Introduction
The response rate of advanced epithelial ovarian
carcinoma treated with standard first-line platinum/taxane-based
chemotherapy is ~80% (1–5). However, the majority of patients will
relapse within 18–24 months (2–5).
Decision-making regarding the treatment of recurrent ovarian cancer
has been a continuous challenge. Since 1990, treatment selection
has been based on whether patients have platinum-sensitive or
platinum-resistant disease (6,7). For
patients relapsing >6 months after the completion of the initial
platinum-based chemotherapy, platinum-containing regimens are
given, as long as the patients have platinum-sensitive disease
(8). By contrast, for patients with
platinum-resistant or platinum-refractory disease, single-drug
regimens, including pegylated liposomal doxorubicin, gemcitabine or
topotecan are indicated (9).
In addition to cytotoxic drugs, the development of
molecular-targeted agents has emerged based on the increasing
knowledge of key biological pathways driving tumor progression
(10). Among the several targeted
therapies investigated, the most promising approach for treating
ovarian cancer is the inhibition of angiogenesis by bevacizumab, a
monoclonal antibody directed against vascular endothelial growth
factor (VEGF). Accordingly, the level of VEGF expression in ovarian
cancer has been associated with ascites formation and poor
prognosis (11–15).
Promising data regarding efficacy have emerged from
trials that have evaluated bevacizumab, alone or in combination,
for the management of patients with recurrent ovarian cancer
(16–20). Two phase III trials were recently
conducted and led to the approval of bevacizumab by the European
authorities for treating the first recurrence of platinum-sensitive
or platinum-resistant ovarian cancer (21,22).
It is well known that the administration of
bevacizumab is frequently associated with adverse events (AEs),
including hypertension and proteinuria. In patients who are
extensively pretreated or who exhibit pelvic disease or bowel
obstructive symptoms, bevacizumab may also result in bowel
perforation or fistula formation (17).
Importantly, the toxicities associated with the use
of bevacizumab may also be observed outside of clinical trials and
may be prominent, particularly when the drug is used in
non-approved regimens. To date, limited data are available
regarding the safety of this treatment in daily clinical practice
(23–30). Thus, the present study was designed to
assess the tolerance of bevacizumab in the management of recurrent
ovarian cancer in routine clinical practice. A retrospective
analysis was conducted using data from patients who were treated
for ovarian cancer in five French referral centers. The safety of
the treatment and its outcomes were evaluated from a cohort of
heavily pretreated patients, the majority of whom were ineligible
for inclusion in clinical trials.
Methods
Patient population
A total of 156 women with recurrent ovarian cancer
who had received bevacizumab between January 2006 and June 2009
were retrospectively identified from the institutional records of
five centers: Hôpital Tenon (Paris, France); Centre Léon Bérard
(Lyon, France); Institut Gustave Roussy (Villejuif, France);
Hôpital Cochin (Paris, France); and Hôtel-Dieu (Paris, France).
This study was approved by the French authority Commission
Nationale d'Informatique et des Libertés.
Data were collected using case report forms designed
for the current study. Detailed information regarding the history
of the disease and its management began at the time of clinical
presentation and diagnosis. Following first-line platinum-based
chemotherapy, patients were categorized as having
platinum-resistant or platinum-sensitive disease, depending on
whether recurrence was detected within 6 months or not,
respectively.
Bevacizumab was administered to patients who
relapsed following alternative chemotherapy. Bevacizumab was given
as a second-line therapy (in patients following a first relapse) or
as a subsequent line of treatment. It was given up to the eighth
line for patients who went through seven previous lines of
chemotherapy and underwent a seventh relapse at the time of
bevacizumab introduction.
Bevacizumab was either administered in combination
with other chemotherapy, or as a single agent. For certain
patients, bevacizumab was initially combined with alternative
chemotherapy, and subsequently used as a maintenance monotherapy
following the completion of the initial therapy.
Endpoints assessment
The safety profile of bevacizumab was the primary
endpoint of the study. Secondary endpoints included the usage
conditions of bevacizumab (e.g., dose schedule, concurrent
chemotherapy) and survival rates. During bevacizumab therapy, AEs
potentially attributable to the monoclonal antibody were described
according to the Common Terminology Criteria for Adverse Events,
Version 3.0 (31). The AEs of
particular interest in the present study were defined prior to data
collection, and focused on the following: Hypertension,
proteinuria, epistaxis, bleeding or hemorrhage, venous
thromboembolic event, arterial thromboembolic event, wound healing
complication, intestinal perforation, gastrointestinal (GI)
fistula, reversible posterior leak-encephalopathy syndrome and
pulmonary hypertension.
