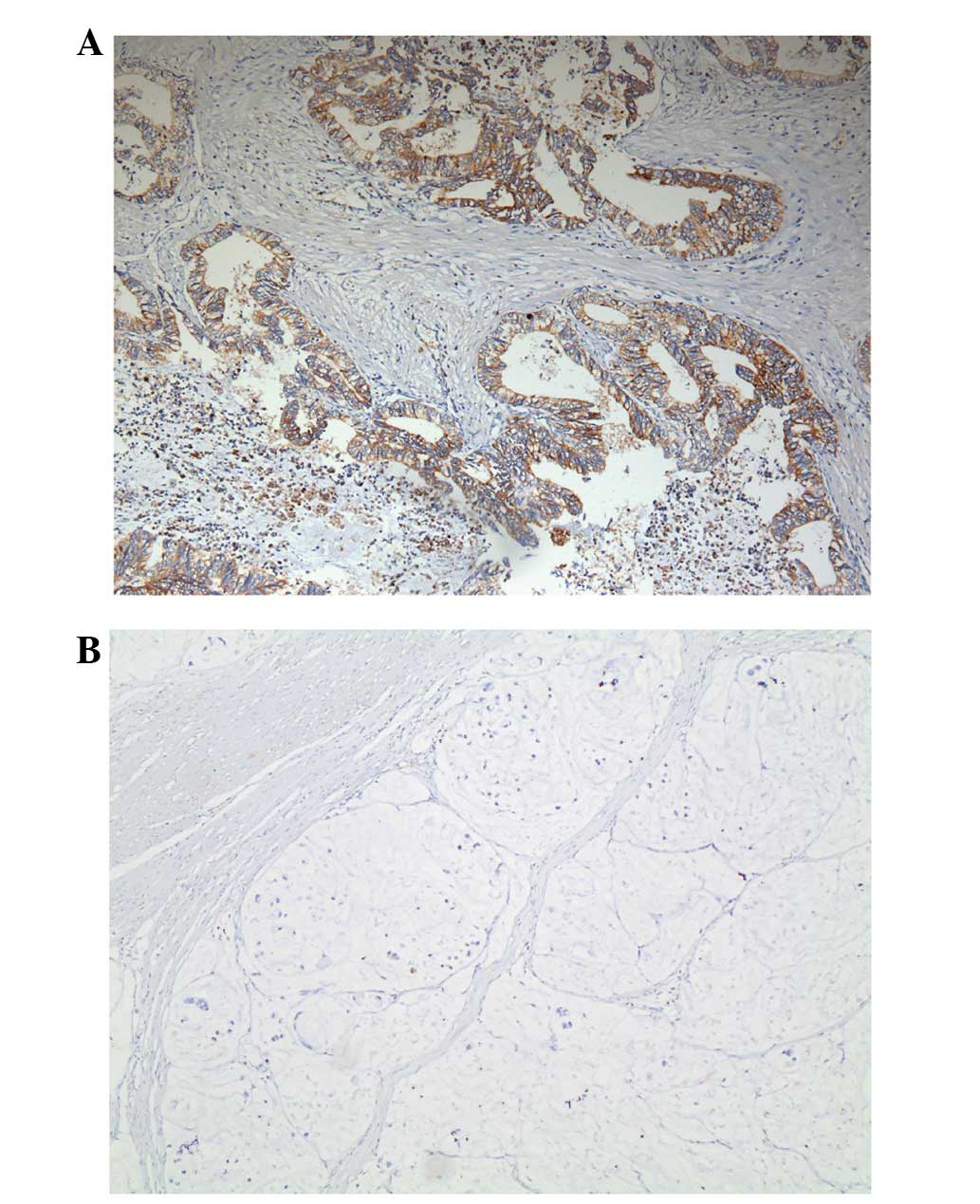
To examine the distribution of TIPE, tumors from 30
patients were tested using immunohistochemistry. The results
indicate that TIPE was expressed in the tumor tissues of the
majority of the patients. TIPE expression was indicated in the
cytoplasm and/or nucleolus of GC cells, and was not indicated in
normal gastric mucosa (Fig. 1).