Overall survival (OS) was determined from the time
of bevacizumab introduction to the time of the mortality of the
patients (due to any cause). Progression-free survival (PFS) was
determined from the time of bevacizumab introduction to disease
progression or patient mortality. The data for patients who were
alive without undergoing disease progression were censored at the
date of their last assessment.
During bevacizumab treatment, disease progression
was evaluated by each treating physician through clinical
examination and/or carbohydrate antigen 125 (CA125) levels and/or
radiological examination. Biological progression was defined,
according to the Gynecological Cancer Intergroup criteria (32), as an increase of CA125 levels.
Determination of radiological and clinical progression relied on
physician judgement.
Statistical analysis
OS and PFS Kaplan-Meier estimates were determined
for the entire cohort and for various subgroups. The long-rank test
was used to compare data between subgroups.
The population who received bevacizumab for only one
relapse (n=136) served to identify predictive factors using the Cox
proportional hazards regression analysis. Predictive factors for
AEs were explored for all grades (grades 1–5) or for only severe
grades (grades 3–5). The same population served to establish
predictive factors for PFS and OS. The factors taken into account
for the univariate analysis of PFS and OS were platinum
sensitivity, first (or unique) line of bevacizumab, combination of
bevacizumab with other chemotherapy and bevacizumab dose scheduling
at the time of the first (or unique) bevacizumab administration.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using Statistical
Analysis System version 9.1 (SAS France, Brie-Comte-Robert,
France).
Results
Patients and study treatment
The majority of the patients who were included in
this study presented advanced disease (stage III or IV) at
diagnosis. Chemotherapy was the most common first-line treatment;
>70% of the patients received the standard chemotherapy based on
platinum and taxane, while only 2 patients were treated with
bevacizumab in this setting. The majority of the patients presented
platinum-sensitive disease at the time of their first relapse.
Platinum-sensitive disease was defined as recurrent disease
occurring >6 months following the end of the first-line of
platinum based chemotherapy.
Bevacizumab was administered to the 156 patients who
relapsed following chemotherapy. At the time of bevacizumab
introduction, the median number of previous lines of chemotherapy
received by patients was two. At that time, the majority of the
patients (for example, 95% of the treated patients in the second
line and 58.3% of the treated patients in the eighth line) had a
favorable performance status, corresponding to an Eastern
Cooperative Oncology Group/World Health Organization grade <2
(33) or a Karnofsky performance
status ≥70% (34). Only 9 patients
presented GI sub-obstructive disease when bevacizumab was
introduced.
The majority of patients (n=136) who received
bevacizumab were treated for a single relapse. Given that some
patients received bevacizumab for more than one relapse, a total of
181 cycles of bevacizumab were administered to the 156 patients.
The median number of relapses per patient was 4, with 33.3% of
patients having ≥6 relapses.
Bevacizumab was administered in combination with
alternative chemotherapy to 118 patients and continued as a
maintenance monotherapy for 42 patients. The median duration of the
maintenance therapy was 4 months (range, 0.2–27 months).
The median duration of bevacizumab treatment (alone
or in combination) was 6.3 months for patients treated in the
second line, and 3.4 months for patients treated in the fifth
line.
The doses of bevacizumab used were 2.5 and 5
mg/kg/week in 36.5% and 45.3% of the cases studied, respectively.
Various other bevacizumab regimens were used for the remaining
cases (18.2%). The median duration of follow-up after bevacizumab
introduction was 15.3 months (range, 0.3–47.9 months).
The clinical and demographic characteristics of the
studied patients are summarized in Table
I.
 | Table I.Clinical and demographic
characteristics of the study population (n=156). |
Table I.
Clinical and demographic
characteristics of the study population (n=156).
| A, At baseline |
|
|---|
|
|---|
| Characteristic | Value |
|---|
| Median age, years
(range) [n=156] | 55 (22–81) |
| FIGO stage, n (%)
[n=152] |
|
| I | 6
(3.9) |
| II | 5
(3.3) |
|
III | 111 (73.0) |
| IV | 30
(19.7) |
| Histological type
at diagnosis, n (%) [n=148] |
|
|
Serous | 115 (77.7) |
|
Mucinous | 3
(2.0) |
|
Endometrioid | 14 (9.5) |
| Clear
cell | 5
(3.4) |
|
Other | 11 (7.4) |
| Histological grade
at diagnosis, n (%) [n=100] |
|
| I | 12 (12.0) |
| II | 36 (36.0) |
|
III | 52 (52.0) |
| Initial surgery, n
(%) [n=154] |
|
| Initial
debulking | 97 (63.0) |
| Optimal
[n=91] | 52 (57.1) |
|
Suboptimal [n=91] | 39 (42.8) |
|
Intestinal resection | 36 (30.5) |
| First-line
chemotherapy, n (%) [n=154] |
|
|
Paclitaxel/platinum | 113 (73.4) |
| Platinum
sensitivity, n (%) [n=148] |
|
|
Resistant | 54
(36.5) |
|
Sensitive | 94
(63.5) |
| Prior medical
history, n (%) |
|
| GI
[n=156] | 31 (19.9) |
|
Cardiovascular [n=156] | 44 (28.2) |
|
Hypertension [n=154] | 30 (19.5) |
|
Proteinuria [n=52] | 1
(1.9) |
|
| B, At bevacizumab
introduction |
|
| Median previous
chemotherapies, n (range) [n=156] | 2
(0–12) |
| Median relapses per
patient, n (range) [n=156] | 4
(1–15) |
| Site of relapse, n
(%) [n=181] |
|
|
Peritoneum | 101 (55.8) |
| Lymph
node | 64
(35.5) |
|
Liver | 25
(13.8) |
|
Lung | 17
(9.4) |
|
Pelvis | 12
(6.6) |
|
Other | 11
(6.1) |
| Ascites, n (%)
[n=181] | 48 (26.5) |
| Pleural effusion, n
(%) [n=181] | 21 (11.6) |
| GI obstructive
syndrome, n (%) [n=181] | 9
(5.0) |
| Chemotherapy
combined with bevacizumab, n (%) [n=181] | 151 (83.4) |
|
Taxane | 67 (37.2) |
|
Platinum | 60 (33.3) |
|
Gemcitabine | 28 (15.5) |
|
Pegylated liposomal
doxorubicin | 27 (15.0) |
|
Other | 22 (12.2) |
| Bevacizumab alone,
n (%) | 30 (16.6) |
Safety
At least one AE (all grades included) that was
possibly due to bevacizumab was observed for 110 patients (70.5%)
among the 156 patients participating to the study and during the
181 cycles administered. AEs of grades 3–5 were observed in 43
cases (29.5%; Table II).
 | Table II.Adverse events of particular interest
potentially associated with bevacizumab treatment (n=156). |
Table II.
Adverse events of particular interest
potentially associated with bevacizumab treatment (n=156).
|
| Patients, n
(%) |
|---|
|
|
|
|---|
| Event type | Any grade | Grade 3–5 |
|---|
| Hypertension | 65 (41.7) | 18
(11.3)a |
| Proteinuria | 42 (27.9) | 7
(4.5) |
| Epistaxis | 43 (27.6) | 1
(0.6)a |
| Bleeding or
hemorrhage | 15 (9.6) | 4
(2.6) |
| Venous
thromboembolic event | 5
(3.2) | 3
(1.9) |
| Arterial
thromboembolic event | 2
(1.3) | 2
(1.3) |
| Wound healing
complication | 1
(0.6) | 0
(0.0) |
| Gastrointestinal
fistula | 5
(3.2) | 5
(3.2) |
| Reversible
posterior leak-encephalopathy syndrome | 2
(1.3) | 2
(1.3) |
| Pulmonary
hypertension | 1
(0.6) | 1
(0.6) |
None of the patients experienced congestive heart
failure. There were 4 treatment-related mortalities. Causes of
mortality included pulmonary hypertension (1 patient), bowel
perforation (1 patient), GI hemorrhage (1 patient) and pulmonary
embolism (1 patient). The latter two patients had a history of
deep-vein thrombosis and received anticoagulation therapy. All
mortalities occurred in patients who underwent a fifth or sixth
relapse. There were 2 mortalities (from a GI hemorrhage and from a
venous thromboembolic event) that occurred during concomitant
bevacizumab/taxane therapy, and 2 mortalities (from pulmonary
hypertension and from bowel perforation) during bevacizumab
monotherapy.