TIPE mRNA expression in stage III GC
tissues
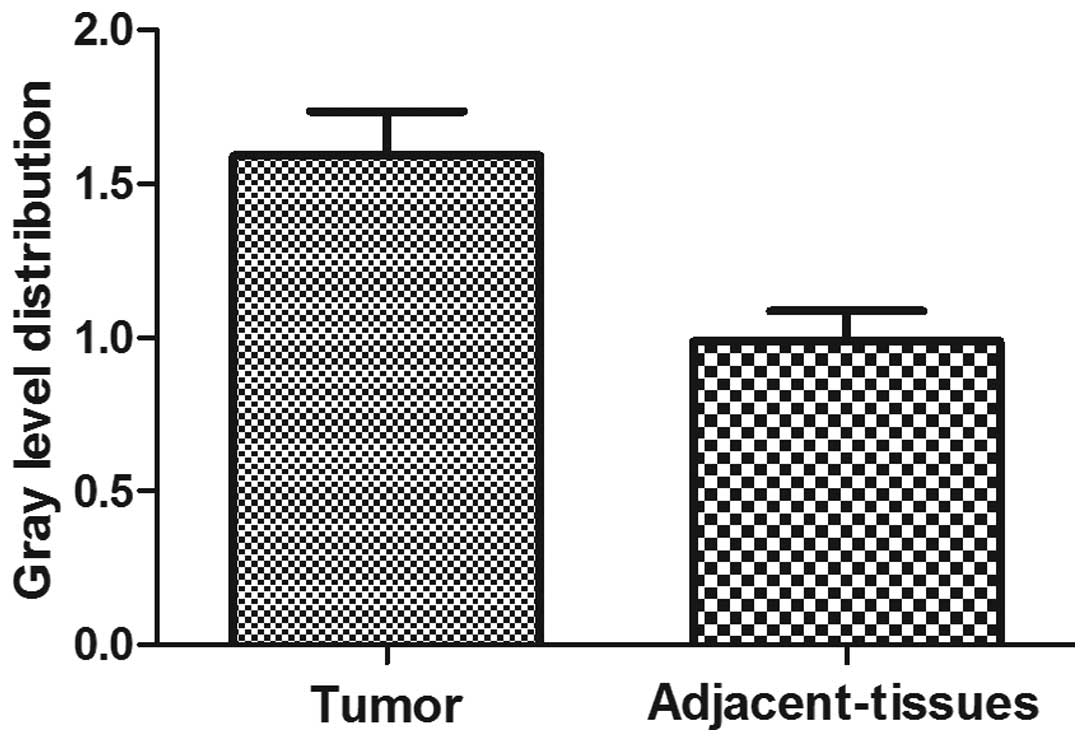
The analyses of the RT-PCR results were used to
confirm the mRNA levels. The mean expression value of TIPE mRNA in
cancer tissues (0.7945±0.1451), which was normalized by β-actin
gene expression, was significantly increased compared with the
value in tumor-adjacent normal gastric tissues (0.5421±0.0990;
P<0.05; comparative t-test). There was a 1.5-fold
difference between the TIPE expression in the tumor and normal
tissues (Fig. 2; Table II).
 | Table II.Distribution of TIPE mRNA in the
gastric tissues of 30 patients. |
Table II.
Distribution of TIPE mRNA in the
gastric tissues of 30 patients.
| Type of tissue | TIPE mRNA value |
|---|
| Gastric cancer | 0.7945±0.1451 |
| Adjacent | 0.5421±0.0990 |
Association between TIPE mRNA
expression and the clinicopathological characteristics of GC
In the present study, all patients were diagnosed
with pathological stage III GC; so the association of other
pathological stages with TIPE was not analyzed. The distribution of
TIPE status in GC according to the clinicopathological tests is
reported in Table II. Table III shows that no strong association
was observed between TIPE and gender, age, nodal status or
differentiation (P>0.05).
 | Table III.Distribution of TIPE mRNA in gastric
cancer patients, according to clinicopathological
characteristics. |
Table III.
Distribution of TIPE mRNA in gastric
cancer patients, according to clinicopathological
characteristics.