On univariate analysis (performed using a cut-off
point of P<0.15) identified three predictive parameters for
bevacizumab-associated AEs: Bevacizumab dose [odds ratio (OR),
1.143; 95% confidence interval (CI), 1.034–1.264; P=0.0091],
peritoneal relapse (OR, 1.829; 95% CI, 0.835–4.005; P=0.1310) and
history of hypertension (OR, 3.377; 95% CI, 0.944–12.082;
P=0.0613). However, using a cut-off point of P<0.05, only
bevacizumab dose remained a significant predictive factor on
multivariate analysis (OR, 1.190; 95% CI, 1.065–1.330; P=0.0021),
while peritoneal relapse (OR, 1.424; 95% CI, 0.616–3.294; P=0.4087)
and history of hypertension (OR, 3.517; 95% CI, 0.923–13.396;
P=0.0654) did not.
Considering severe AEs (grade 3–5), history of
hypertension (OR, 4.875; 95% CI, 1.906–12.472; P=0.0009) and
peritoneal relapse (OR, 3.224; 95% CI, 1.218–8.538; P=0.0185) were
significant predictive factors in univariate analysis. Both history
of hypertension (OR, 3.959; 95% CI, 1.482–10.575; P=0.0060) and
peritoneal relapse (OR, 2.782; 95% CI, 1.024–7.560; P=0.0448)
remained significant on multivariate analysis.
Efficacy
At the end of the bevacizumab therapy, patients
underwent clinical and/or biological and/or radiological evaluation
of the disease. For the global cohort of patients (n=156), the
median PFS was 8.3 months (95% CI, 6.5–10.1 months) and the median
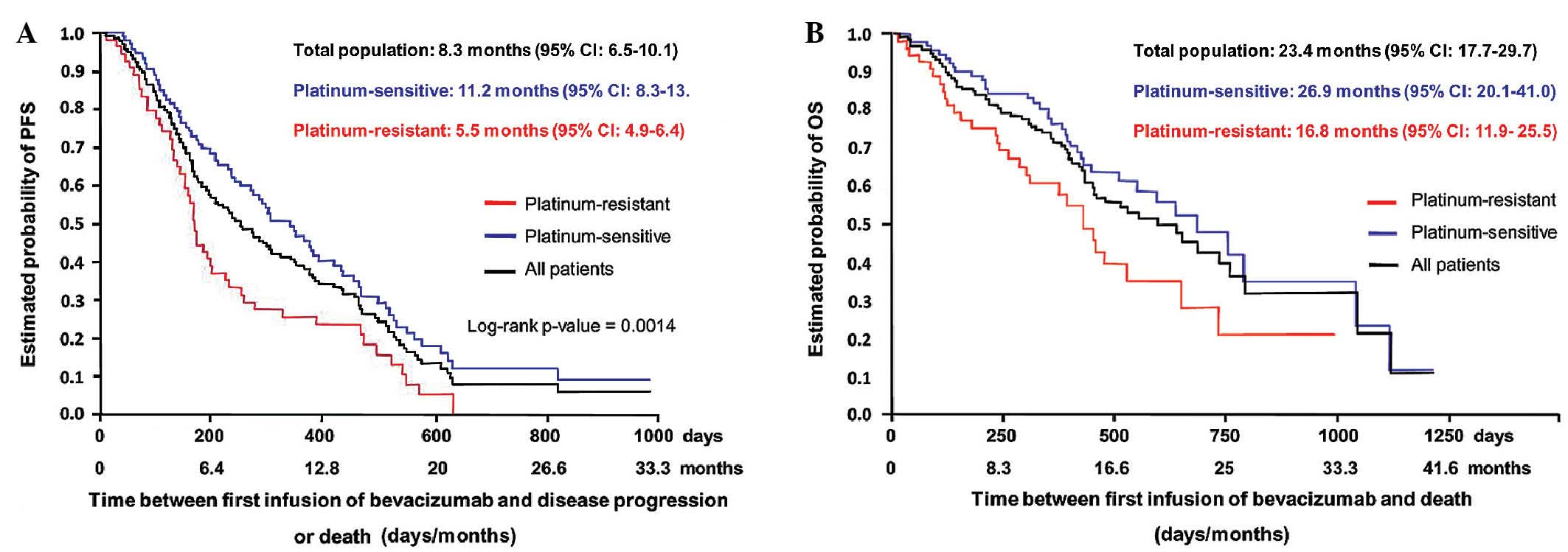
OS was 23.4 months (95% CI, 17.7–29.7 months) (Fig. 1). The 6-month PFS rate was 60.0% for
the entire cohort. The 6-month PFS rate was 79.1% for patients
treated for a first relapse, and 63.0, 44.4, 47.1, 42.9 and 58.0%
for patients treated for a second, third, fourth, fifth, and sixth
or more relapses, respectively. Median PFS and OS were 11.2 months
(95% CI, 8.3–13.8 months) and 26.9 months (95% CI, 20.1–41.0
months), respectively, in patients with platinum-sensitive disease,
while these values were 5.5 months (95% CI, 4.9–6.4 months) and
16.8 months (95% CI, 11.9–25.5 months), respectively, in patients
with platinum-resistant disease.
The significant factors predictive of longer PFS
time on univariate Cox regression analysis that were also confirmed
on multivariate analysis were platinum sensitivity [hazard ratio
(HR), 0.53; 95% CI, 0.36–0.77; P=0.001], early introduction of
bevacizumab as second- or third-line therapy (HR, 0.67; 95% CI,
0.46–0.99; P=0.042) and combination with chemotherapy (HR, 0.52;
95% CI, 0.31–0.86; P=0.011). The two significant factors for longer
OS time on multivariate analysis were platinum sensitivity (HR,
0.44; 95% CI, 0.27–0.73; P=0.002) and early introduction of
bevacizumab as second- or third-line therapy (HR, 0.37; 95% CI,
0.21–0.65; P<0.001).
Discussion
The present multi-center observational retrospective
study, designed to analyze the safety profile of bevacizumab in
relapsed ovarian cancer, identified predictive factors for the
development of severe AEs during bevacizumab treatment. The results
presented here are of particular interest, as this study included
patients treated with bevacizumab in clinical practice. Indeed,
data regarding treatment and outcomes of patients outside of
clinical trials remains scarce, even though it may more accurately
reflect the events that occur in the management and outcomes of
patient with ovarian cancer in normal clinical practice.
Patients included in the current study, in contrast
to those selected for clinical trials, did not conform to strict
mandatory inclusion criteria, diagnostic procedures, management and
follow-up protocols. The patients had a heterogeneous profile
according to their previous medical history, lines of treatment
prior to bevacizumab introduction and number of relapses. However,
the clinical profiles of the patients at relapse were relatively
homogeneous with regard to the sites of relapse and the general
conditions of the patients. A majority of them had
platinum-sensitive disease, and a low fraction exhibited GI
obstructive disease.
The primary aim of the present study was to describe
the safety profile of bevacizumab in routine practice. The most
common AEs observed were hypertension, proteinuria and epistaxis,
which are known side effects of bevacizumab treatment. The risk of
the occurrence of such events may be dose-associated, as indicated
by the multivariate Cox regression analysis and by previously
published data (35–37).
Hypertension is a frequent side effect of anti-VEGF
therapy (38). However, the impact of
baseline hypertension on the development of high-grade hypertension
during bevacizumab therapy is less well documented. For instance,
in a phase III trial of bevacizumab treatment in ovarian cancer
(39), and the studies included in
the meta-analyses conducted by Zhu et al (35) and by Ranpura et al (40), an increased risk of high-grade
hypertension associated with bevacizumab treatment was reported;
however, the histories of the hypertensive patients were not
analyzed. The OCEANS 20 trial reported similar findings (21). Indeed, while the baseline incidence of
hypertension in enrolled patients was similar in the different
groups of the study (37.6 vs. 39.7% for placebo and bevacizumab
arms, respectively), and while grade ≥3 was only reported for 1
patient in the placebo arm (compared to 43 patients in the
bevacizumab arm) the increased incidence of hypertension observed
during bevacizumab treatment was not analyzed with regard to the
hypertensive history of the patients.
In the current study, the incidence of history of
hypertension for the entire cohort was 19.5%. On multivariate
analysis, this feature was identified as an independent predictive
risk factor for the development of high-grade hypertension during
treatment. Therefore, previous history of hypertension must be
taken into account for the management of patients receiving
bevacizumab treatment for recurrent ovarian cancer.
All treatment-related mortalities in the current
cohort occurred in patients who were previously treated with ≥4
lines of chemotherapy. This observation confirms that heavy
pretreatment is an important factor involved in the occurrence of
serious and fatal AEs, in clinical practice or in clinical trials
(17,41).
GI perforation has been associated with the use of
bevacizumab in various types of cancer (36,37,42). In
trials where only ovarian cancer patients experiencing a first
relapse (21) or patients treated
with ≤2 regimens (43) were included,
GI perforation were reported. In the present study, the rates of GI
perforation (<1%) and GI fistula (3.2%) were low compared with
that of other studies conducted in relapsed patients heavily
pretreated with bevacizumab (17,44). This
is likely due to the good performance status and relatively low
incidence of pre-existing obstructive disease at the time of
bevacizumab introduction in the current patients. Indeed,
obstructive disease and peritoneal relapse have been reported to be
the primary risk factors for GI perforation (45,46). Thus,
the present results reveal that treating physicians are considering
these known risk factors for bevacizumab toxicity before
introducing the drug. Accordingly, multivariate analysis indicated
peritoneal relapse as an independent predictive factor for grade
3–5 AEs.