| Characteristics | No. of patients | TIPE mRNA value | P-value |
|---|
| Gender |
|
| 0.532 |
| Male | 27 | 1.5218±0.6817 |
|
|
Female | 3 | 2.2047±1.5754 |
|
| Age, years |
|
| 0.595 |
|
<65 | 17 | 1.6322±1.0004 |
|
|
65–75 | 9 | 1.6806±0.4414 |
|
|
>75 | 4 | 1.2069±0.1187 |
|
| Nodal status |
|
| 0.559 |
| N1 | 7 | 1.3865±0.4333 |
|
| N2 | 8 | 1.8311±1.4112 |
|
| N3 | 15 | 1.5565±0.4200 |
|
|
Differentiation |
|
| 0.337 |
|
Moderate | 11 | 1.4038±0.4502 |
|
|
Poor | 19 | 1.6979±0.9333 |
|
The association of TIPE with DcR3 and
ERK in GC
In order to identify the signaling molecules that
are associated with TIPE, the expression of DcR3, ERK1 and ERK2 in
GC tissues and tumor-adjacent normal gastric tissues was measured
using RT-PCR. DcR3 mRNA and ERK1/2 mRNA was detected in tumor
samples, and the mean expression value of DcR3 mRNA and ERK1/2 mRNA
in cancer tissues was significantly increased compared with the
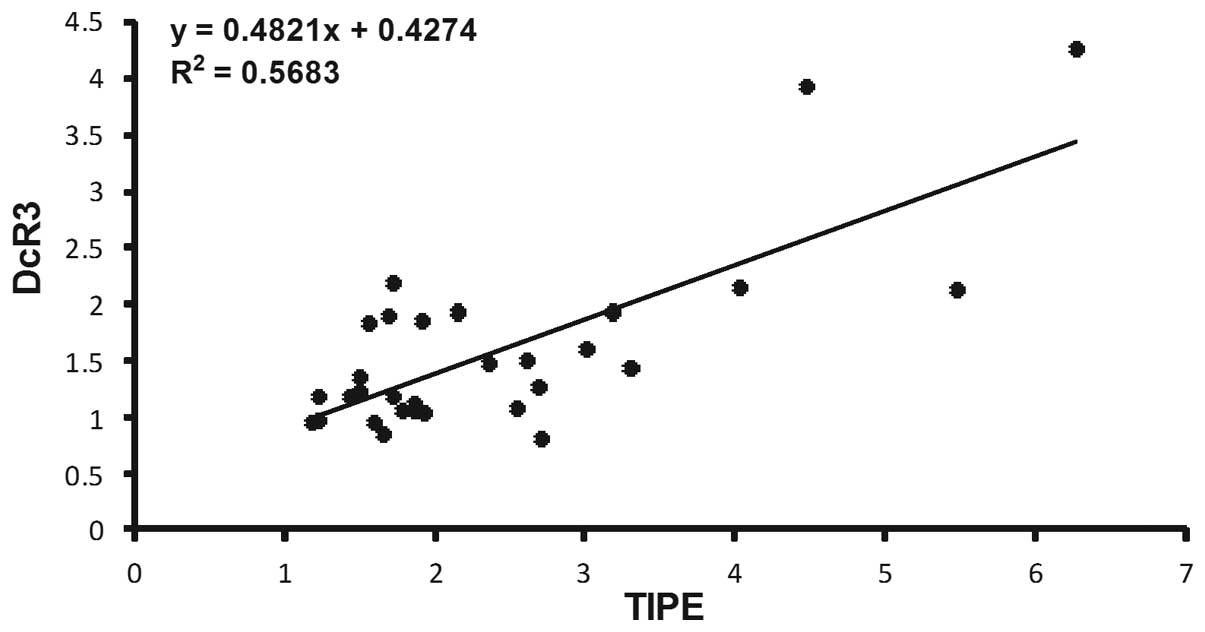
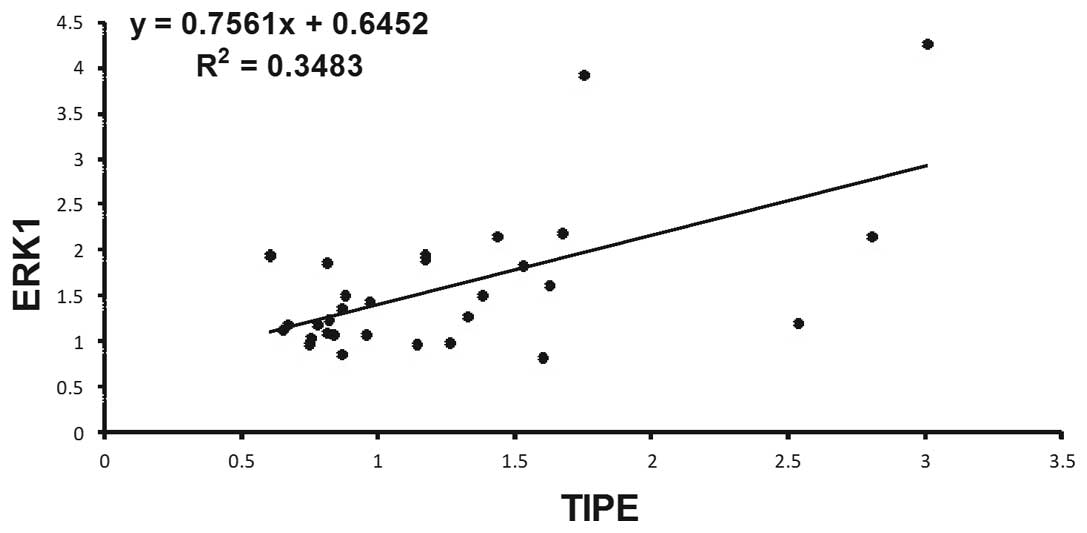
value in non-cancerous tissues (P<0.05; Table IV). Spearman's rank correlation
coefficient analysis (Figs. 3 and
4) revealed that TIPE expression was
positively correlated with DcR3 (r=0.733, P<0.05) and ERK1
(r=0.590, P<0.05) expression, but was not significantly
correlated with ERK2 expression (P>0.05). In every case of high
TIPE expression, DcR3 and ERK1 expression was increased compared
with non-tumor tissues.