The secondary endpoints for the present study
included PFS and OS rates. Median PFS and OS were better for
patients with platinum-sensitive disease compared with those having
platinum-resistant disease. Accordingly, multivariate analysis
revealed that patients who benefitted the most from bevacizumab
were those treated in the second or third lines with the antibody,
and who presented a platinum-sensitive disease.
In summary, as previously reported by clinical
trials and other retrospective studies, the current findings
confirm the impact of heavy pre-treatment on the occurrence of
serious and fatal adverse events in patients treated with
bevacizumab in daily practice. Notably, the present study
demonstrated that a medical history of hypertension is an
independent predictive risk factor for the development of
high-grade hypertension during bevacizumab treatment. The current
findings confirm the feasibility and toxic acceptability of the use
of bevacizumab for treating relapsed ovarian cancer patients.
Although these results are of importance and contribute to improved
understanding of the management of adverse events attributable to
the use of bevacizumab in ovarian cancer, more studies in this
field are required. In particular, studies aimed at characterizing
and applying biomarkers that could contribute to safer
administration of bevacizumab, through the identification of
patients with ovarian cancer most likely to benefit from the
treatment, should be performed. Thus, retrospective analysis of
patient cohorts may be of interest to validate such biomarkers and
to determine whether they can be applied in clinical trials.
Acknowledgements
Third-party writing assistance for this manuscript
was supported by Roche France and was performed by Lee Miller of
Miller Medical Communications.
References
|
1
|
National Comprehensive Cancer Network:
Clinical Practice Guidelines in Oncology - Ovarian Cancer version
3. 2014.http://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdfAccessed.
September 01–2014
|
|
2
|
McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piccart NJ, Bertelsen K, James K, Cassidy
J, Mangioni C, Simonsen E, Stuart G, Kaye S, Vergote I, Blom R, et
al: Randomized intergroup trial of cisplatin-paclitaxel versus
cisplatin-cyclophosphamide in women with advance epithelial ovarian
cancer: Three-year results. J Natl Cancer Inst. 92:699–708. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
du Bois A, Lück HJ, Meier W, Adams HP,
Möbus V, Costa S, Bauknecht T, Richter B, Warm M, Schröder W, et
al: A randomized clinical trial of cisplatin/paclitaxel versus
carboplatin/paclitaxel as first-line treatment of ovarian cancer. J
Natl Cancer Inst. 95:1320–1329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R: Gynecologic Oncology Group: Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
gynecologic oncology group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Markman M, Rothman R, Hakes T, Reichman B,
Hoskins W, Rubin S, Jones W, Almadrones L and Lewis JL Jr:
Second-line platinum therapy in patients with ovarian cancer
previously treated with cisplatin. J Clin Oncol. 9:389–393.
1991.PubMed/NCBI
|
|
7
|
Eisenhauer EA, Vermorken JB and van
Glabbeke M: Predictors of response to subsequent chemotherapy in
platinum pretreated ovarian cancer: A multivariate analysis of 704
patients [seecomments]. Ann Oncol. 8:963–968. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferrero JM, Weber B, Geay JF, Lepille D,
Orfeuvre H, Combe M, Mayer F, Leduc B, Bourgeois H, Paraiso D and
Pujade-Lauraine E: Second-line chemotherapy with pegylated
liposomal doxorubicin and carboplatin is highly effective in
patients with advanced ovarian cancer in late relapse: A GINECO
phase II trial. Ann Oncol. 18:263–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sawyers C: Targeted cancer therapy.
Nature. 432:294–297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamamoto S, Konishi I, Mandai M, Kuroda H,
Komatsu T, Nanbu K, Sakahara H and Mori T: Expression of vascular
endothelial growth factor (VEGF) in epithelial ovarian neoplasms:
Correlation with clinicopathology and patient survival and analysis
of serum VEGF levels. Br J Cancer. 76:1221–1227. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cooper BC, Ritchie JM, Broghammer CL,
Coffin J, Sorosky JI, Buller RE, Hendrix MJ and Sood AK:
Preoperative serum vascular endothelial growth factor levels:
Significance in ovarian cancer. Clin Cancer Res. 8:3193–3197.
2002.PubMed/NCBI
|
|
13
|
Hefler LA, Zeillinger R, Grimm C, Sood AK,
Cheng WF, Gadducci A, Tempfer CB and Reinthaller A: Preoperative
serum vascular endothelial growth factor as a prognostic parameter
in ovarian cancer. Gynecol Oncol. 103:512–517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gerber HP and Ferrara N: Pharmacology and
pharmacodynamics of bevacizumab as monotherapy or in combination
with cytotoxic therapy in preclinical studies. Cancer Res.
65:671–680. 2005.PubMed/NCBI
|
|
15
|
Smolle E, Taucher V and Haybaeck J:
Malignant ascites in ovarian cancer and the role of targeted
therapeutics. Anticancer Res. 34:1553–1561. 2014.PubMed/NCBI
|
|
16
|
Burger RA, Sill MW, Monk BJ, Greer BE and
Sorosky JI: Phase II trial of bevacizumab in persistent or
recurrent epithelial ovarian cancer or primary peritoneal cancer: A
gynecologic oncology group study. J Clin Oncol. 25:5165–5171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cannistra SA, Matulonis UA, Penson RT,
Hambleton J, Dupont J, Mackey H, Douglas J, Burger RA, Armstrong D,
Wenham R and McGuire W: Phase II study of bevacizumab in patients
with platinum-resistant ovarian cancer or peritoneal serous cancer.
J Clin Oncol. 25:5180–5186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garcia AA, Hirte H, Fleming G, Yang D,
Tsao-Wei DD, Roman L, Groshen S, Swenson S, Markland F, Gandara D,
et al: Phase II clinical trial of bevacizumab and low-dose
metronomic oral cyclophosphamide in recurrent ovarian cancer: A
trial of the California, Chicago and princess Margaret hospital
phase II consortia. J Clin Oncol. 26:76–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nimeiri HS, Oza AM, Morgan RJ, Friberg G,
Kasza K, Faoro L, Salgia R, Stadler WM, Vokes EE and Fleming GF:
Chicago Phase II Consortium; PMH Phase II Consortium; California
Phase II Consortium: Efficacy and safety of bevacizumab plus
erlotinib for patients with recurrent ovarian, primary peritoneal
and fallopian tube cancer: A trial of the Chicago, PMH and
California phase II consortia. Gynecol Oncol. 110:49–55. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McGonigle KF, Muntz HG, Viky J, Paley PJ,
Veljovich DS, Greer BE, Goff BA, Gray HJ and Malpass TW: Combined
weekly topotecan and biweekly bevacizumab in women with
platinum-resistant ovarian, peritoneal, or fallopian tube cancer:
Results of a phase 2 Study. Cancer. 117:3731–3740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aghajanian C, Blanck SV, Goff BA, Judson
PL, Teneriello MG, Husain A, Sovak MA, Yi J and Nycum LR: OCEANS: A
randomized, double-blind, placebo-controlled phase III trial of
chemotherapy with or without bevacizumab in patients with
platinum-sensitive recurrent epithelial ovarian, primary
peritoneal, or fallopian tube cancer. J Clin Oncol. 30:2039–2045.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohn DE, Valmadre S, Resnick KE, Eaton LA,
Copeland LJ and Fowler JM: Bevacizumab and weekly taxane
chemotherapy demonstrates activity in refractory ovarian cancer.
Gynecol Oncol. 102:134–139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Monk BJ, Han E, Josephs-Cowan CA, Pugmire
G and Burger RA: Salvage bevacizumab (rhuMAB VEGF)-based therapy
after multiple prior cytotoxic regimens in advanced refractory
epithelial ovarian cancer. Gynecol Oncol. 102:140–144. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wright JD, Hagemann A, Rader JS, Viviano
D, Gibb RK, Norris L, Mutch DG and Powell MA: Bevacizumab
combination therapy in recurrent, platinum-refractory, epithelial
ovarian carcinoma: A retrospective analysis. Cancer. 107:83–89.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Richardson DL, Backes FJ, Seamon LG,
Zanagnolo V, O'Malley DM, Cohn DE, Fowler JM and Copeland LJ:
Combination gemcitabine, platinum and bevacizumab for the treatment
of recurrent ovarian cancer. Gynecol Oncol. 111:461–466. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wright JD, Secord AA, Numnum TM, Rocconi
RP, Powell MA, Berchuck A, Alvarez RD, Gibb RK, Trinkaus K, Rader
JS and Mutch DG: A multi-institutional evaluation of factors
predictive of toxicity and efficacy of bevacizumab for recurrent
ovarian cancer. Int J Gynecol Cancer. 18:400–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hurt JD, Richardson DL, Seamon LG, Fowler
JF, Copeland LJ, Cohn DE, Eisenhauer E, Salani R and O'Malley DM:
Sustained progression-free survival with weekly paclitaxel and
bevacizumab in recurrent ovarian cancer. Gynecol Oncol.