 | Table IV.Distribution of DcR3, ERK1 and ERK2
mRNA in the gastric tissues of 30 patients. |
Table IV.
Distribution of DcR3, ERK1 and ERK2
mRNA in the gastric tissues of 30 patients.
| Type of mRNA | Gastric tissue | mRNA value | P-value |
|---|
| DcR3 | Cancer | 2.1411±1.2424 | 0.023 |
|
| Normal | 1.8169±0.4850 |
|
| ERK1 | Cancer | 1.2497±0.6202 | 0.033 |
|
| Normal | 0.9656±0.6086 |
|
| ERK2 | Cancer | 1.1598±0.5080 | 0.012 |
|
| Normal | 0.8489±0.3659 |
|
Discussion
To the best of our knowledge, the present study is
the first to report that, unlike in normal gastric tissues, TIPE
expression is upregulated at the protein and mRNA levels in GC
tissues. In the present study, the cytoplasmic and nuclear
localization and high expression levels of TIPE were not
significantly associated with gender, age, nodal status or
differentiation. In addition, TIPE overexpression was correlated
with DcR3 and ERK1 expression in GC. The present study revealed a
concomitant increase in TIPE and DcR3, ERK1 expression in cancer
tissues, suggesting that TIPE may be important in the occurrence
and progression of GC.
The increasing incidence, delayed detection and poor
prognosis of advanced GC underline the requirement for novel
diagnosis strategies, follow-up and treatment. The TIPE family is a
novel subfamily of death effector domain proteins that consists of
TIPE, TIPE1, TIPE2 and TIPE3, with TIPE being the first identified
member (17,18). TIPE was indicated to be expressed in
various human cancer cell lines; in increased levels in A549 lung
carcinoma, K562 chronic myelogenous leukemia and MOLT-4
lymphoblastic leukemia cells, and in lower levels in SW480
colorectal adenocarcinoma cells (19). TIPE overexpression was detected in
several human malignant tumors at the mRNA and protein level.
Positive correlations between TIPE and the histological grade,
residual tumor size, recurrence and metastases of the peritoneum
and lymph node have been demonstrated in the present study. In a
previous study, TIPE overexpression was indicated to be an
independent predictor of recurrent prostate cancer (8). In another study, functional variants of
TIPE were hypothesized to result in tumor progression and poor
outcomes for cervical cancer, by inhibiting the response of tumor
cells to platinum drugs, particularly cisplatin and nedaplatin
(13). Collectively, the
aforementioned studies indicate that TIPE may be important in
oncogenesis. However, the expression of TIPE and the correlation
with clinical pathological factors has not been defined in GC.
In the present study, protein expression in TIPE was
examined using immunohistochemistry, and mRNA levels were examined
using RT-PCR in 30 GC tissues. The results indicated that TIPE was
expressed in the cytoplasm and nucleolus of GC cells, and that TIPE
mRNA expression was significantly increased in GC compared with
normal gastric tissues. The results were consistent with a previous
finding that TIPE is overexpressed in human malignant tumors
(8–10,14).
However, no association was observed between TIPE mRNA expression
and nodal status or differentiation in the present study.
Considering that the participants of the present study were all
patients with stage III GC, additional studies of patients at
various pathological stages of the disease are of paramount
importance to confirming this conclusion, and if the sample size
was increased, the findings may vary.
DcR3, also termed TR6, is a member of the tumor
necrosis factor receptor superfamily, and is the decoy receptor for
Fas ligand, tumor necrosis factor superfamily member 14 and
TNF-like ligand 1A (20,21). The DcR3 gene is expressed at a low
levels in the human embryo, lung, brain, liver, spleen, stomach,
colon, lymph nodes and spinal cord, and is expressed at a high
levels in cancers, including gastrointestinal cancer,
hepatocellular carcinoma and pancreatic cancer (22–24. DcR3
overexpression is associated with cell proliferation, lymph node
metastasis, pathological stage and a significantly shortened
survival rate (13,25). Mitogen-activated protein kinases
(MAPKs) are an important group of serine and threonine signaling
kinases that activate certain transcription factors by causing a
cascade of protein phosphorylation through a variety of
extracellular stimuli. In addition, the extracellular
signal-regulated kinase (ERK) pathway is linked to cellular
proliferation and differentiation and the proinflammatory cellular
response. Previously, evidence has indicated that the expression of
ERK1/2 was increased in certain cancers, which correlated with the
TNM stages and prognosis (26,27). In
the present study, the expression of DcR3 and ERK1/2 mRNA was
detected in the same samples using RT-PCR. The results indicated
that the level of DcR3 and ERK1/2 mRNA in GC tissues is
significantly increased compared with normal gastric tissues.
Additionally, TIPE expression positively correlated with DcR3 and
ERK1 when analyzed with Spearman's rank correlation coefficient.
Therefore, the samples that demonstrated high TIPE mRNA expression
also demonstrated high DcR3 and ERK1 expression in stage III GC
tissues.
The present findings indicate that TIPE may be
important in the oncogenesis and progression of GC. TIPE may,
therefore, be considered as a novel marker and promising
therapeutic target in GC patients. However, the present study has a
number of limitations. The present study is a retrospective
analysis and studies a limited number of patients. The protein
expression was not examined using western blot analysis, and the
mechanism behind the findings was not examined due to limited time.
Therefore additional studies are required in order to examine a
larger sample of patients, and to explore the possible mechanism of
GC oncogenesis and progression caused by the overexpression of
TIPE.
Acknowledgements
This study was supported by the grants from the
National Natural Science Foundation of China (grant no. 81272720),
Public Projects of Fujian Province (grant no. 2016R1102) Fujian
Province Health Planning Commission medical innovation subject
(grant no. 2014-CXB-43).
References
|
1
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patel S, Wang FH, Whiteside TL and Kasid
U: Identification of seven differentially displayed transcripts in
human primary and matched metastatic head and neck squamous cell
carcinoma cell lines: Implications in metastasis and/or radiation
response. Oral Oncol. 33:197–203. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar D, Gokhale P, Broustas C,
Chakravarty D, Ahmad I and Kasid U: Expression of SCC-S2, an
antiapoptotic molecule, correlates with enhanced proliferation and
tumorigenicity of MDA-MB 435 cells. Oncogene. 23:612–616. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
You Z, Ouyang H, Lopatin D, Polver PJ and
Wang CY: Nuclear factor-kappa B-inducible death effector
domain-containing protein suppresses tumor necrosis factor-mediated
apoptosis by inhibiting caspase-8 activity. J Biol Chem.
276:26398–26404. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laliberté B, Wilson AM, Nafisi H, Mao H,
Zhou YY, Daigle M and Albert PR: TNFAIP8: A new effector for
Galpha(i) coupling to reduce cell death and induce cell
transformation. J Cell Physiol. 225:865–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang C, Kallakury BV, Ross JS, Mewani RR,
Sheehan CE, Sakabe I, Luta G, Kumar D, Yadavalli S, Starr J, et al:
The significance of TNFAIP8 in prostate cancer response to
radiation and docetaxel and disease recurrence. Int J Cancer.
133:31–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong QZ, Zhao Y, Liu Y, Wang Y, Zhang PX,
Jiang GY, Dong XJ, Cui QZ and Wang EH: Overexpression of SCC-S2
correlates with lymph node metastasis and poor prognosis in
patients with non-small-cell lung cancer. Cancer Sci.