115:396–400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peitzner K, Richter R, Chekerov R, Erol E,
Oskay-Özcelik G, Lichtenegger W and Sehouli J: Bevacizumab in
heavily pre-treated and platinum resistant ovarian cancer: A
retrospective study of the North-Eastern German society of
gynaecologic oncology (NOGGO) ovarian cancer study group.
Anticancer Res. 31:2679–2682. 2011.PubMed/NCBI
|
|
30
|
Asmane I, Kurtz JE, Bajard A, Guastalla
JP, Meeus P, Tredan O, Galy Labidi I, Moullet I, Ardisson P,
Vincent L, et al: Bevacizumab plus microtubule targeting agents in
heavily pre-treated ovarian cancer patients: A retrospective study.
Bull Cancer. 98:80–89. 2011.PubMed/NCBI
|
|
31
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events v3.0 (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdfAccessed.
September 01–2014
|
|
32
|
Rustin GJ, Quinn M, Thigpen T, du Bois A,
Pujade-Lauraine E, Jakobsen A, Eisenhauer E, Sagae S, Greven K,
Vergote I, et al: Re: New guidelines to evaluate the response to
treatment in solid tumors (ovarian cancer). J Natl Cancer Inst.
96:487–488. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schag CC, Heinrich RL and Ganz PA:
Karnofsky performance status revisited: Reliability, validity, and
guidelines. J Clin Oncol. 2:187–193. 1984.PubMed/NCBI
|
|
35
|
Zhu X, Wu S, Dahut WL and Parikh CR: Risks
of proteinuria and hypertension with bevacizumab, an antibody
against vascular endothelial growth factor: Systematic review and
meta-analysis. Am J Kidney Dis. 49:186–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hapani S, Chu D and Wu S: Risk of
gastrointestinal perforation in patients with cancer treated with
bevacizumab: A meta-analysis. Lancet Oncol. 10:559–568. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ranpura V, Hapani S and Wu S:
Treatment-related mortality with bevacizumab in cancer patients: A
meta-analysis. JAMA. 305:487–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Jesus-Gonzalez N, Robinson E, Moslehi J
and Humphreys BD: Management of antiangiogenic therapy-induced
hypertension. Hypertension. 60:607–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al: Incorporation of bevacizumab in the primary treatment of
ovarian cancer. N Engl J Med. 365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ranpura V, Pulipati B, Chu D, Zhu X and Wu
S: Increased risk of high-grade hypertension with bevacizumab in
cancer patients: A meta-analysis. Am J Hypertens. 23:460–468. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Randall LM and Monk BJ: Bevacizumab
toxicities and their management in ovarian cancer. Gynecol Oncol.
117:497–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qi WX, Shen Z, Tang LN and Yao Y:
Bevacizumab increases the risk of gastrointestinal perforation in
cancer patients: A meta-analysis with a focus on different
subgroups. Eur J Clin Pharmacol. 70:893–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pujade-Lauraine E, Hilpert F and Weber B:
AURELIA: A randomized phase III trial evaluating bevacizumab (BEV)
plus chemotherapy (CT) for platinum (PT)-resistant recurrent
ovarian cancer (OC). J Clin Oncol. 30:(suppl; abstr LBA5002).
2012.PubMed/NCBI
|
|
44
|
Wright JD, Secord AA, Numnum TM, Rocconi
RP, Powell MA, Berchuck A, Alvarez RD, Gibb RK, Trinkaus K, Rader
JS and Mutch DG: A multi-institutional evaluation of factors
predictive of toxicity and efficacy of bevacizumab for recurrent
ovarian cancer. Int J Gynecol Cancer. 18:400–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Randall LM and Monk BJ: Bevacizumab
toxicities and their management in ovarian cancer. Gynecol Oncol.
117:497–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tanyi JL, McCann G, Hagemann AR, Coukos G,
Rubin SC, Liao JB and Chu CS: Clinical predictors of
bevacizumab-associated gastrointestinal perforation. Gynecol Oncol.
120:464–469. 2011. View Article : Google Scholar : PubMed/NCBI
|