101:1562–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan D, Zhu YQ, Guan LL and Wang J:
Upregulation of SCC-S2 in immune cells and tumor tissues of
papillary thyroid carcinoma. Tumour Biol. 35:4331–4337. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laliberté B, Wilson AM, Nafisi H, Mao H,
Zhou YY, Daigle M and Albert PR: TNFAIP8: A new effector for
Galpha(i) coupling to reduce cell death and induce cell
transformation. J Cell Physiol. 225:865–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang D, Ding Y, Luo WM, Bender S, Qian
CN, Kort E, Zhang ZF, VandenBeldt K, Duesbery NS, Resau JH and Teh
BT: Inhibition of MAPK kinase signaling pathways suppressed renal
cell carcinoma growth and angiogenesis in vivo. Cancer Res.
68:81–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Guo E, Yu J, Xie Q and Chen J: High
DcR3 expression predicts stage pN2 in GC. Hepatogastroenterology.
54:2172–2176. 2007.PubMed/NCBI
|
|
14
|
Yang D, Fan X, Yin P, Wen Q, Yan F, Yuan
S, Liu B, Zhuang G and Liu Z: Significance of decoy receptor 3
(Dcr3) and external-signal regulated kinase 1/2 (Erk1/2) in gastric
cancer. BMC Immunol. 13:282012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kleihues P and Sobin LH: World Health
Organization classification of tumours. Cancer. 88:28872000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sobin LH and Wittekind C: International
Union Against Cancer (UICC). TNM Classification of Malignant
Tumours (6th). (New Jersey). Wiley Blackwell, Hoboken. 2002.
|
|
17
|
Freundt EC, Bidere N and Lenardo MJ: A
different TIPE of immune homeostasis. Cell. 133:401–402. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun H, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, Shen H, Yang X and Chen
YH: TIPE2, a negative regulator of innate and adaptive immunity
that maintains immune homeostasis. Cell. 133:415–426. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar D, Whiteside TL and Kasid U:
Identification of a novel tumor necrosis factor-alpha-inducible
gene, SCC-S2, containing the consensus sequence of a death effector
domain of fas-associated death domain-like interleukin-
1beta-converting enzyme-inhibitory protein. J Biol Chem.
275:2973–2978. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Migone TS, Zhang J, Luo X, Zhuang L, Chen
C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, et al: TL1A is a
TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell
costimulator. Immunity. 16:479–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roth W, Isenmann S, Nakamura M, Platten M,
Wick W, Kleihues P, Bähr M, Ohgaki H, Ashkenazi A and Weller M:
Soluble decoy receptor 3 is expressed by malignant gliomas and
suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer
Res. 61:2759–2765. 2001.PubMed/NCBI
|
|
22
|
Pitti RM, Marsters SA, Lawrence DA, Roy M,
Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT,
et al: Genomic amplification of a decoy receptor for Fas ligand in
lung and colon cancer. Nature. 396:699–703. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bai C, Connolly B, Metzker ML, Hilliard
CA, Liu X, Sandig V, Soderman A, Galloway SM, Liu Q, Austin CP and
Caskey CT: Overexpression of M68/DcR3 in human gastrointestinal
tract tumors independent of gene amplification and its location in
a four-gene cluster. Proc Natl Acad Sci USA. 97:1230–1235. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsuji S, Hosotani R, Yonehara S, Masui T,
Tulachan SS, Nakajima S, Kobayashi H, Koizumi M, Toyoda E and Ito
D: Endogenous decoy receptor 3 blocks the growth inhibition signals
mediated by Fas ligand in human pancreatic adenocarcinoma. Int J
Cancer. 106:17–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu KY, Kwon B, Ni J, Zhai Y, Ebner R and
Kwon BS: A newly identified member of tumor necrosis factor
receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J
Biol Chem. 274:13733–13736. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song H, Lee AY, Jung H, Choi JH, Roh K, Ha
S, Kim KD, Bae KB, Kang MS, Park S, et al: A8, an anti-uPA
agonistic antibody, promotes metastasis of cancer cells via ERK
pathway. Monoclon Antib Immunodiagn Immunother. 33:312–318. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jerjees DAI, Alabdullah M, Alkaabi M,
Abduljabbar R, Muftah A, Nolan C, Green AR, Ellis IO and Rakha EA:
ERK1/2 is related to oestrogen receptor and predicts outcome in
hormone-treated breast cancer. Breast Cancer Res Treat. 147:25–37.
2014. View Article : Google Scholar : PubMed/NCBI
|